Sorption of CO2, CH4 and Their Mixtures in Amorphous Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO)
Abstract
1. Introduction
2. Theoretical Background
2.1. The NETGP-NR Model
2.2. Solution Diffusion Model of Small Molecules in Polymers
3. Materials and Methods
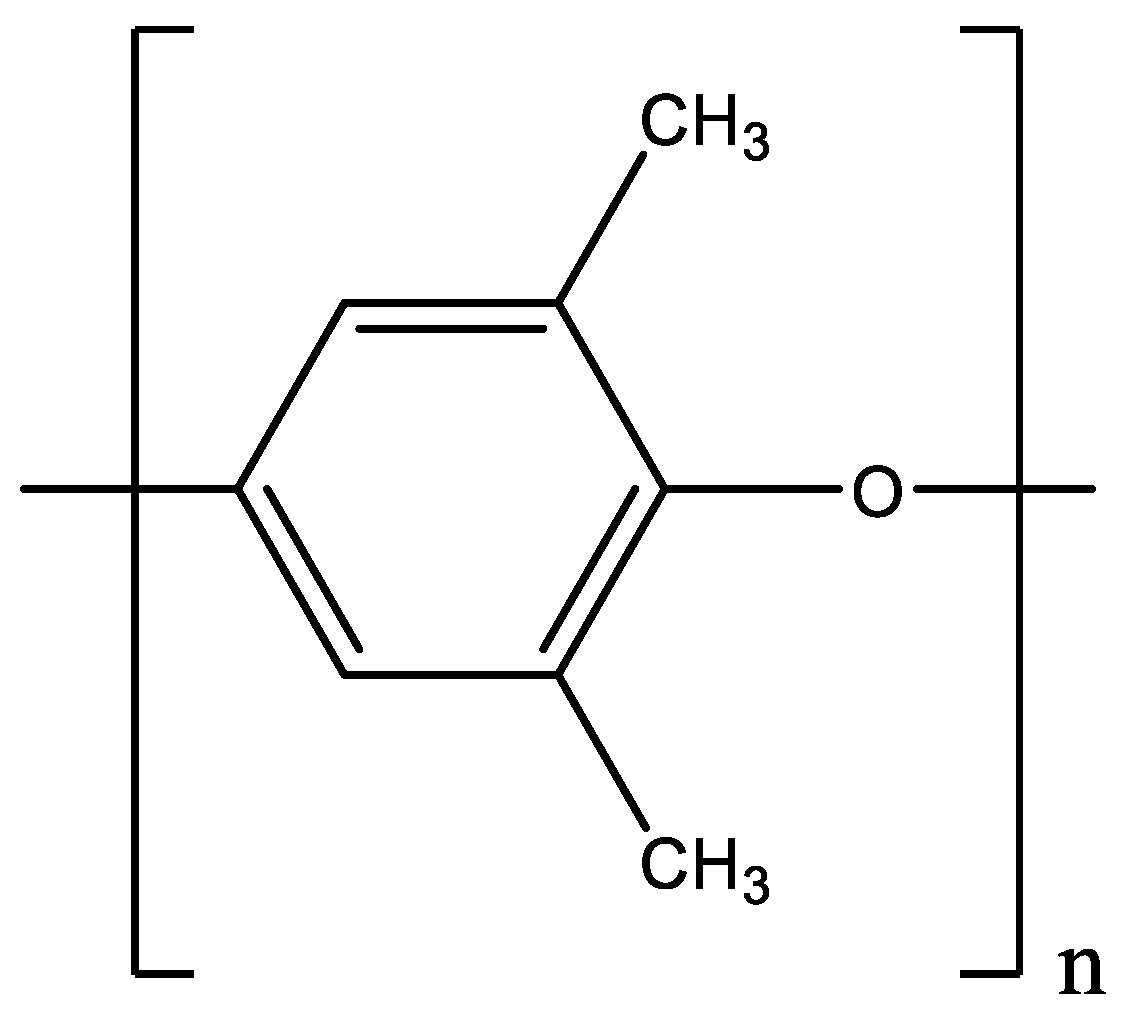
3.1. Materials
3.2. Methods
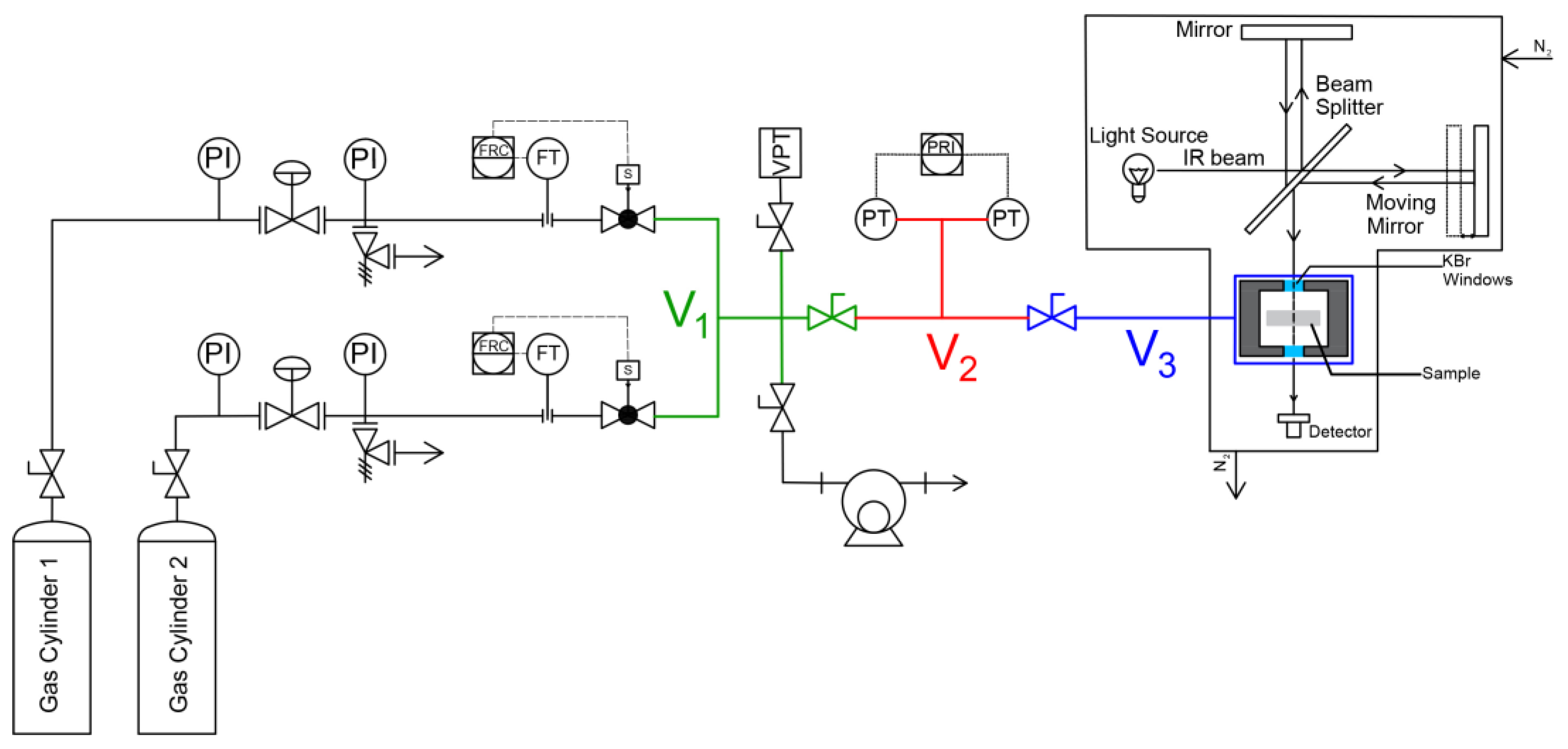
3.2.1. Closed Volume–Variable Pressure Apparatus
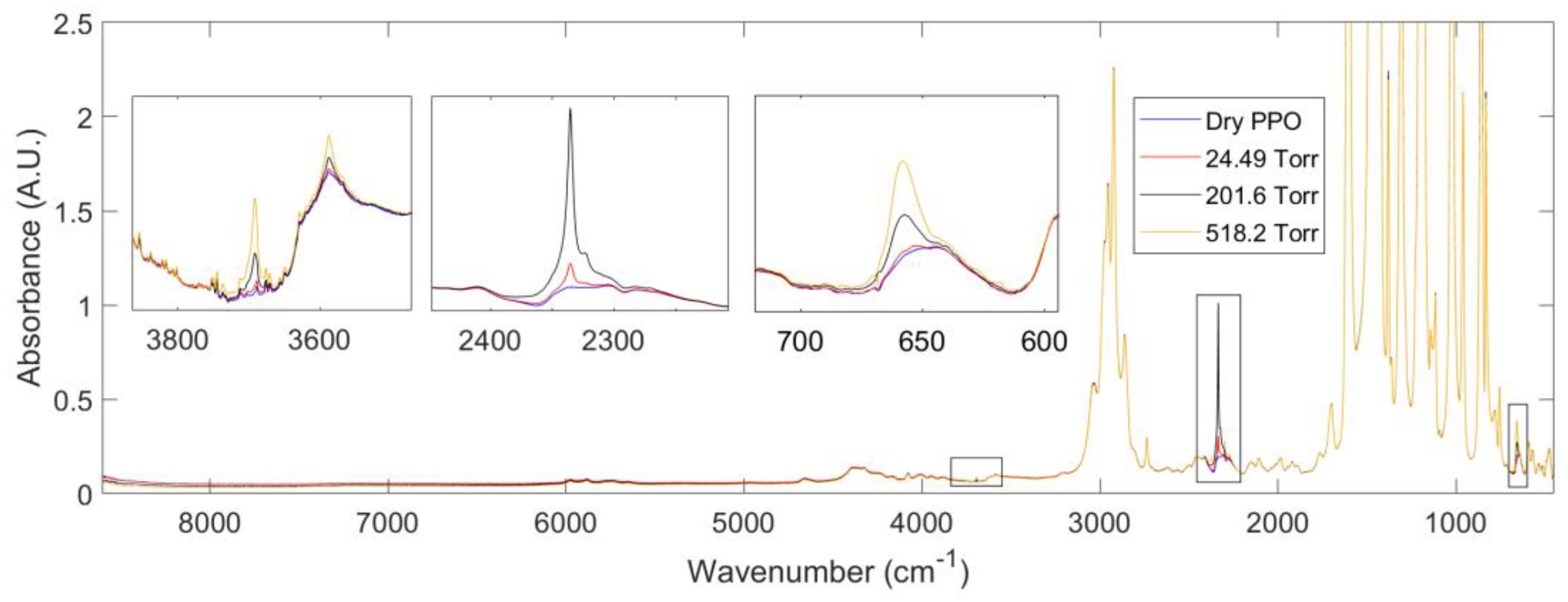
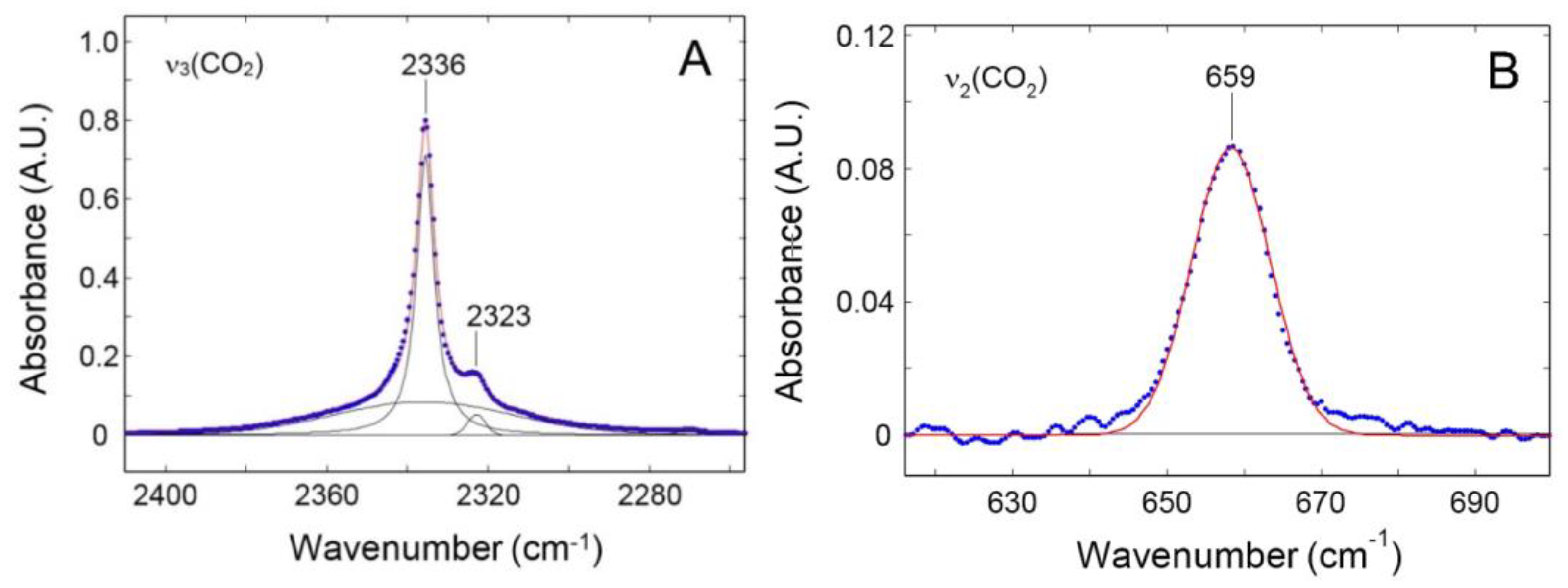
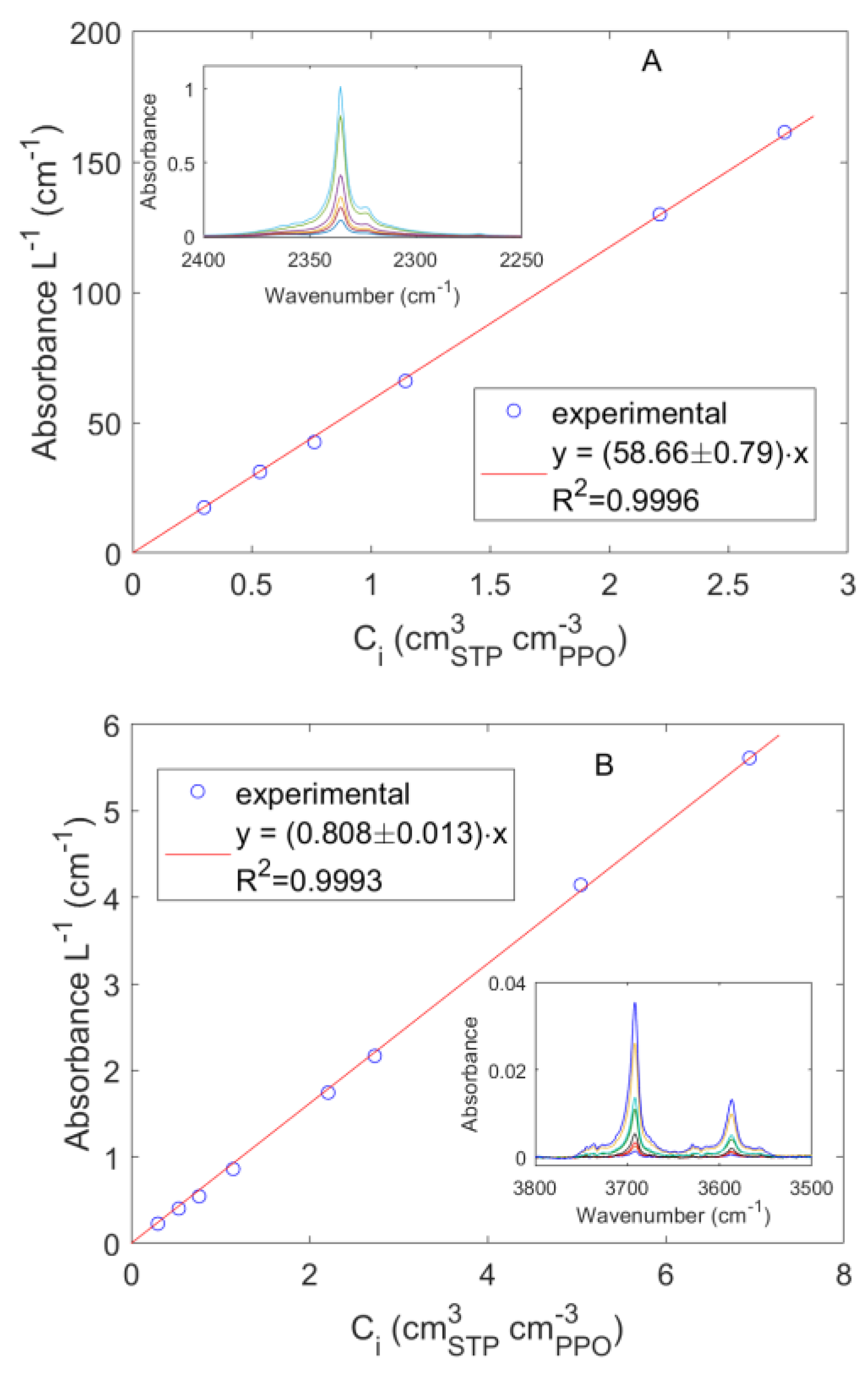
3.2.2. FTIR-Spectroscopy in the Transmission Mode
3.2.3. Performing a Sorption Experiment
4. Results and Discussion
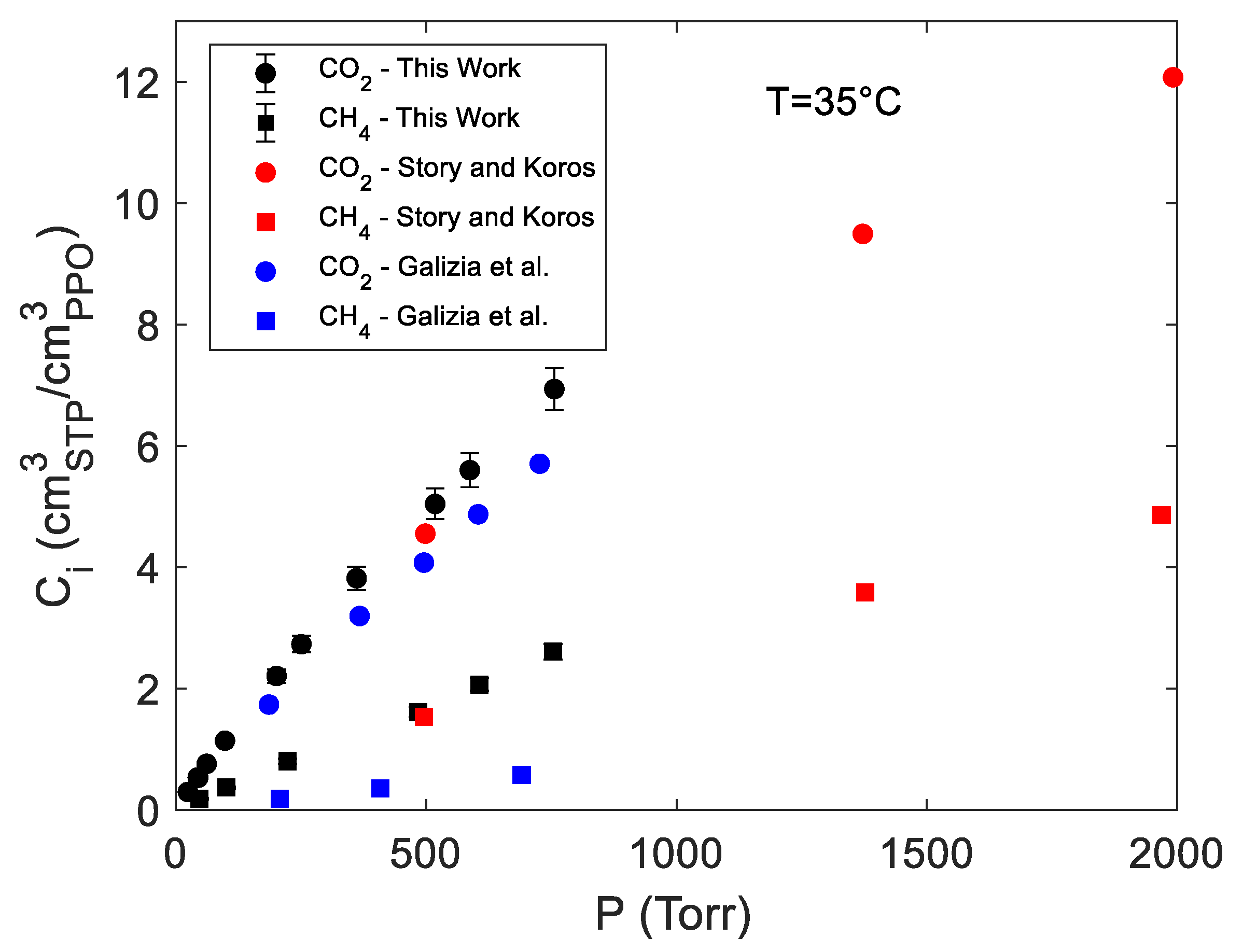
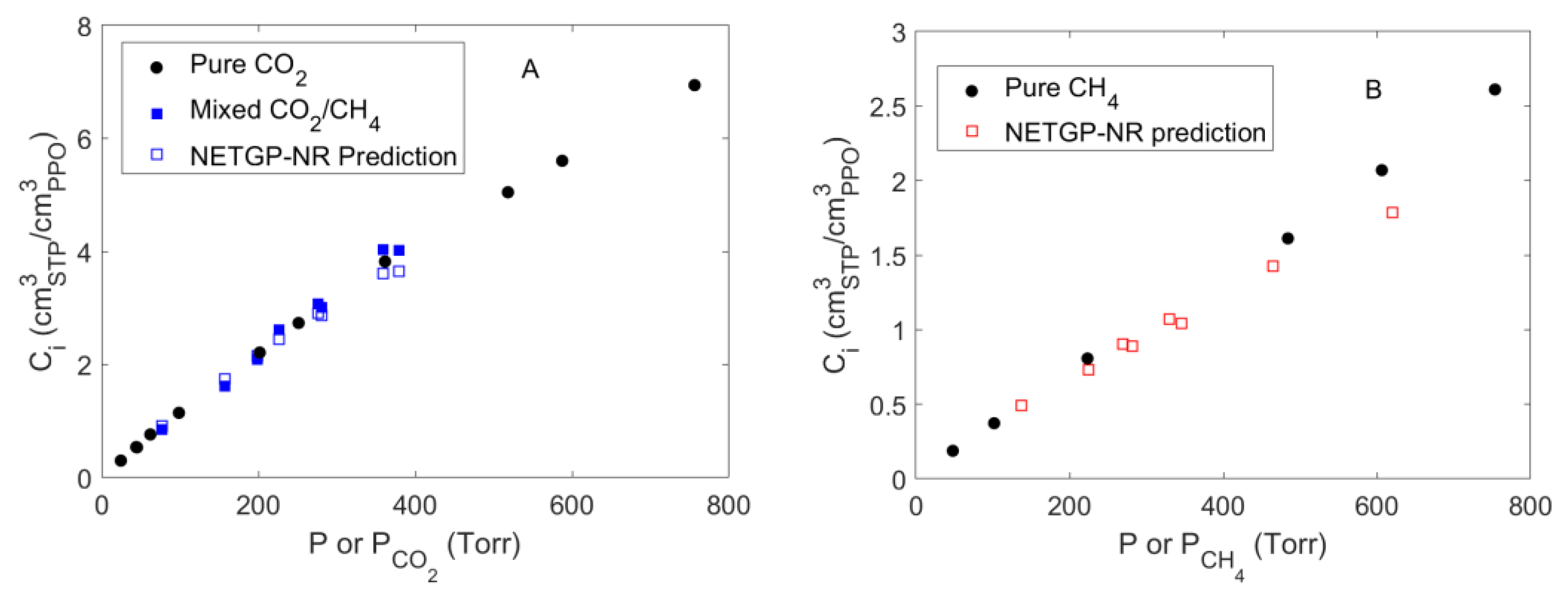
4.1. Sorption of Pure CO2 and CH4 in PPO
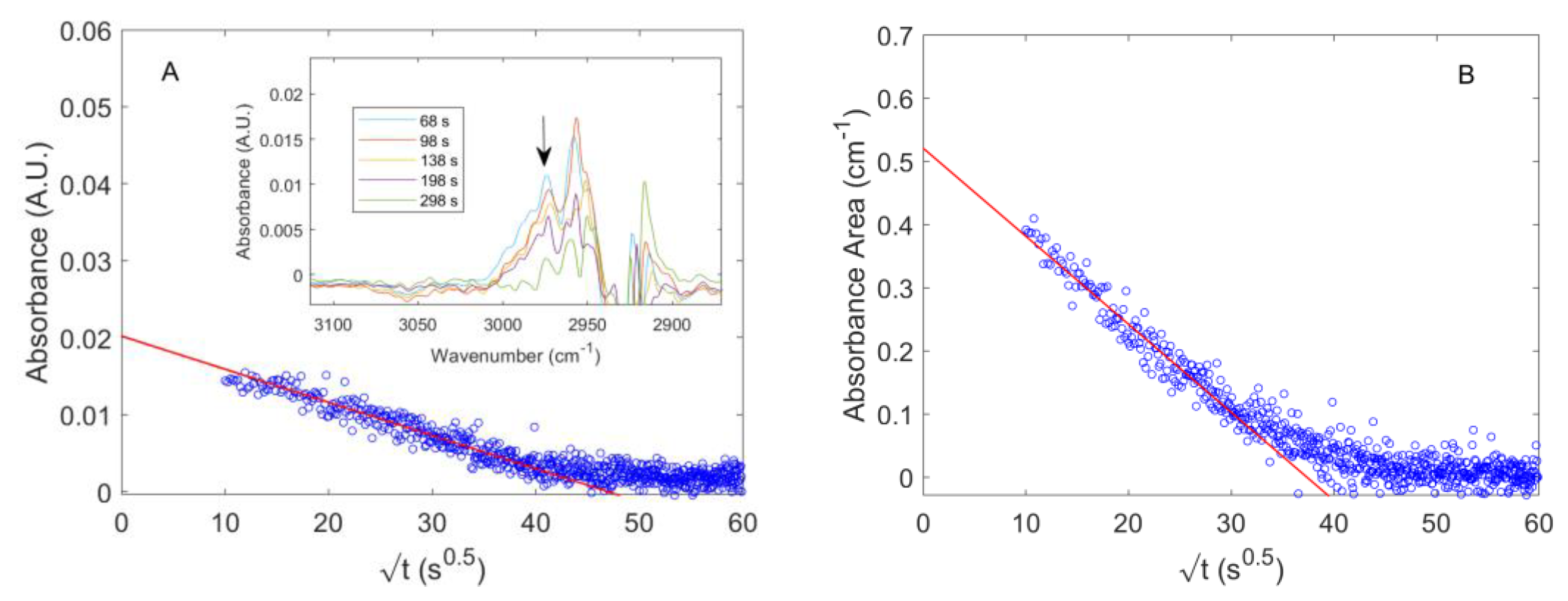
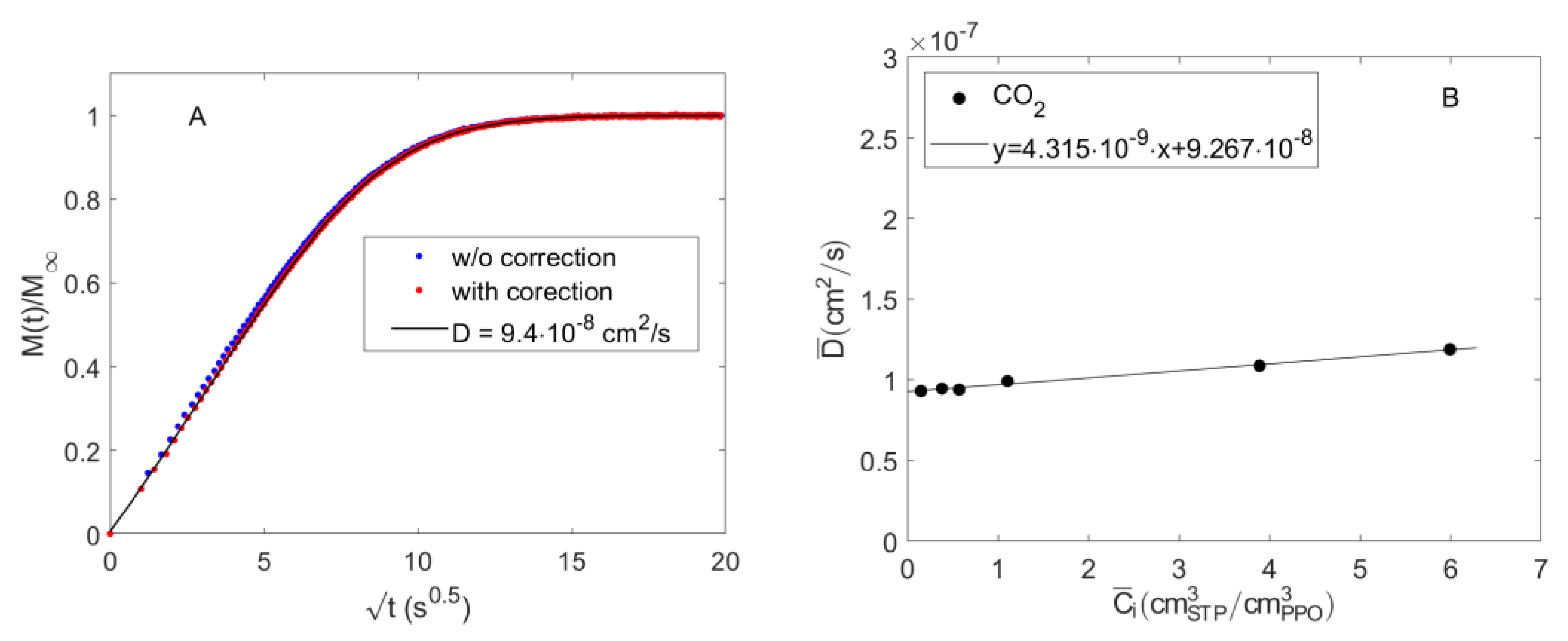
4.2. Diffusion of Pure CO2 in PPO
4.3. Modelling Sorption of Light Gases in PPO
(Jmol−1) | (Jmol−1K−1) | (cm3g−1) | Ref. | ||
|---|---|---|---|---|---|
| CO2 | 3468.4 | −4.5855 | 0.79641 | 0.909 | [38] |
| CH4 | 1956.2 | −0.9181 | 2.12519 | 0.961 | [38] |
| PPO | 5320 | 3.440 | 0.862 | 0.748 | [39] |
| CO2 | CH4 | Ref. |
|---|---|---|
| −0.091 | −0.292 | This Work |
| −0.087 | −0.278 | [16] |
4.4. Mixed Gas Sorption in PPO
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols
| gas phase contribution to the overall absorbance spectrum at time t | |
| gas phase contribution to the overall absorbance spectrum at sorption equilibrium state | |
| C | molar concentration of the pure penetrant |
| molar concentration of the pure penetrant in the polymer phase at the downstream side in a permeation experiment | |
| molar concentration of the pure penetrant in the polymer phase at the upstream side in a permeation experiment | |
| concentration of component i within the polymer phase in a sorption experiment | |
| average concentration of component i within the polymer phase in a sorption experiment | |
| initial uniform concentration of component i within the polymer phase in a sorption experiment | |
| equilibrium concentration of component i within the polymer phase in a sorption experiment | |
| molar gas phase concentration at time t | |
| molar gas phase concentration at sorption equilibrium state | |
| D | mutual diffusion coefficient |
| Di | mutual diffusion coefficient of the component i |
| effective diffusivity coefficient | |
| defined in Equation (17) and calculated from Equation (18) | |
| mean field lattice fluid binary interactional parameter between the species i and j | |
| the swelling factor associated with the polymer-penetrant couple | |
| defined as | |
| L | membrane thickness in a sorption experiment |
| m | number of components |
| mass of absorbed penetrant at time t in a sorption experiment | |
| equilibrium mass of absorbed penetrant in a sorption experiment | |
| partial pressure of the component i at the upstream side in a permeation test | |
| P | pressure |
| scaled pressure of the pure component or of the mixture | |
| mean steady-state permeability | |
| steady-state permeability of component i | |
| q | average number of lattice contacts per molecule in the mixture and it is equal to |
| qi | number of external contacts made by one molecule of species i |
| R | universal gas constant |
| r | average number of sites occupied by one molecule in the mixture and it is equal to |
| ri | number of sites occupied by one molecule of species i |
| S | apparent solubility coefficient |
| solubility coefficient of component i in the polymer–penetrant mixture | |
| t | time |
| T | temperature |
| Tg | glass-to-rubber transition temperature |
| scaled temperature of the pure component or of the mixture | |
| scaled temperature of the pure component i in the mixture | |
| the temperature-independent contribution to the close packed specific volume of the pure component i | |
| close packed specific volume of the pure component i | |
| scaled lattice fluid volume of the pure components or of the mixture and it is equal to | |
| the ratio of molar surface to molar volume of component i | |
| xi | molar fraction of species i |
| molar fraction of species i at Pseudo-Equilibrium of phase condition | |
| z | lattice coordination number |
| Greek letters | |
| solubility selectivity defined by Equation (15) | |
| αid | ideal selectivity |
| solubility contribution to the ideal selectivity | |
| diffusivity contribution to the ideal selectivity | |
| non-random factor | |
| exchanged interaction energy determined by breaking one i-ì and one j-j interaction for the formation of two i–j interactions in the lattice | |
| flexibility factor of species i | |
| mean field interaction energy within the mixture | |
| mean field interaction energy per molar segment | |
| mean interaction energy per molar segment, enthalpic contribution | |
| mean interaction energy per molar segment, entropic contribution | |
| surface fraction of component i, | |
| molar chemical potential of the species i | |
| equilibrium molar chemical potential of the species i | |
| non-equilibrium molar chemical potential of the species i within the glassy polymer–penetrant phase | |
| non-equilibrium polymer mass density within the mixture | |
| closed-packed density of the polymer–penetrant mixture | |
| polymer mass density induced by elastic instantaneous swelling | |
| the value of the unpenetrated polymer mass density right before the start of the sorption process | |
| scaled lattice fluid density of a pure component or of the mixture ( | |
| “close-packed” volumetric fraction of component i | |
| polymer mass fraction | |
References
- Eurostat. Available online: https://Ec.Europa.Eu/Eurostat/ (accessed on 13 October 2022).
- Short-Term Energy Outlook. 2022. Available online: https://www.Eia.Gov/Todayinenergy/Detail.Php?Id=53839 (accessed on 26 October 2022).
- Baker, R.W.; Lokhandwala, A. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- White, L.S. Effect of Operating Environment on Membrane Performance. Curr. Opin. Chem. Eng. 2020, 28, 105–111. [Google Scholar] [CrossRef]
- Genduso, G.; Ghanem, B.S.; Pinnau, I. Experimental Mixed-Gas Permeability, Sorption and Diffusion of CO2-CH4 Mixtures in 6FDA-MPDA Polyimide Membrane: Unveiling the Effect of Competitive Sorption on Permeability Selectivity. Membranes 2019, 9, 10. [Google Scholar] [CrossRef]
- Pourafshari Chenar, M.; Soltanieh, M.; Matsuura, T.; Tabe-Mohammadi, A.; Khulbe, K.C. The Effect of Water Vapor on the Performance of Commercial Polyphenylene Oxide and Cardo-Type Polyimide Hollow Fiber Membranes in CO2/CH4 Separation Applications. J. Membr. Sci. 2006, 285, 265–271. [Google Scholar] [CrossRef]
- Story, B.J.; Koros, W.J. Sorption of CO2/CH4 Mixtures in Poly(Phenylene Oxide) and a Carboxylated Derivative. J. Appl. Polym. Sci. 1991, 42, 2613–2626. [Google Scholar] [CrossRef]
- Sanders, E.S.; Koros, W.J.; Hopfenberg, H.B.; Stannett, V.T. Mixed Gas Sorption in Glassy Polymers: Equipment Design Considerations and Preliminary Results. J. Membr. Sci. 1983, 13, 161–174. [Google Scholar] [CrossRef]
- Ricci, E.; Benedetti, F.M.; Noto, A.; Merkel, T.C.; Jin, J.; De Angelis, M.G. Enabling Experimental Characterization and Prediction of Ternary Mixed-Gas Sorption in Polymers: C2H6/CO2/CH4 in PIM-1. Chem. Eng. J. 2021, 426, 130715. [Google Scholar] [CrossRef]
- Genduso, G.; Litwiller, E.; Ma, X.; Zampini, S.; Pinnau, I. Mixed-Gas Sorption in Polymers via a New Barometric Test System: Sorption and Diffusion of CO2-CH4 Mixtures in Polydimethylsiloxane (PDMS). J. Membr. Sci. 2019, 577, 195–204. [Google Scholar] [CrossRef]
- Loianno, V.; Mensitieri, G.; Baldanza, A.; Scherillo, G.; Musto, P. Combining FTIR Spectroscopy and Pressure-Decay Techniques to Analyze Sorption Isotherms and Sorption Kinetics of Pure Gases and Their Mixtures in Polymers: The Case of CO2 and CH4 Sorption in Polydimethylsiloxane. J. Membr. Sci. 2022, 652, 120445. [Google Scholar] [CrossRef]
- Hong, S.U.; Barbari, T.A.; Sloan, J.M. Multicomponent Diffusion of Methyl Ethyl Ketone and Toluene in Polyisobutylene from Vapor Sorption FTIR-ATR Spectroscopy. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 337–344. [Google Scholar] [CrossRef]
- Shade, D.; Bout, B.W.S.; Sholl, D.S.; Walton, K.S. Opening the Toolbox: 18 Experimental Techniques for Measurement of Mixed Gas Adsorption. Ind. Eng. Chem. Res. 2022, 61, 2367–2391. [Google Scholar] [CrossRef]
- Minelli, M.; Campagnoli, S.; De Angelis, M.G.; Doghieri, F.; Sarti, G.C. Predictive Model for the Solubility of Fluid Mixtures in Glassy Polymers. Macromolecules 2011, 44, 4852–4862. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Modeling Mass Transport in Dense Polymer Membranes: Cooperative Synergy among Multiple Scale Approaches. Curr. Opin. Chem. Eng. 2020, 28, 43–50. [Google Scholar] [CrossRef]
- Baldanza, A.; Loianno, V.; Mensitieri, G.; Scherillo, G. Predictive Approach for the Solubility and Permeability of Binary Gas Mixtures in Glassy Polymers Based on an NETGP-NRHB Model. Ind. Eng. Chem. Res. 2022, 61, 3439–3456. [Google Scholar] [CrossRef]
- Panayiotou, C.; Pantoula, M.; Stefanis, E.; Tsivintzelis, I.; Economou, I.G. Nonrandom Hydrogen-Bonding Model of Fluids and Their Mixtures. 1. Pure Fluids. Ind. Eng. Chem. Res. 2004, 43, 6592–6606. [Google Scholar] [CrossRef]
- Panayiotou, C.; Tsivintzelis, I.; Economou, I.G. Nonrandom Hydrogen-Bonding Model of Fluids and Their Mixtures. 2. Multicomponent Mixtures. Ind. Eng. Chem. Res. 2007, 46, 2628–2636. [Google Scholar] [CrossRef]
- Mensitieri, G.; Scherillo, G.; Panayiotou, C.; Musto, P. Towards a Predictive Thermodynamic Description of Sorption Processes in Polymers: The Synergy between Theoretical EoS Models and Vibrational Spectroscopy. Mater. Sci. Eng. R Rep. 2020, 140, 100525. [Google Scholar] [CrossRef]
- Neau, E. A consistent method for phase equilibrium calculation using the Sanchez–Lacombe lattice–fluid equation-of-state. Fluid Phase Equilibria 2002, 203, 133–140. [Google Scholar] [CrossRef]
- Von Konigslow, K.; Park, C.B.; Thompson, R.B. Polymeric Foaming Predictions from the Sanchez-Lacombe Equation of State: Application to Polypropylene-Carbon Dioxide MixturesPhys. Rev. Appl. 2017, 8, 044009. [Google Scholar] [CrossRef]
- Von Konigslow, K.; Park, C.B.; Thompson, R.B. Application of a Constant Hole Volume Sanchez–Lacombe Equation of State to Mixtures Relevant to Polymeric Foaming. Soft Matter 2018, 14, 4603–4614. [Google Scholar] [CrossRef]
- Baldanza, A.; Loianno, V.; Mensitieri, G.; Panayiotou, C.; Scherillo, G. On the Thermodynamic Consistency of Non-Random Hydrogen Bonding Lattice-Fluid Model for Multicomponent Mixtures. Fluid Phase Equilibria 2022, 553, 113302. [Google Scholar] [CrossRef]
- Doghieri, F.; Sarti, G.C. Nonequilibrium Lattice Fluids: A Predictive Model for the Solubility in Glassy Polymers. Macromolecules 1996, 29, 7885–7896. [Google Scholar] [CrossRef]
- Sarti, G.C.; Doghieri, F. Predictions of the Solubility of Gases in Glassy Polymers Based on the NELF Model. Chem. Eng. Sci. 1998, 53, 3435–3447. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Graham, T.L.V. On the Absorption and Dialytic Separation of Gases by Colloid Septa. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1866, 32, 401–420. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Loianno, V.; Mensitieri, G. A Novel Dynamic Method for the Storage of Calibration Gas Mixtures Based on Thermal Mass Flow Controllers. Meas. Sci. Technol. 2022, 33, 065017. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://Webbook.Nist.Gov/Chemistry/Fluid/ (accessed on 21 July 2022).
- Galizia, M.; Daniel, C.; Fasano, G.; Guerra, G.; Mensitieri, G. Gas Sorption and Diffusion in Amorphous and Semicrystalline Nanoporous Poly(2,6-Dimethyl-1,4-Phenylene)Oxide. Macromolecules 2012, 45, 3604–3615. [Google Scholar] [CrossRef]
- Story, B.J.; Koros, W.J. Sorption and Transport of CO2 and CH4 in Chemically Modified Poly(Phenylene Oxide). J. Membr. Sci. 1992, 67, 191–210. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Turner, J.J. Bandwidths. In Handbook of Vibrational Spectroscopy; Chalmers, J., Griffiths, P., Eds.; John Wiley & Son: Chichester, UK, 2002; Volume 1, pp. 101–127. [Google Scholar]
- Clarke, J.H.R. Band Shapes and Molecular Dynamics in Liquids. In Advances in Infrared and Raman Spectroscopy; Clarke, J.H.R., Hester, R.E., Eds.; Hey Den: London, UK, 1978; Volume 4, pp. 109–193. [Google Scholar]
- Li, C.; Xin, Q. FT-IR Spectroscopic Investigation of Methane Adsorption on Cerium Oxide. J. Phys. Chem. 1992, 96, 7714–7718. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamazaki, T.; Ozawa, S. IR Spectra of CH4 Physisorbed on an Active Carbon at Low Temperature. J. Colloid Interface Sci. 2000, 224, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Tsivintzelis, I.; Spyriouni, T.; Economou, I.G. Modeling of Fluid Phase Equilibria with Two Thermodynamic Theories: Non-Random Hydrogen Bondin g (NRHB) and Statistical Associating Fluid Theory (SAFT). Fluid Phase Equilibria 2007, 253, 19–28. [Google Scholar] [CrossRef]
- Musto, P.; Loianno, V.; Scherillo, G.; La Manna, P.; Galizia, M.; Guerra, G.; Mensitieri, G. Benzene-Induced Crystallization of PPO: A Combined Thermodynamic and Vibrational Spectroscopy Study. Ind. Eng. Chem. Res. 2020, 59, 5402–5411. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loianno, V.; Baldanza, A.; Scherillo, G.; Musto, P.; Mensitieri, G. Sorption of CO2, CH4 and Their Mixtures in Amorphous Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO). Polymers 2023, 15, 1144. https://doi.org/10.3390/polym15051144
Loianno V, Baldanza A, Scherillo G, Musto P, Mensitieri G. Sorption of CO2, CH4 and Their Mixtures in Amorphous Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO). Polymers. 2023; 15(5):1144. https://doi.org/10.3390/polym15051144
Chicago/Turabian StyleLoianno, Valerio, Antonio Baldanza, Giuseppe Scherillo, Pellegrino Musto, and Giuseppe Mensitieri. 2023. "Sorption of CO2, CH4 and Their Mixtures in Amorphous Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO)" Polymers 15, no. 5: 1144. https://doi.org/10.3390/polym15051144
APA StyleLoianno, V., Baldanza, A., Scherillo, G., Musto, P., & Mensitieri, G. (2023). Sorption of CO2, CH4 and Their Mixtures in Amorphous Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO). Polymers, 15(5), 1144. https://doi.org/10.3390/polym15051144









