Extensional Rheology of Poly(vinylidene fluoride)/N,N-dimethylformamide Solutions
Abstract
:1. Introduction
2. Theoretical Background
2.1. Extensional Viscosity
2.2. Shear Viscosity
2.3. Trouton Ratio
3. Solution Preparation and Experiments
3.1. Solution Preparation
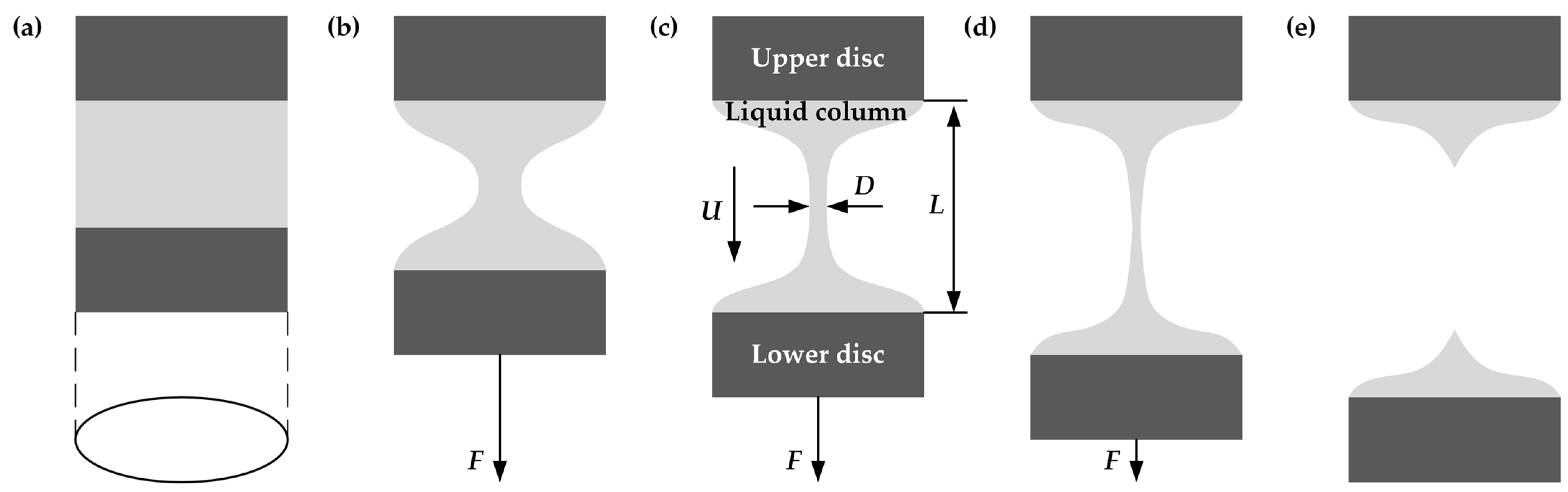
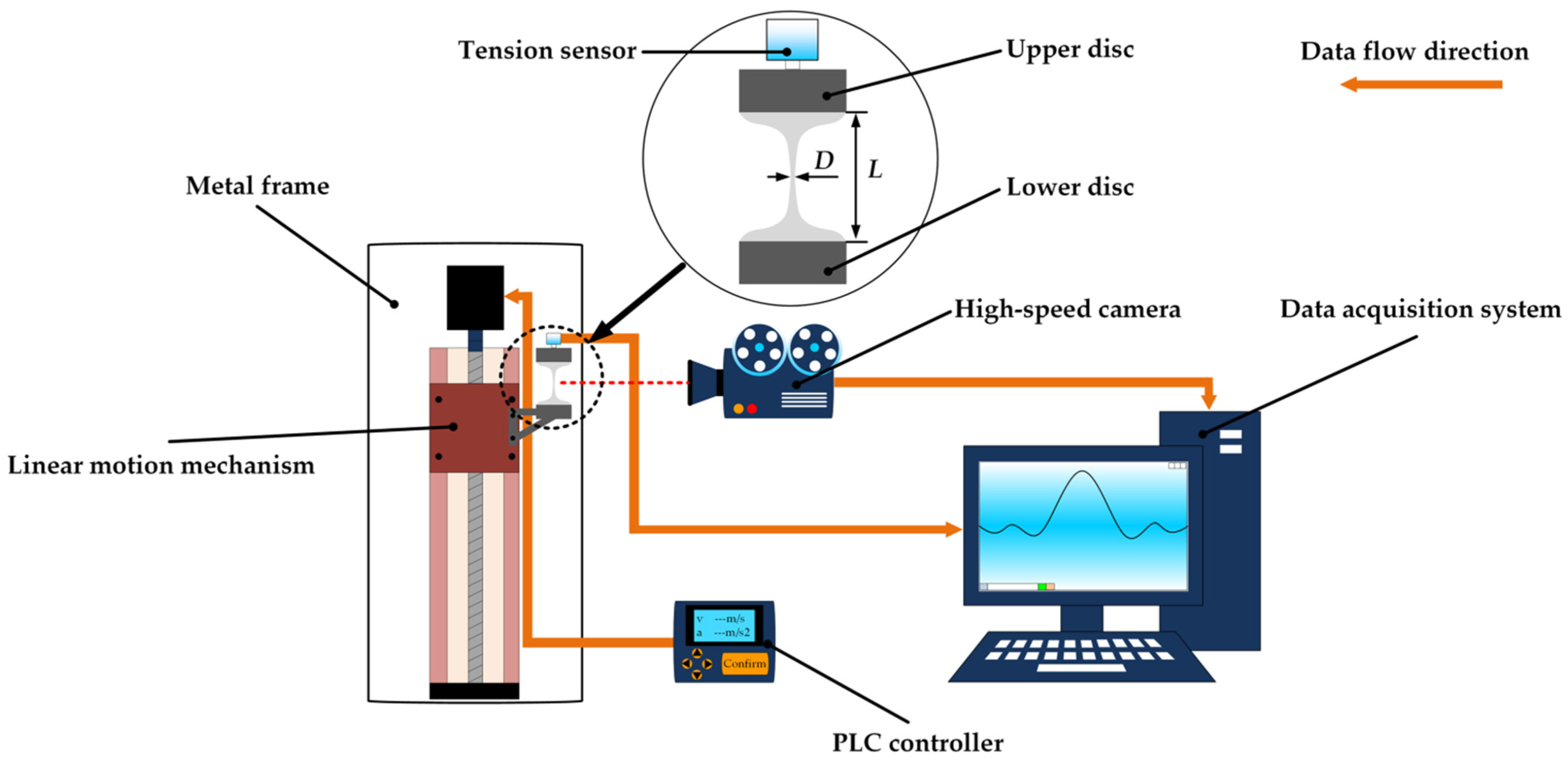
3.2. Experimental Methods
4. Results and Discussion
4.1. Tensile Evolution of PVDF/DMF Solutions
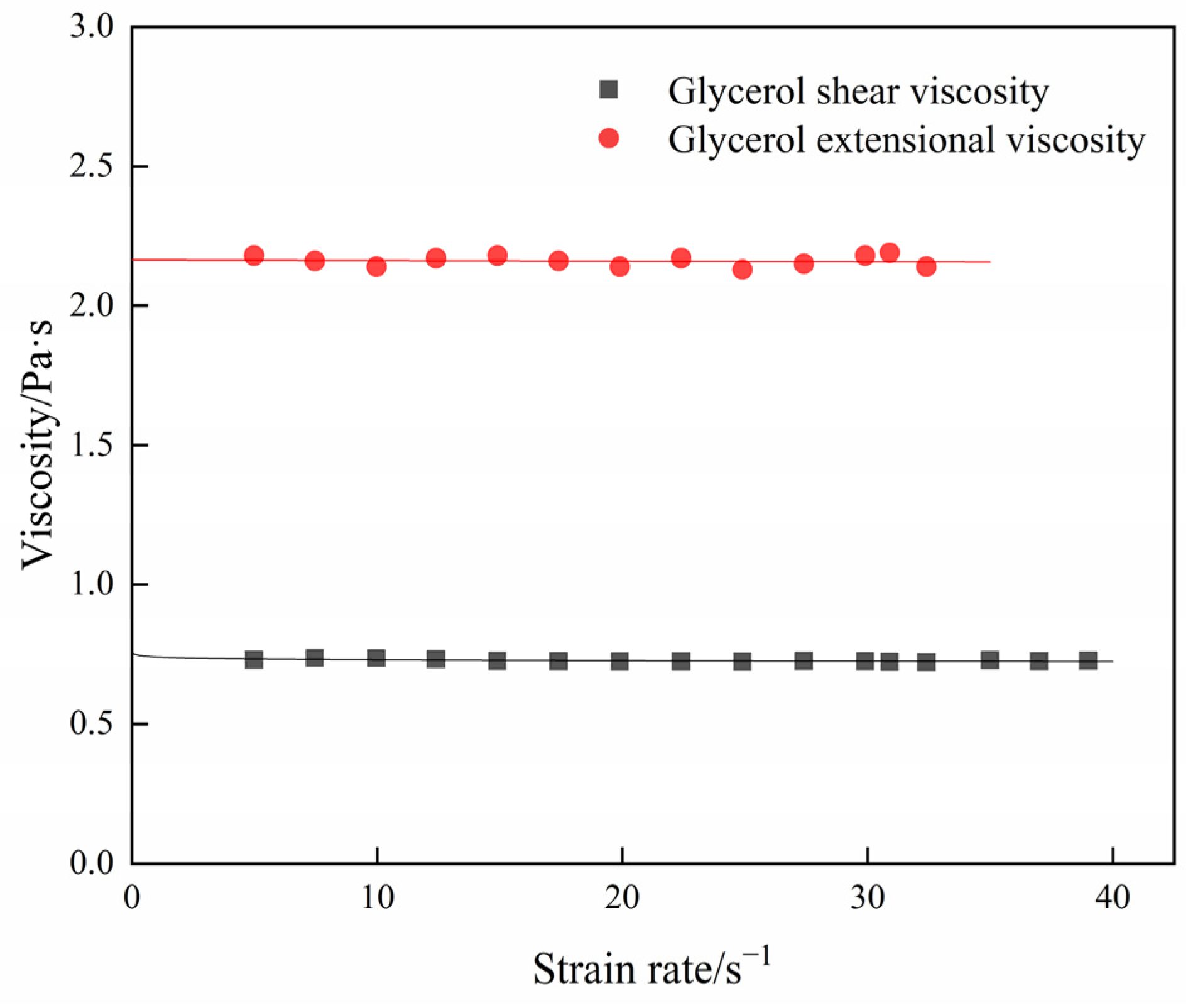
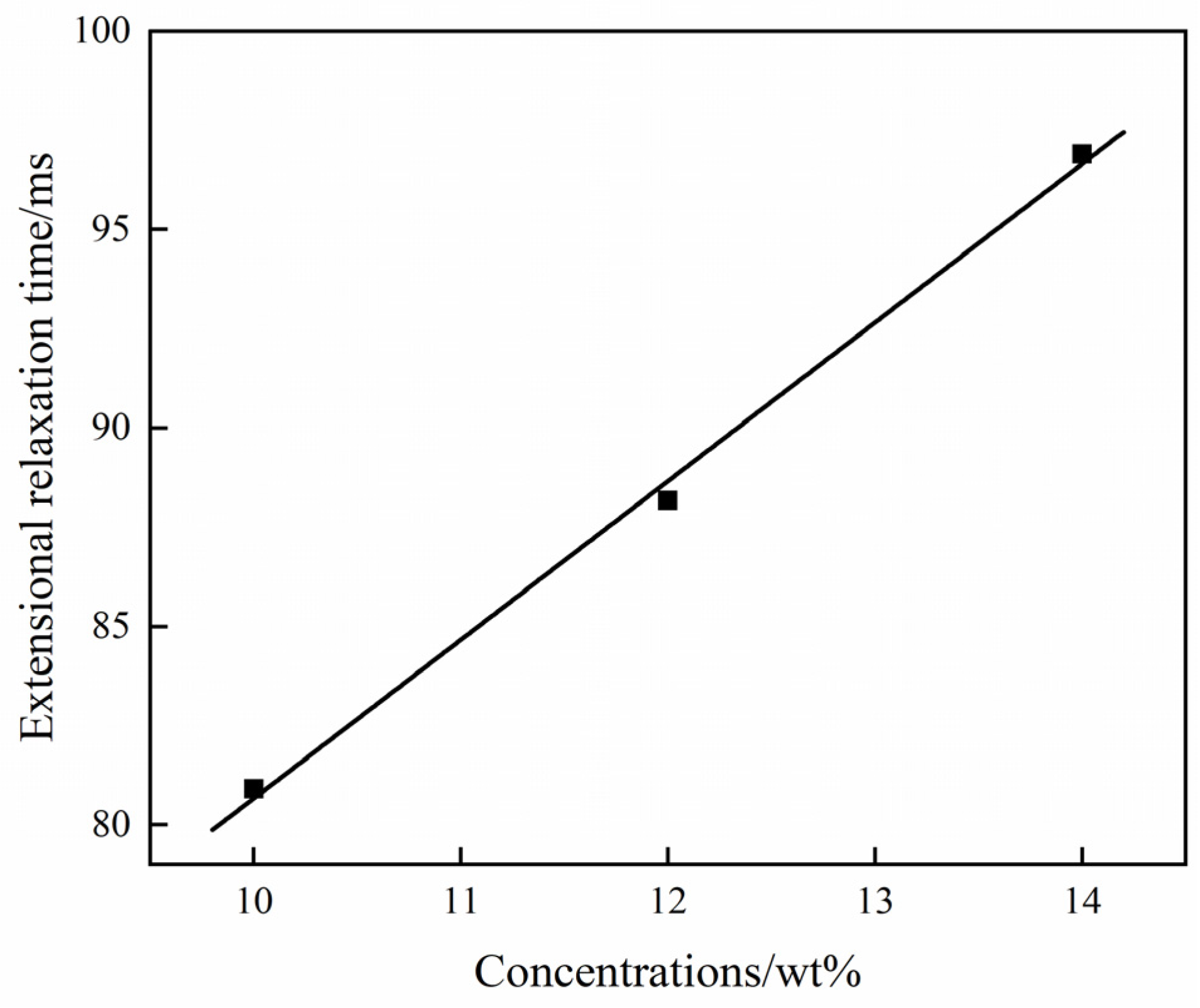
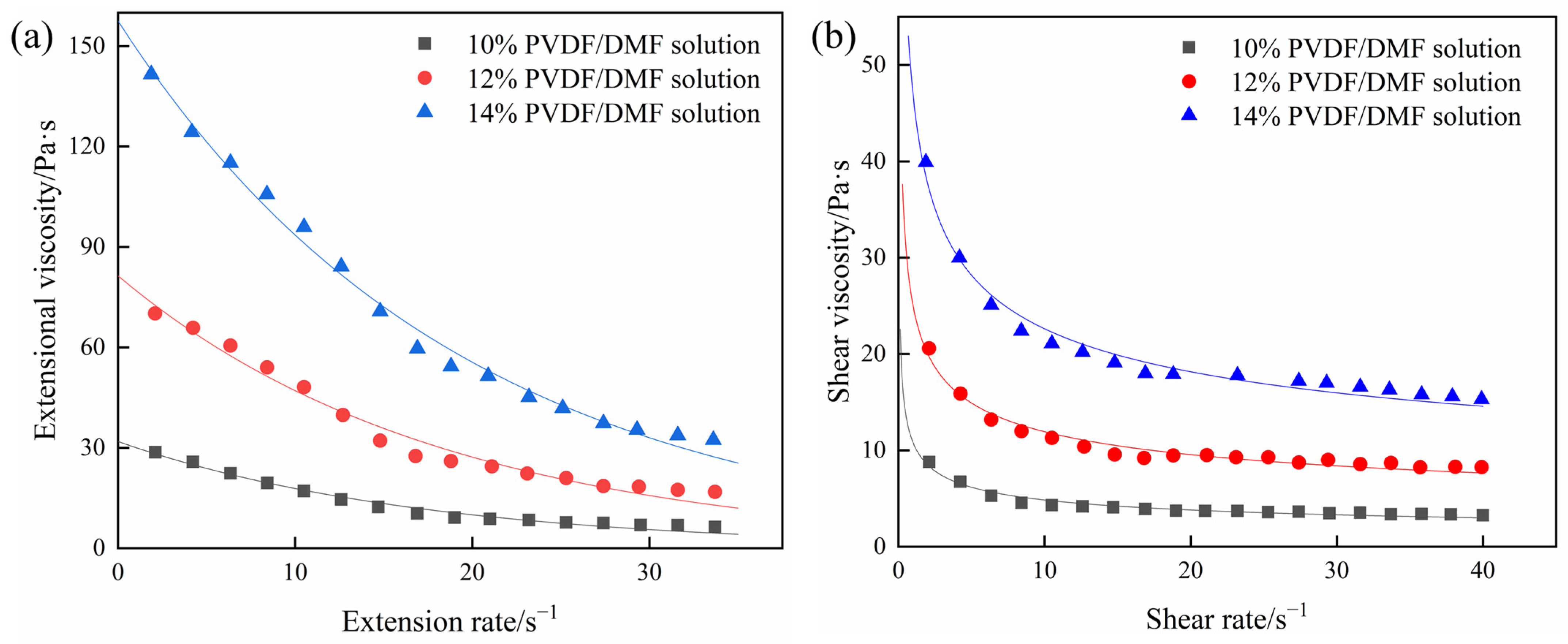
4.2. Extensional Viscosity and Shear Viscosity of PVDF/DMF Solution
4.3. Trouton Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chola, N.M.; Sreenath, S.; Dave, B. A non-gassing electroosmotic pump with sandwich of poly (2-ethyl aniline)-Prussian blue nanocomposite and PVDF membrane. Electrophoresis 2019, 40, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.U.; Ismail, M.C.; Nosbi, N. Permeation damage of polymer liner in oil and gas pipelines: A review. Polymers 2020, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Li, B.; Guo, Z.; Feng, Y. A multi-functional photothermal-catalytic foam for cascade treatment of saline wastewater. J. Mater. Chem. A 2021, 9, 16510–16521. [Google Scholar] [CrossRef]
- Goldbach, J.T.; Amin-Sanayei, R.; He, W. Commercial synthesis and applications of poly(vinylidene fluoride). In Fluorinated Polymers, 2nd ed.; Ameduri, B., Sawada, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; Volume 2, pp. 127–157. [Google Scholar]
- Gardiner, J. Fluoropolymers: Origin, production, and industrial and commercial applications. Aust. J. Chem. 2014, 68, 13–22. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, H.; Deng, H. “Toolbox” for the processing of functional polymer composites. Nanomicro. Lett. 2022, 14, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Dominick, V.; Rosato, P.E. Plastics Processing Data Handbook, 2nd ed.; Chapman & Hall: Chatham, MA, USA, 2012; pp. 1–119. [Google Scholar]
- Liang, Z.J. Elongation Rheology of Polymer Fluids; South China University of Technology Press: Guangzhou, China, 2015; pp. 1–228. [Google Scholar]
- Sadeghi, F.; Tabatabaei, S.; Ajji, A. Effect of PVDF characteristics on extruded film morphology and porous membranes feasibility by stretching. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1219–1229. [Google Scholar] [CrossRef]
- Hedhli, L.; Mekhilef, N.; Moyses, S. Characterization of randomly branched poly(vinylidene fluoride). Macromolecules 2008, 41, 2011–2021. [Google Scholar] [CrossRef]
- Guo, Z.; Nilsson, E.; Rigdahl, M. Melt spinning of PVDF fibers with enhanced β phase structure. J. Appl. Polym. Sci. 2013, 130, 2603–2609. [Google Scholar] [CrossRef]
- Bairagi, S.; Ali, S.W. A unique piezoelectric nanogenerator composed of melt-spun PVDF/KNN nanorod-based nanocomposite fibre. Eur. Polym. J. 2019, 116, 554–561. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M. Electrospun PVDF nanofibers for piezoelectric applications: A review of the influence of electrospinning parameters on the β phase and crystallinity enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Cai, X. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Yao, X.; Chang, Y. Properties and applications of the β phase poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Kumarasinghe, H.; Bandara, L.; Bandara, T. Fabrication of β-phase poly(vinylidene fluoride) piezoelectric film by electrospinning for nanogenerator preparations. Ceylon. J. Sci. 2021, 50, 357–363. [Google Scholar] [CrossRef]
- Bera, B.; Sarkar, M.D. PVDF based Piezoelectric Nanogenerator as a new kind of device for generating power from renewable resources. IOSR-JPTE 2017, 4, 01–05. [Google Scholar] [CrossRef]
- Gao, K.; Hu, X.; Dai, C. Crystal structures of electrospun PVDF membranes and its separator application for rechargeable lithium metal cells. Mater. Sci. Eng. B-Adv. 2006, 131, 100–105. [Google Scholar] [CrossRef]
- Yang, C.; Jia, Z.; Guan, Z. Polyvinylidene fluoride membrane by novel electrospinning system for separator of Li-ion batteries. J. Power Sources 2009, 189, 716–720. [Google Scholar] [CrossRef]
- Liu, K.; Deng, L.; Zhang, T. Facile fabrication of environmentally friendly, waterproof, and breathable nanofibrous membranes with high UV-resistant performance by one-step electrospinning. Ind. Eng. Chem. Res. 2020, 59, 4447–4458. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, M.; Shen, J. All-fiber structured electronic skin with high elasticity and breathability. Adv. Funct. Mater. 2020, 30, 1908411. [Google Scholar] [CrossRef]
- Xin, Y.; Zhu, J.; Sun, H. A brief review on piezoelectric PVDF nanofibers prepared by electrospinning. Ferroelectrics 2018, 526, 140–151. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Yin, G. Tactile-sensing based on flexible PVDF nanofibers via electrospinning: A review. Sensors 2018, 18, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Zhou, Z.; Zhang, Z. Porous, multi-layered piezoelectric composites based on highly oriented PZT/PVDF electrospinning fibers for high-performance piezoelectric nanogenerators. J. Adv. Ceram. 2022, 11, 331–344. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Lu, Y. PDMS/PVDF Electrospinning Membranes for Water-in-Oil Emulsion Separation and UV Protection. Biomimetics 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.Y.; Chen, S.; He, W. Fabrication of high-performance graphene oxide doped PVDF/CuO/Al nanocomposites via electrospinning. Chem. Eng. J. 2019, 368, 129–137. [Google Scholar] [CrossRef]
- Ng, H.C.H.; Corker, A.; García-Tuñón, E. GO CaBER: Capillary breakup and steady-shear experiments on aqueous graphene oxide (GO) suspensions. J. Rheol. 2020, 64, 81–93. [Google Scholar] [CrossRef]
- Yuan, B.; Ritzoulis, C.; Chen, J. Extensional and shear rheology of a food hydrocolloid. Food. Hydrocoll. 2018, 74, 296–306. [Google Scholar] [CrossRef]
- Ochowiak, M.; Broniarz-Press, L.; Rozanska, S. The effect of extensional viscosity on the effervescent atomization of polyacrylamide solutions. J. Ind. Eng. Chem. 2012, 18, 2028–2035. [Google Scholar] [CrossRef]
- Li, B.; Yu, W.; Cao, X. Horizontal extensional rheometry (HER) for low viscosity polymer melts. J. Rheol. 2020, 64, 177–190. [Google Scholar] [CrossRef]
- Hodder, P.; Franck, A. A new tool for measuring extensional viscosity. Ann. Trans. Nord. Rheol. Soc. 2005, 13, 227–232. [Google Scholar]
- Sentmanat, M.L. Miniature universal testing platform: From extensional melt rheology to solid-state deformation behavior. Rheol. Acta 2004, 43, 657–669. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; Sridhar, T. A filament stretching device for measurement of extensional viscosity. J. Rheol. 1993, 37, 1081–1102. [Google Scholar] [CrossRef]
- Luger, H.J.; Miethlinger, J. Development of an online rheometer for simultaneous measurement of shear and extensional viscosity during the polymer extrusion process. Polym. Test. 2019, 77, 105914. [Google Scholar] [CrossRef]
- Jones, D.; Walters, K.; Williams, P.R. On the extensional viscosity of mobile polymer solutions. Rheol. Acta 1987, 26, 20–30. [Google Scholar] [CrossRef]
- Różańska, S.; Różański, J. Extensional flow of carboxymethylcellulose sodium salt measured on the opposed-nozzle device. Soft Mater. 2017, 15, 302–314. [Google Scholar] [CrossRef]
- Coquand, O.; Sperl, M. Rheology of granular liquids in extensional flows: Beyond the μ (I)-law. Phys. Rev. E 2021, 104, 014604. [Google Scholar] [CrossRef]
- Haward, S.J. Characterization of hyaluronic acid and synovial fluid in stagnation point elongational flow. Biopolymers 2014, 101, 287–305. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Turdakyn, N.; Abay, I. A review of piezoelectric PVDF film by electrospinning and its applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef]
- Abbas, I.; Hobiny, A.; Alshehri, H. Analysis of Thermoelastic Interaction in a Polymeric Orthotropic Medium Using the Finite Element Method. Polymers 2022, 14, 2112. [Google Scholar] [CrossRef]
- Dinic, J.; Zhang, Y.; Jimenez, L.N. Extensional relaxation times of dilute, aqueous polymer solutions. ACS Macro Lett. 2015, 4, 804–808. [Google Scholar] [CrossRef]
- Sousa, P.C.; Vega, E.J.; Sousa, R.G. Measurement of relaxation times in extensional flow of weakly viscoelastic polymer solutions. Rheol. Acta 2017, 56, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.G. The Structure and Rheology of Complex Fluids; Oxford University Press: New York, NY, USA, 1999; pp. 137–140. [Google Scholar] [CrossRef]
- Wang, D.P.; Zhao, Z.H.; Li, C.H. Universal Self-Healing Poly (dimethylsiloxane) Polymer Crosslinked Predominantly by Physical Entanglements. ACS Appl. Mater. Inter. 2021, 13, 31129–31139. [Google Scholar] [CrossRef] [PubMed]



| Chemical Name | Abbreviation | Chemical Structure | Purity | CAS Number | Molecular Weight (g/mol) | Supplier |
|---|---|---|---|---|---|---|
| Poly(vinylidene fluoride) | PVDF | 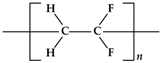 | 99% | 24937-79-9 | 1,100,000 | Arkema |
| N,N-dimethylformamide | DMF | 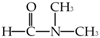 | 99.5% | 68-12-2 | 73.9 | Macklin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Ju, M.; Guo, W.; Yu, S. Extensional Rheology of Poly(vinylidene fluoride)/N,N-dimethylformamide Solutions. Polymers 2023, 15, 1119. https://doi.org/10.3390/polym15051119
Xu L, Ju M, Guo W, Yu S. Extensional Rheology of Poly(vinylidene fluoride)/N,N-dimethylformamide Solutions. Polymers. 2023; 15(5):1119. https://doi.org/10.3390/polym15051119
Chicago/Turabian StyleXu, Lei, Mingxiang Ju, Wentai Guo, and Shengrui Yu. 2023. "Extensional Rheology of Poly(vinylidene fluoride)/N,N-dimethylformamide Solutions" Polymers 15, no. 5: 1119. https://doi.org/10.3390/polym15051119




