RETRACTED: The Dosidicus gigas Collagen for Scaffold Preparation and Cell Cultivation: Mechanical and Physicochemical Properties, Morphology, Composition and Cell Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Collagen
2.2. Preparation Collagen Scaffold
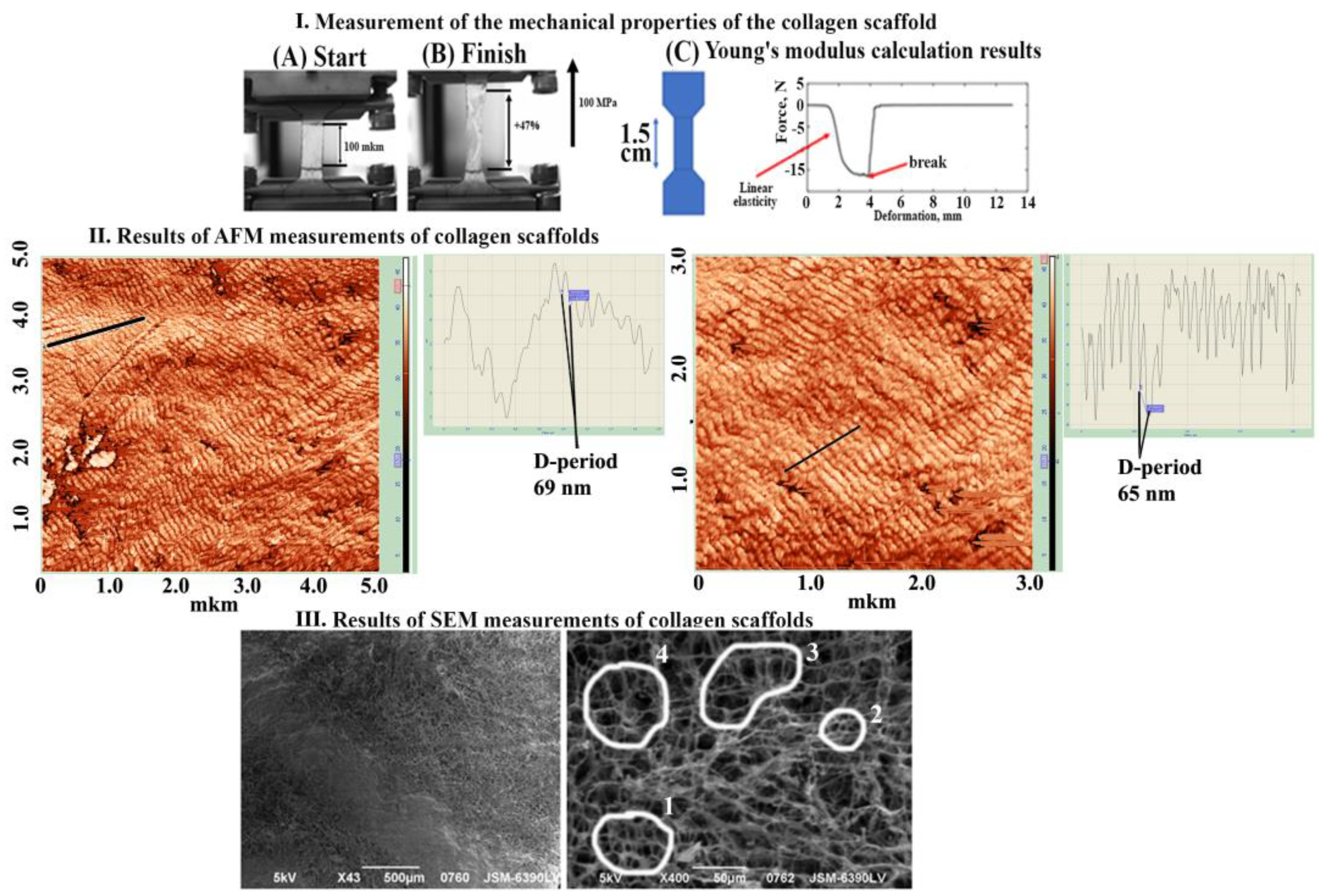
2.3. Mechanical Properties of Collagen Scaffold Investigation
2.4. Atomic Force Microscopy
2.5. Scanning Electron Microscopy
2.6. Amino Acid Content by HPLC
2.7. Size Exclusion Chromatography
2.8. SDS-PAGE
2.9. FTIR Spectra Registration
2.10. MALDI-TOF
2.11. Cell Culture
2.12. MTT Assay for Toxic Effect Determination
2.13. Fluorescent Dying
2.14. Visualization of Extracellular Matrix
2.15. Study of Cell Culture on the Surface of a Collagen Matrix at a Laboratory Complex, “SynchrotronLike”
2.16. Image Processing of High-Resolution X-ray Radiography and Microscopy
3. Results
3.1. Scaffolds’ Mechanical Properties, Surface Topography, and Composition
3.2. Fractional Composition Investigation of a Collagen Proteins Mixture
3.3. Visualization of Cell Compartments, Extracellular Matrix, and Estimation of Biocompatibility with Collagen Materials
3.4. High-Resolution X-ray Radiography on the Surface of the Collagen Matrix
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen Structure–Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2009, 339, 247. [Google Scholar] [CrossRef]
- Antipova, L.V.; Sukhov, I.V.; Slobodyanik, V.S.; Kotov, I.I.; Antipov, S.S. Improving the technology of collagen substance from raw fish materials for obtaining porous materials for cosmetology and medicine. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 32044. [Google Scholar] [CrossRef]
- Sarkar, B.; O’Leary, L.E.R.; Hartgerink, J.D. Self-Assembly of Fiber-Forming Collagen Mimetic Peptides Controlled by Triple-Helical Nucleation. J. Am. Chem. Soc. 2014, 136, 14417–14424. [Google Scholar] [CrossRef] [PubMed]
- Köster, S.; Evans, H.M.; Wong, J.Y.; Pfohl, T. An In Situ Study of Collagen Self-Assembly Processes. Biomacromolecules 2008, 9, 199–207. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef]
- Antipova, L.V.A. Study of the Use of Modified Collagen of Freshwater Fish as a Material for Personal Care Products; Storublevtsev, S.A., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78985-958-4. [Google Scholar]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Belamie, E.; Mosser, G.; Gobeaux, F.; Giraud-Guille, M.M. Possible transient liquid crystal phase during the laying out of connective tissues: α-chitin and collagen as models. J. Phys. Condens. Matter 2006, 18, S115–S129. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Rivera-Martínez, C.A.; Quiñones-Colón, B.A.; Almodóvar, J. Electrospun Collagen Scaffolds BT—Electrospun Biomaterials and Related Technologies; Almodovar, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–55. ISBN 978-3-319-70049-6. [Google Scholar]
- Lowe, C.J.; Reucroft, I.M.; Grota, M.C.; Shreiber, D.I. Production of Highly Aligned Collagen Scaffolds by Freeze-drying of Self-assembled, Fibrillar Collagen Gels. ACS Biomater. Sci. Eng. 2016, 2, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Nitti, P.; Kunjalukkal Padmanabhan, S.; Cortazzi, S.; Stanca, E.; Siculella, L.; Licciulli, A.; Demitri, C. Enhancing Bioactivity of Hydroxyapatite Scaffolds Using Fibrous Type I Collagen. Front. Bioeng. Biotechnol. 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Appel, A.; Anastasio, M.A.; Brey, E.M. Potential for Imaging Engineered Tissues with X-Ray Phase Contrast. Tissue Eng. Part B Rev. 2011, 17, 321–330. [Google Scholar] [CrossRef]
- Shearer, T.; Bradley, R.S.; Hidalgo-Bastida, L.A.; Sherratt, M.J.; Cartmell, S.H. Three-dimensional visualisation of soft biological structures by X-ray computed micro-tomography. J. Cell Sci. 2016, 129, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Rawson, S.D.; Maksimcuka, J.; Withers, P.J.; Cartmell, S.H. X-ray computed tomography in life sciences. BMC Biol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Papantoniou, I.; Sonnaert, M.; Geris, L.; Luyten, F.P.; Schrooten, J.; Kerckhofs, G. Three-Dimensional Characterization of Tissue-Engineered Constructs by Contrast-Enhanced Nanofocus Computed Tomography. Tissue Eng. Part C Methods 2013, 20, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Iturri, J.; Toca-Herrera, J.L. Characterization of Cell Scaffolds by Atomic Force Microscopy. Polymers 2017, 9, 383. [Google Scholar] [CrossRef]
- Ahmed, M.; Ramos, T.A.d.S.; Damanik, F.; Quang Le, B.; Wieringa, P.; Bennink, M.; van Blitterswijk, C.; de Boer, J.; Moroni, L. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci. Rep. 2015, 5, 14804. [Google Scholar] [CrossRef]
- Kukreti, U.; Belkoff, S.M. Collagen fibril D-period may change as a function of strain and location in ligament. J. Biomech. 2000, 33, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Liou, H.; Lin, H.; Chen, W.; Liou, W.; Hwu, Y. Tomography Observations of Osteoblast Seeding on 3-D Collagen Scaffold by Synchrotron Radiation Hard X-Ray. J. Biomim. Biomater. Tissue Eng. 2009, 3, 93–101. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Campi, G.; Cancedda, R.; Cedola, A. Synchrotron radiation techniques boost the research in bone tissue engineering. Acta Biomater. 2019, 89, 33–46. [Google Scholar] [CrossRef]
- Duan, X.; Li, N.; Chen, X.; Zhu, N. Characterization of Tissue Scaffolds Using Synchrotron Radiation Microcomputed Tomography Imaging. Tissue Eng. Part C Methods 2021, 27, 573–588. [Google Scholar] [CrossRef]
- Bukreeva, I.; Fratini, M.; Campi, G.; Pelliccia, D.; Spanò, R.; Tromba, G.; Brun, F.; Burghammer, M.; Grilli, M.; Cancedda, R.; et al. High-Resolution X-ray Techniques as New Tool to Investigate the 3D Vascularization of Engineered-Bone Tissue. Front. Bioeng. Biotechnol. 2015, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Macmillan, J.; Shrewry, P.R.; Tatham, A.S.; Puxkandl, R.; Zizak, I.; Paris, O.; Keckes, J.; Tesch, W.; Bernstorff, S.; et al. Viscoelastic properties of collagen: Synchrotron radiation investigations and structural model. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2002, 357, 191–197. [Google Scholar] [CrossRef]
- Anokhova, V.D.; Chupakhin, E.G.; Pershina, N.A.; Storublevtsev, S.A.; Antipova, L.V.; Matskova, L.V.; Antipov, S.S. Sensitivity of Different Collagens to Proteolytic Enzyme Treatment BT—Proceedings of the International Conference “Health and wellbeing in modern society” (ICHW 2020); Atlantis Press: Paris, France, 2020; pp. 44–49. [Google Scholar]
- Xu, N.; Peng, X.-L.; Li, H.-R.; Liu, J.-X.; Cheng, J.-S.-Y.; Qi, X.-Y.; Ye, S.-J.; Gong, H.-L.; Zhao, X.-H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Jency, G.; Manjusha, W.A. Extraction and Purification of Collagen from Marine Squid Uroteuthis Duvauceli. Int. J. Pharma Bio Sci. 2020, 10. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef]
- Watanabe-Nakayama, T.; Itami, M.; Kodera, N.; Ando, T.; Konno, H. High-speed atomic force microscopy reveals strongly polarized movement of clostridial collagenase along collagen fibrils. Sci. Rep. 2016, 6, 28975. [Google Scholar] [CrossRef] [PubMed]
- Toroian, D.; Lim, J.E.; Price, P.A. The Size Exclusion Characteristics of Type I Collagen: Implications for the role of noncollagenous bone constituents in mineralization *. J. Biol. Chem. 2007, 282, 22437–22447. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Z.; Yuan, X.; Wang, P.; Liu, Y.; Wang, H. Extraction and isolation of type I, III and V collagens and their SDS-PAGE analyses. Trans. Tianjin Univ. 2011, 17, 111. [Google Scholar] [CrossRef]
- Kanta, J. Collagen matrix as a tool in studying fibroblastic cell behavior. Cell Adh. Migr. 2015, 9, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Koohestani, F.; Braundmeier, A.G.; Mahdian, A.; Seo, J.; Bi, J.; Nowak, R.A. Extracellular Matrix Collagen Alters Cell Proliferation and Cell Cycle Progression of Human Uterine Leiomyoma Smooth Muscle Cells. PLoS ONE 2013, 8, e75844. [Google Scholar] [CrossRef]
- Acuna, A.; Drakopoulos, M.A.; Leng, Y.; Goergen, C.J.; Calve, S. Three-dimensional visualization of extracellular matrix networks during murine development. Dev. Biol. 2018, 435, 122–129. [Google Scholar] [CrossRef]
- Barannikov, A.; Shevyrtalov, S.; Zverev, D.; Narikovich, A.; Sinitsyn, A.; Panormov, I.; Snigireva, I.; Snigirev, A. Laboratory complex for the tests of the X-ray optics and coherence-related techniques. In Proceedings of the Proc.SPIE, Online, 19–29 April 2021; Volume 11776. [Google Scholar]
- Hållstedt, J.; Espes, E.; Lundström, U.; Hansson, B. Liquid-metal-jet X-ray technology for nanoelectronics characterization and metrology. In Proceedings of the 2018 29th Annual SEMI Advanced Semiconductor Manufacturing Conference (ASMC), New York, NY, USA, 30 April 2018–3 May 2018; pp. 151–154. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Montgomery, H.; Rustogi, N.; Hadjisavvas, A.; Tanaka, K.; Kyriacou, K.; Sutton, C.W. Proteomic Profiling of Breast Tissue Collagens and Site-specific Characterization of Hydroxyproline Residues of Collagen Alpha-1-(I). J. Proteome Res. 2012, 11, 5890–5902. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Rizk, M.A.; Mostafa, N.Y. Extraction and characterization of collagen from buffalo skin for biomedical applications. Orient. J. Chem. 2016, 32, 1601–1609. [Google Scholar] [CrossRef]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, H.S.; Lee, O.J.; Chao, J.R.; Park, H.J.; Lee, J.M.; Ju, H.W.; Moon, B.M.; Park, Y.R.; Song, J.E.; et al. Fabrication of duck’s feet collagen–silk hybrid biomaterial for tissue engineering. Int. J. Biol. Macromol. 2016, 85, 442–450. [Google Scholar] [CrossRef]
- Dogan, A.; Lasch, P.; Neuschl, C.; Millrose, M.K.; Alberts, R.; Schughart, K.; Naumann, D.; Brockmann, G.A. ATR-FTIR spectroscopy reveals genomic loci regulating the tissue response in high fat diet fed BXD recombinant inbred mouse strains. BMC Genom. 2013, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, F.; Giordano, M.; Marchini, M. A model for type II collagen fibrils: Distinctive D-band patterns in native and reconstituted fibrils compared with sequence data for helix and telopeptide domains. Biopolymers 2000, 54, 448–463. [Google Scholar] [CrossRef]





| Amino Acid | % | Amino Acid | % |
|---|---|---|---|
| Hydroxyproline (OHPro) | 10.13 | Methionine (Met) | 1.39 |
| Asparagines (Asp) | 7.49 | Isoleucine (Ile) | 1.64 |
| Threonine (Thr) | 2.97 | Leucine (Leu) | 3.47 |
| Serine (Ser) | 3.86 | Threonine (Tyr) | 1.04 |
| Glutamine (Glu) | 11.38 | Phenylalanine (Phe) | 1.64 |
| Proline (Pro) | 9.40 | Hydroxy lysine (OH-L) | 1.50 |
| Glycine (Gly) | 22.18 | lysine (Lys) | 1.83 |
| Alanine (Ala) | 6.87 | histidine (His) | 1.07 |
| Cysteine (Cys) | 0.74 | arginine (Arg) | 8.79 |
| Valine (Val) | 2.61 |
| Collagen Type | ||
|---|---|---|
| Collagen I | Collagen II | Collagen III |
| P*G | P*GPP*P*GE | P*GG* |
| KG | P*GI/LGE | KG |
| APTGGTTA | TAPP* | GHI/L |
| GPAG*AKDG*GYK* | P*HDP | I/LGCI/L |
| P*GDK* | P*P*EP*VG | CAG*I/L |
| PGMK* | P*P*EP*G*GGE | SMKG*PG |
| DK*I/LK*G*GG | P*P*EP*G*GEGM | SMMG*PG |
| peptide - GVG P* M C PI/L | G*GP*G* | |
| P*G*GCK* | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anohova, V.; Asyakina, L.; Babich, O.; Dikaya, O.; Goikhman, A.; Maksimova, K.; Grechkina, M.; Korobenkov, M.; Burkova, D.; Barannikov, A.; et al. RETRACTED: The Dosidicus gigas Collagen for Scaffold Preparation and Cell Cultivation: Mechanical and Physicochemical Properties, Morphology, Composition and Cell Viability. Polymers 2023, 15, 1220. https://doi.org/10.3390/polym15051220
Anohova V, Asyakina L, Babich O, Dikaya O, Goikhman A, Maksimova K, Grechkina M, Korobenkov M, Burkova D, Barannikov A, et al. RETRACTED: The Dosidicus gigas Collagen for Scaffold Preparation and Cell Cultivation: Mechanical and Physicochemical Properties, Morphology, Composition and Cell Viability. Polymers. 2023; 15(5):1220. https://doi.org/10.3390/polym15051220
Chicago/Turabian StyleAnohova, Veronika, Lyudmila Asyakina, Olga Babich, Olga Dikaya, Aleksandr Goikhman, Ksenia Maksimova, Margarita Grechkina, Maxim Korobenkov, Diana Burkova, Aleksandr Barannikov, and et al. 2023. "RETRACTED: The Dosidicus gigas Collagen for Scaffold Preparation and Cell Cultivation: Mechanical and Physicochemical Properties, Morphology, Composition and Cell Viability" Polymers 15, no. 5: 1220. https://doi.org/10.3390/polym15051220
APA StyleAnohova, V., Asyakina, L., Babich, O., Dikaya, O., Goikhman, A., Maksimova, K., Grechkina, M., Korobenkov, M., Burkova, D., Barannikov, A., Narikovich, A., Chupakhin, E., Snigirev, A., & Antipov, S. (2023). RETRACTED: The Dosidicus gigas Collagen for Scaffold Preparation and Cell Cultivation: Mechanical and Physicochemical Properties, Morphology, Composition and Cell Viability. Polymers, 15(5), 1220. https://doi.org/10.3390/polym15051220












