Developing Post-Consumer Recycled Flexible Polypropylene and Fumed Silica-Based Nanocomposites with Improved Processability and Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCPP/NS Nanocomposites
2.3. Characterization of the Nanocomposites
2.3.1. Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Rheological Analysis
2.3.5. Melt Flow Index
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.2. Thermal Stability of the Nanocomposites
3.3. Differential Scanning Calorimetry (DSC)
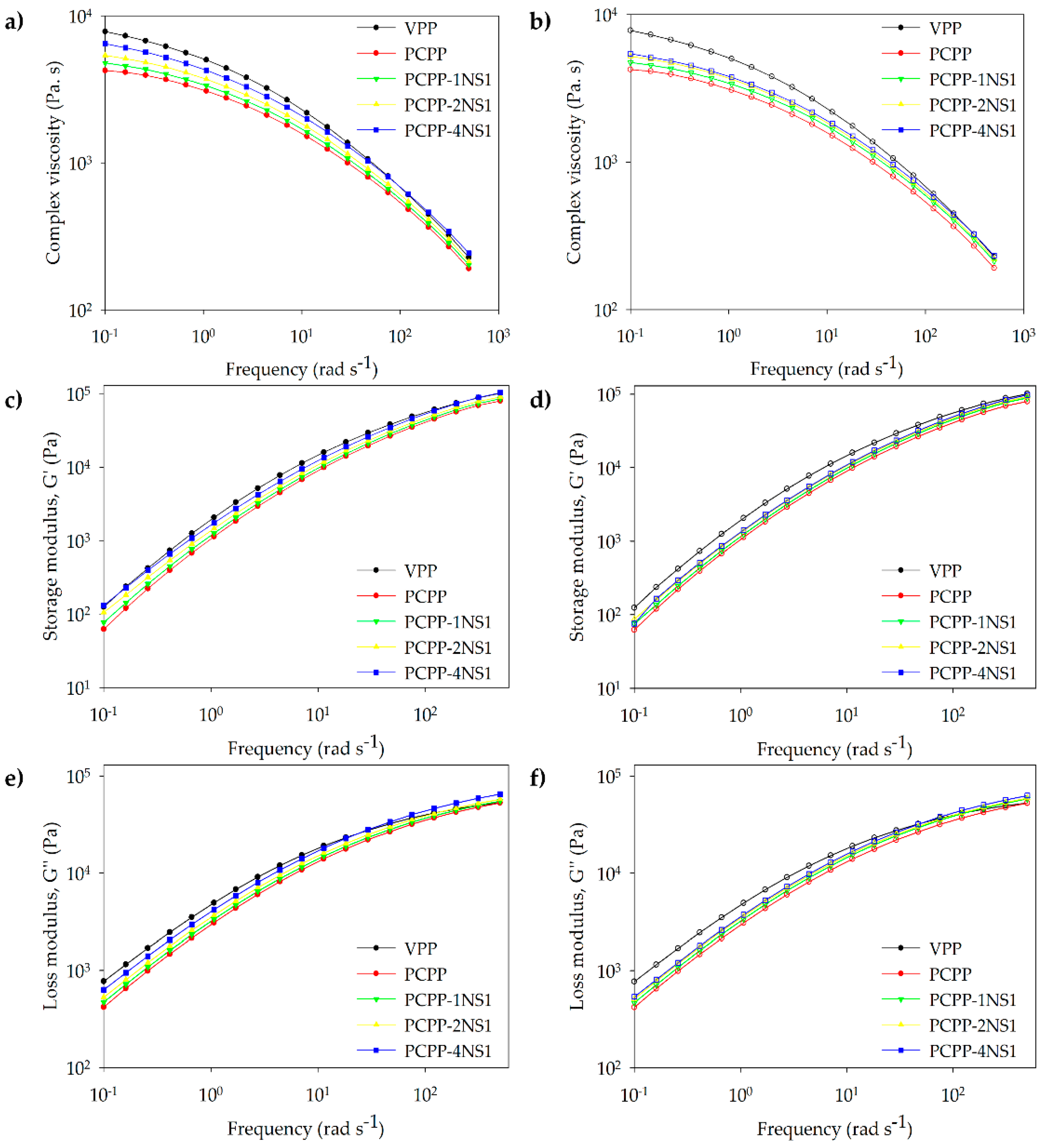
3.4. Rheological Analysis
3.5. Melt Flow Index (MFI)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe Plastics-The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 29 December 2022).
- Calhoun, A. 3-Polypropylene. In Plastics Design Library; Wagner, J.R.B.T.-M.F.P., Second, E., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 35–45. ISBN 978-0-323-37100-1. [Google Scholar]
- Chen, Y.; Yang, H.; Yang, S.; Ren, P.; Zhang, Q.; Li, Z. Polypropylene films with high barrier performance via crystal morphology manipulation. J. Mater. Sci. 2017, 52, 5449–5461. [Google Scholar] [CrossRef]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef] [Green Version]
- Spicker, C.; Rudolph, N.; Kühnert, I.; Aumnate, C. The use of rheological behavior to monitor the processing and service life properties of recycled polypropylene. Food Packag. Shelf Life 2019, 19, 174–183. [Google Scholar] [CrossRef]
- Velásquez, E.; Guerrero Correa, M.; Garrido, L.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Food Packaging Plastics: Identification and Recycling BT. In Recent Developments in Plastic Recycling; Parameswaranpillai, J., Mavinkere Rangappa, S., Gulihonnehalli Rajkumar, A., Siengchin, S., Eds.; Springer: Singapore, 2021; pp. 311–341. ISBN 978-981-16-3627-1. [Google Scholar]
- Phuong, N.T.; Gilbert, V.; Chuong, B. Preparation of Recycled Polypropylene/ Organophilic Modified Layered Silicates Nanocomposites Part I: The Recycling Process of Polypropylene and the Mechanical Properties of Recycled Polypropylene/Organoclay Nanocomposites. J. Reinf. Plast. Compos. 2008, 27, 1983–2000. [Google Scholar] [CrossRef]
- Barbosa, L.G.; Piaia, M.; Ceni, G.H. Analysis of Impact and Tensile Properties of Recycled Polypropylene. Int. J. Mater. Eng. 2017, 7, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, E.; Espinoza, S.; Valenzuela, X.; Garrido, L.; Galotto, M.; Guarda, A.; de Dicastillo, C.L. Effect of Organic Modifier Types on the Physical–Mechanical Properties and Overall Migration of Post-Consumer Polypropylene/Clay Nanocomposites for Food Packaging. Polymers 2021, 13, 1502. [Google Scholar] [CrossRef]
- Jmal, H.; Bahlouli, N.; Wagner-Kocher, C.; Leray, D.; Ruch, F.; Munsch, J.-N.; Nardin, M. Influence of the grade on the variability of the mechanical properties of polypropylene waste. Waste Manag. 2018, 75, 160–173. [Google Scholar] [CrossRef] [Green Version]
- De Dicastillo, C.L.; Velásquez, E.; Rojas, A.; Guarda, A.; Galotto, M.J. The use of nanoadditives within recycled polymers for food packaging: Properties, recyclability, and safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1760–1776. [Google Scholar] [CrossRef]
- Velasquez, E.; Garrido, L.; Valenzuela, X.; Galotto, M.J.; Guarda, A.; de Dicastillo, C.L. Physical properties and safety of 100% post-consumer PET bottle -organoclay nanocomposites towards a circular economy. Sustain. Chem. Pharm. 2020, 17, 100285. [Google Scholar] [CrossRef]
- Velasquez, E.; Garrido, L.; Guarda, A.; Galotto, M.; de Dicastillo, C.L. Increasing the incorporation of recycled PET on polymeric blends through the reinforcement with commercial nanoclays. Appl. Clay Sci. 2019, 180, 105185. [Google Scholar] [CrossRef]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Azinfar, B.; Ramazani, S.A.; Jafariesfad, N. In situ preparation and property investigation of polypropylene/fumed silica nanocomposites. Polym. Compos. 2013, 35, 37–44. [Google Scholar] [CrossRef]
- Titone, V.; Mistretta, M.; Botta, L.; Mantia, F. Investigation on the Properties and on the Photo-Oxidation Behaviour of Polypropylene/Fumed Silica Nanocomposites. Polymers 2021, 13, 2673. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.N.; Vasileiou, A.; Pavlidou, E.; Karayannidis, G.P. Compatibilisation effect of PP-g-MA copolymer on iPP/SiO2 nanocomposites prepared by melt mixing. Eur. Polym. J. 2005, 41, 1965–1978. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A. Reprocessing effects on polypropylene/silica nanocomposites. J. Appl. Polym. Sci. 2013, 131, 40242. [Google Scholar] [CrossRef]
- Fambri, L.; Dabrowska, I.; Ceccato, R.; Pegoretti, A. Effects of Fumed Silica and Draw Ratio on Nanocomposite Polypropylene Fibers. Polymers 2017, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- FDA Code of Federal Regulations Title 21CFR172.480 Food additives permitted for direct addition to food for human consumption. Title 21, Volume 3. Subpart B Anticaking agents 172.480. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-E/section-172.480 (accessed on 28 January 2023).
- Winkler, H.C.; Suter, M.; Naegeli, H. Critical review of the safety assessment of nano-structured silica additives in food. J. Nanobiotechnology 2016, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Pegoretti, A. (Re)processing effects on linear low-density polyethylene/silica nanocomposites. J. Polym. Res. 2013, 20, 92. [Google Scholar] [CrossRef]
- Fambri, L.; Dorigato, A.; Pegoretti, A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Appl. Sci. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Xian, J.; Li, M.; Lin, Z.; Deng, S. Crystallization and thermal behavior of recycled polypropylene composites containing nonmetallic printed circuit board powder and β-nucleating agents. J. Therm. Anal. Calorim. 2017, 130, 869–878. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaaba, N.F.; Ismail, H.; Jaafar, M. Recycled polypropylene/peanut shell powder (RPP/PSP) composites: Property comparison before and after electron beam irradiation. Polym. Compos. 2017, 39, 3048–3056. [Google Scholar] [CrossRef]
- Gall, M.; Freudenthaler, P.; Fischer, J.; Lang, R. Characterization of Composition and Structure–Property Relationships of Commercial Post-Consumer Polyethylene and Polypropylene Recyclates. Polymers 2021, 13, 1574. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El Saeed, A.M.; El-Ghazawy, R.A. Chemical interaction of different sized fumed silica with epoxy via ultrasonication for improved coating. Prog. Org. Coat. 2018, 129, 1–9. [Google Scholar] [CrossRef]
- Zebardastan, N.; Khanmirzaei, M.; Ramesh, S.; Ramesh, K. Novel poly(vinylidene fluoride-co-hexafluoro propylene)/polyethylene oxide based gel polymer electrolyte containing fumed silica (SiO2) nanofiller for high performance dye-sensitized solar cell. Electrochimica Acta 2016, 220, 573–580. [Google Scholar] [CrossRef]
- Bikiaris, D.; Vasileiou, A. Fumed Silica Reinforced Nanocomposites. Chapter 4. In Nanocomposite Coatings and Nanocomposite Materials; Ochsner, A., Ahmed, W., Ali, N., Eds.; Rans Tech Publications Ltd.: Zurich, Switzerland, 2009. [Google Scholar]
- Pakizeh, M.; Moghadam, A.N.; Omidkhah, M.R.; Namvar-Mahboub, M. Preparation and characterization of dimethyldichlorosilane modified SiO2/PSf nanocomposite membrane. Korean J. Chem. Eng. 2013, 30, 751–760. [Google Scholar] [CrossRef]
- Chapa-Rodríguez, R.; Avila-de la Rosa, G.; Pérez, E. Thermal stability and ageing properties of PP–PE film modulated by nano-silica particles: Comparison between dry and moist particles. Polym. Bull. 2021, 78, 3071–3088. [Google Scholar] [CrossRef]
- Sipaut, C.; Ahmad, N.; Adnan, R.; Rahman, I.; Bakar, M.; Ismail, J.; Chee, C. Surface Modification of Fumed Nanosilica with Epoxy Molecule. In Proceedings of the International Conference Bioprocess & Engineering, Kota Kinabalu, Malaysia, 8–10 December 2005. [Google Scholar]
- Dikobe, D.G. Comparative study of the morphology and properties of PP/LLDPE/wood powder and MAPP/LLDPE/wood powder polymer blend composites. Express Polym. Lett. 2010, 4, 729–741. [Google Scholar] [CrossRef]
- de Camargo, R.V.; Saron, C. Mechanical–Chemical Recycling of Low-Density Polyethylene Waste with Polypropylene. J. Polym. Environ. 2019, 28, 794–802. [Google Scholar] [CrossRef]
- Dorigato, A.; D’Amato, M.; Pegoretti, A. Thermo-mechanical properties of high density polyethylene–fumed silica nanocomposites: Effect of filler surface area and treatment. J. Polym. Res. 2012, 19, 9889. [Google Scholar] [CrossRef]
- Gall, M.; Steinbichler, G.; Lang, R.W. Learnings about design from recycling by using post-consumer polypropylene as a core layer in a co-injection molded sandwich structure product. Mater. Des. 2021, 202, 109576. [Google Scholar] [CrossRef]
- de Souza, G.P.M.; dos Anjos, E.G.R.; Montagna, L.S.; Ferro, O.; Passador, F.R. A New Strategy for the Use of Post-Processing Vacuum Bags from Aerospace Supplies: Nucleating Agent to LLDPE Phase in PA6/LLDPE Blends. Recycling 2019, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhou, L.; Wang, X.; He, L.; Yang, X. Effect of Crystallinity of Polyethylene with Different Densities on Breakdown Strength and Conductance Property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.; Borsoi, C.; Júnior, M.A.D.; Catto, A.L. Thermal and thermo-mechanical properties of polypropylene composites using yerba mate residues as reinforcing filler. Ind. Crop. Prod. 2019, 140, 111696. [Google Scholar] [CrossRef]
- Kim, H.; Biswas, J.; Choe, S. Effects of stearic acid coating on zeolite in LDPE, LLDPE, and HDPE composites. Polymer 2006, 47, 3981–3992. [Google Scholar] [CrossRef]
- Garcia, P.S.; Scuracchio, C.H.; Cruz, S.A. Effect of residual contaminants and of different types of extrusion processes on the rheological properties of the post-consumer polypropylene. Polym. Test. 2013, 32, 1237–1243. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.-E.; Vaudreuil, S.; Qaiss, A.E.K.; Bousmina, M.; Fassi-Fehri, O. Mechanical, thermal, and rheological properties of graphene-based polypropylene nanocomposites prepared by melt mixing. Polym. Compos. 2012, 33, 733–744. [Google Scholar] [CrossRef]
- Grala, M.; Bartczak, Z.; Rozanski, A. Morphology, thermal and mechanical properties of polypropylene/SiO2 nanocomposites obtained by reactive blending. J. Polym. Res. 2016, 23, 25. [Google Scholar] [CrossRef]
- Chappell, B.; Pramanik, A.; Basak, A.; Sarker, P.; Prakash, C.; Debnath, S.; Shankar, S. Processing household plastics for recycling—A review. Clean. Mater. 2022, 6, 100158. [Google Scholar] [CrossRef]
- Jana, D.K.; Roy, S.; Dey, P.; Bej, B. Utilization of a linguistic response surface methodology to the business strategy of polypropylene in an Indian petrochemical plant. Clean. Chem. Eng. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Haq, S.; Srivastava, R. Wood Polypropylene (PP) Composites Manufactured by Mango Wood Waste with Virgin or Recycled PP: Mechanical, Morphology, Melt Flow Index and Crystalline Behaviour. J. Polym. Environ. 2016, 25, 640–648. [Google Scholar] [CrossRef]
- Melo, P.M.A.; Macêdo, O.B.; Barbosa, G.P.; Santos, A.S.F.; Silva, L.B. Reuse of Natural Waste to Improve the Thermal Stability, Stiffness, and Toughness of Postconsumer Polypropylene Composites. J. Polym. Environ. 2020, 29, 538–551. [Google Scholar] [CrossRef]

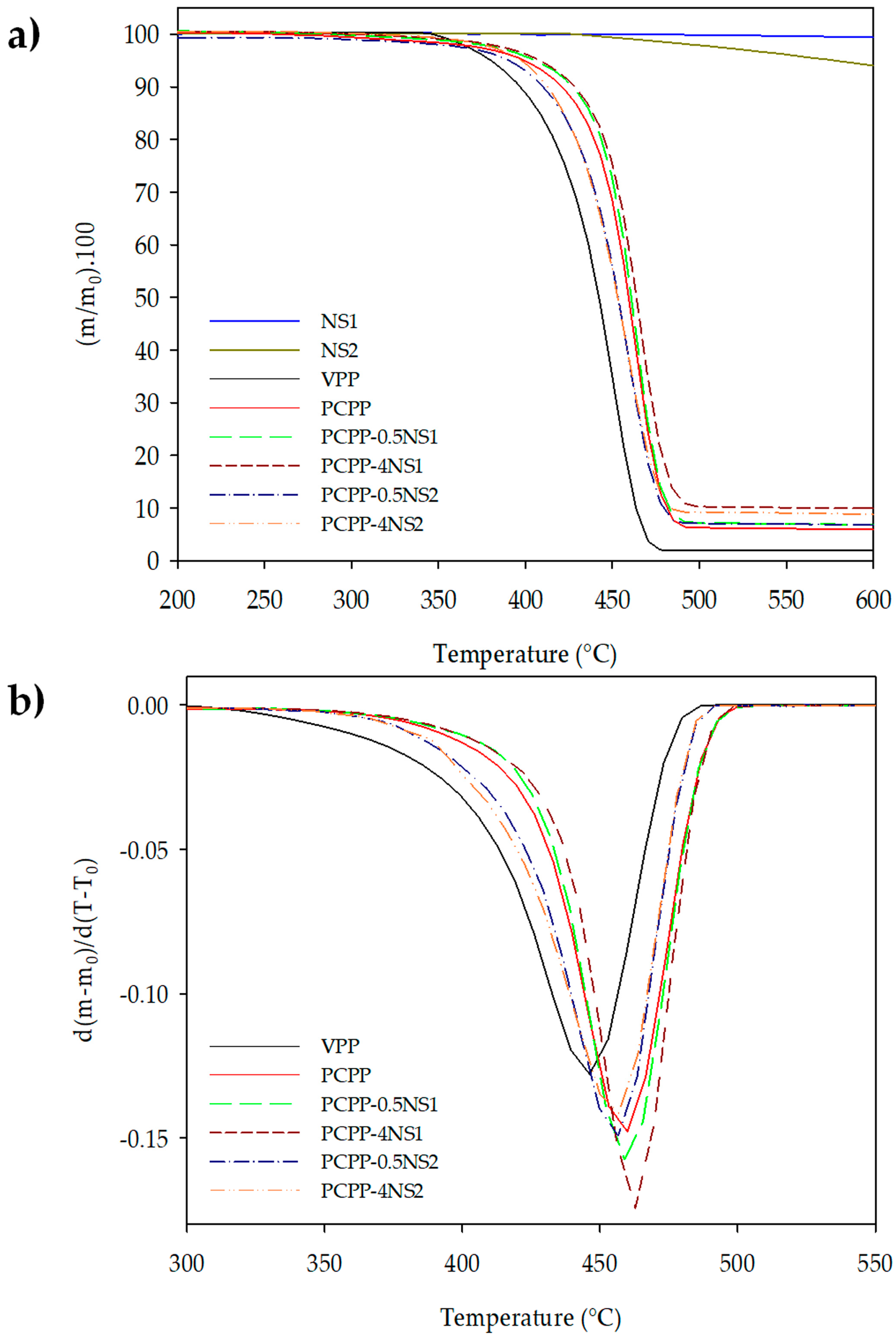


| Film | Tonset (°C) | Td (°C) | Residues at 600 °C (wt%) |
|---|---|---|---|
| VPP | 367.1 | 448.6 | 2.0 |
| PCPP | 372.8 | 461.8 | 5.9 |
| PCPP-0.5NS1 | 382.0 | 462.5 | 6.8 |
| PCPP-1NS1 | 385.5 | 463.9 | 7.2 |
| PCPP-2NS1 | 386.6 | 464.6 | 8.0 |
| PCPP-4NS1 | 387.0 | 465.0 | 9.9 |
| PCPP-0.5NS2 | 363.2 | 457.1 | 6.7 |
| PCPP-1NS2 | 385.4 | 463.5 | 7.4 |
| PCPP-2NS2 | 389.5 | 455.7 | 9.6 |
| PCPP-4NS2 | 380.9 | 456.7 | 8.8 |
| First Heating | ||||||
| Film | Tm1 (°C) | ΔHm1 (J g−1) | Tm2 (°C) | Tm3 (°C) | ΔHm2-3 (J g−1) | Xc (%) |
| VPP | - | - | - | 164.1 ± 1.6 a | 112.4 ± 4.2 c | 54.3 ± 2.1 c |
| PCPP | 124.9 ± 1.4 a | 4.0 ± 0.4 cd | - | 164.3 ± 2.2 a | 74.4 ± 3.2 a | 36.1 ± 1.7 a |
| PCPP-0.5NS1 | 124.2 ± 0.5 a | 2.8 ± 0.1 a | 160.4 ± 0.2 b | 164.4 ± 0.3 a | 80.4 ± 0.3 b | 39.0 ± 0.2 b |
| PCPP-1NS1 | 124.2 ± 0.7 a | 2.9 ± 0.2 a | - | 163.3 ± 0.8 a | 77.3 ± 2.1 ab | 37.7 ± 1.0 ab |
| PCPP-2NS1 | 125.2 ± 0.1 a | 4.4 ± 0.1 d | - | 164.2 ± 1.5 a | 79.3 ± 2.3 ab | 39.1 ± 1.1 b |
| PCPP-4NS1 | 124.3 ± 0.7 a | 4.2 ± 0.3 d | 159.0 ± 1.2 a | 164.2 ± 1.1 a | 77.1 ± 1.8 ab | 38.8 ± 0.9 ab |
| PCPP-0.5NS2 | 125.2 ± 0.4 a | 3.4 ± 0.1 b | - | 164.2 ± 0.1 a | 79.1 ± 2.9 ab | 38.4 ± 1.4 ab |
| PCPP-1NS2 | 125.4 ± 0.5 a | 4.4 ± 0.3 d | - | 164.6 ± 1.5 a | 79.5 ± 3.3 ab | 38.8 ± 1.6 ab |
| PCPP-2NS2 | 125.1 ± 0.1 a | 3.8 ± 0.1 bc | - | 164.3 ± 0.7 a | 77.9 ± 1.4 ab | 38.4 ± 0.7 ab |
| PCPP-4NS2 | 125.0 ± 0.1 a | 4.1 ± 0.1 cd | - | 163.6 ± 1.3 a | 77.1 ± 1.6 ab | 38.8 ± 0.8 ab |
| Cooling | ||||||
| Film | Tc1 (°C) | Tc2 (°C) | ΔHc (J g−1) | |||
| VPP | - | 113.9 ± 0.4 abc | 127.0 ± 11.1 e | |||
| PCPP | 107.5 ± 0.1 abc | 112.8 ± 0.8 a | 103.3 ± 3.1 a | |||
| PCPP-0.5NS1 | 107.3 ± 0.1 ab | 113.8 ± 0.1 ab | 111.4 ± 0.1 abcd | |||
| PCPP-1NS1 | 107.0 ± 0.1 a | 113.5 ± 0.1 ab | 105.8 ± 3.3 ab | |||
| PCPP-2NS1 | 108.1 ± 0.4 c | 115.0 ± 0.5 c | 118.2 ± 1.4 cde | |||
| PCPP-4NS1 | 107.6 ± 0.8 abc | 114.1 ± 1.0 bc | 106.7 ± 1.6 ab | |||
| PCPP-0.5NS2 | 108.2 ± 0.1 c | 114.2 ± 0.1 bc | 114.7 ± 1.3 bcd | |||
| PCPP-1NS2 | 108.2 ± 0.1 c | 114.6 ± 0.5 bc | 119.3 ± 6.6 de | |||
| PCPP-2NS2 | 108.0 ± 0.1 bc | 114.2 ± 0.1 bc | 111.1 ± 1.2 abcd | |||
| PCPP-4NS2 | 108.0 ± 0.1 bc | 114.5 ± 0.1 bc | 109.2 ± 1.7 abc | |||
| Second Heating | ||||||
| Film | Tm1 (°C) | ΔHm1 (J g−1) | Tm2 (°C) | ΔHm2 (J g−1) | Xc (%) | |
| VPP | - | - | 161.5 ± 1.3 b | 111.2 ± 7.3 c | 53.7 ± 3.5 c | |
| PCPP | 123.6 ± 1.3 b | 3.2 ± 0.1 b | 159.6 ± 2.0 a | 73.0 ± 0.6 a | 35.3 ± 0.3 a | |
| PCPP-0.5NS1 | 121.3 ± 2.4 a | 2.8 ± 0.1 a | 159.4 ± 0.3 a | 82.5 ± 0.6 b | 40.1 ± 0.3 b | |
| PCPP-1NS1 | 122.9 ± 0.7 ab | 2.6 ± 0.1 a | 159.1 ± 1.2 a | 79.0 ± 1.7 ab | 38.5 ± 0.8 ab | |
| PCPP-2NS1 | 123.6 ± 0.1 b | 4.3 ± 0.1e | 159.0 ± 0.4 a | 82.3 ± 2.0 b | 40.6 ± 1.0 b | |
| PCPP-4NS1 | 123.3 ± 0.7 ab | 4.1 ± 0.1 de | 159.1 ± 0.3 a | 77.4 ± 0.4 ab | 39.0 ± 0.2 b | |
| PCPP-0.5NS2 | 123.4 ± 0.1 b | 3.6 ± 0.1 bc | 158.7 ± 0.1 a | 77.1 ± 0.8 ab | 37.4 ± 0.4 ab | |
| PCPP-1NS2 | 123.8 ± 0.3 b | 4.1 ± 0.4 de | 159.4 ± 0.2 a | 81.3 ± 6.0 b | 39.7 ± 3.0 b | |
| PCPP-2NS2 | 123.6 ± 0.1 b | 4.2 ± 0.1e | 159.3 ± 0.1 a | 78.9 ± 0.8 ab | 38.9 ± 0.4 b | |
| PCPP-4NS2 | 123.8 ± 0.1 b | 3.8 ± 0.3 cd | 159.8 ± 0.2 ab | 79.4 ± 1.1 ab | 40.0 ± 0.5 b | |
| Sample | ηo (Pa. s) |
|---|---|
| VPP | 7810 |
| PCPP | 4240 |
| PCPP-1NS1 | 4780 |
| PCPP-2NS1 | 5380 |
| PCPP-4NS1 | 6450 |
| PCPP-1NS2 | 4740 |
| PCPP-2NS2 | 5210 |
| PCPP-4NS2 | 5430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, E.; Patiño Vidal, C.; Copello, G.; López de Dicastillo, C.; Pérez, C.J.; Guarda, A.; Galotto, M.J. Developing Post-Consumer Recycled Flexible Polypropylene and Fumed Silica-Based Nanocomposites with Improved Processability and Thermal Stability. Polymers 2023, 15, 1142. https://doi.org/10.3390/polym15051142
Velásquez E, Patiño Vidal C, Copello G, López de Dicastillo C, Pérez CJ, Guarda A, Galotto MJ. Developing Post-Consumer Recycled Flexible Polypropylene and Fumed Silica-Based Nanocomposites with Improved Processability and Thermal Stability. Polymers. 2023; 15(5):1142. https://doi.org/10.3390/polym15051142
Chicago/Turabian StyleVelásquez, Eliezer, Cristian Patiño Vidal, Guillermo Copello, Carol López de Dicastillo, C. J. Pérez, Abel Guarda, and María José Galotto. 2023. "Developing Post-Consumer Recycled Flexible Polypropylene and Fumed Silica-Based Nanocomposites with Improved Processability and Thermal Stability" Polymers 15, no. 5: 1142. https://doi.org/10.3390/polym15051142
APA StyleVelásquez, E., Patiño Vidal, C., Copello, G., López de Dicastillo, C., Pérez, C. J., Guarda, A., & Galotto, M. J. (2023). Developing Post-Consumer Recycled Flexible Polypropylene and Fumed Silica-Based Nanocomposites with Improved Processability and Thermal Stability. Polymers, 15(5), 1142. https://doi.org/10.3390/polym15051142











