Effects of Vinyl Functionalized Silica Particles on Thermal and Mechanical Properties of Liquid Silicone Rubber Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Surface Modification of Silica Particles
2.3. Preparation of SR Nanocomposites
2.4. Characterization
3. Results and Discussion
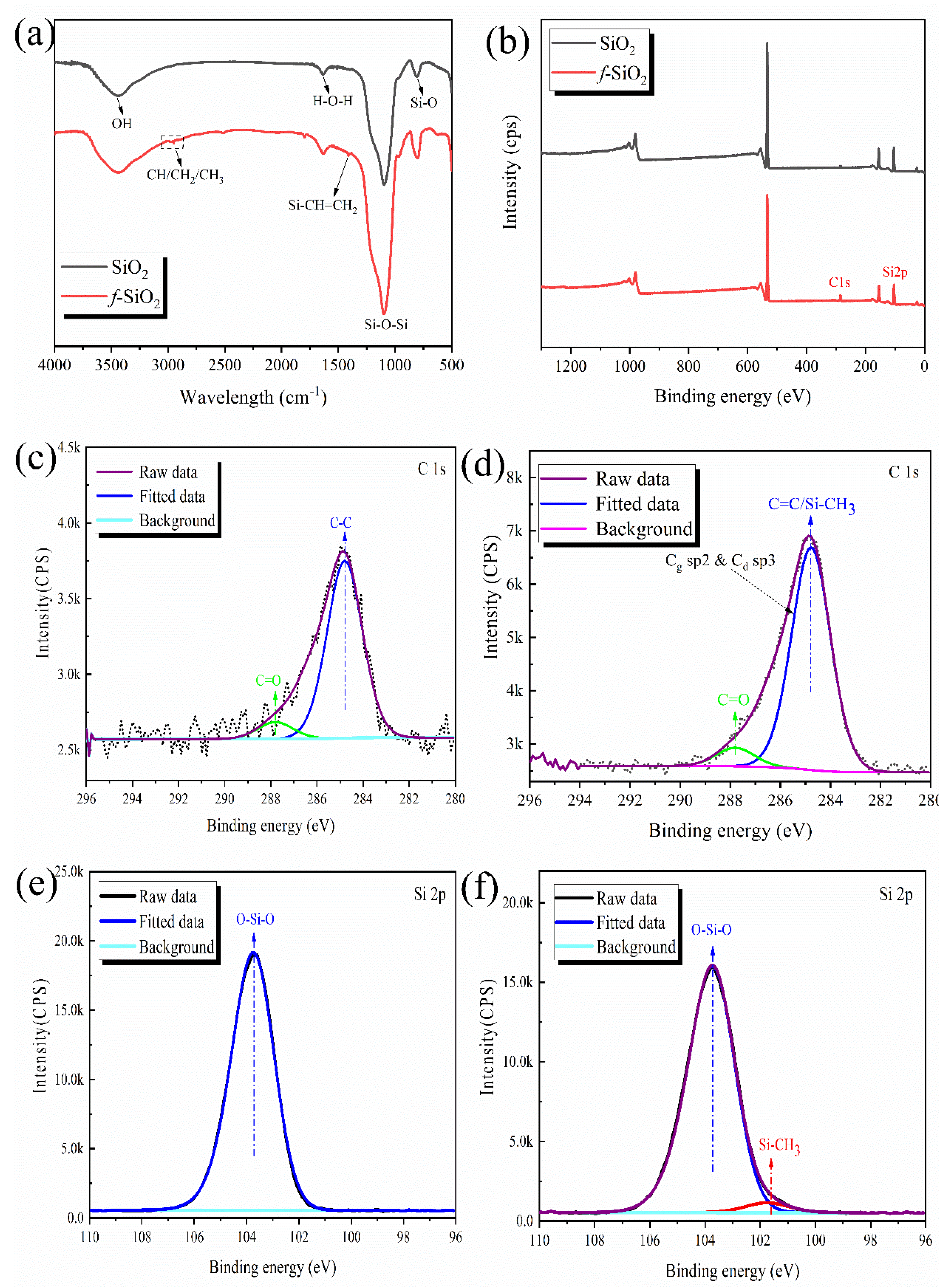
3.1. Surface Chemical Structure Analysis of f-SiO2
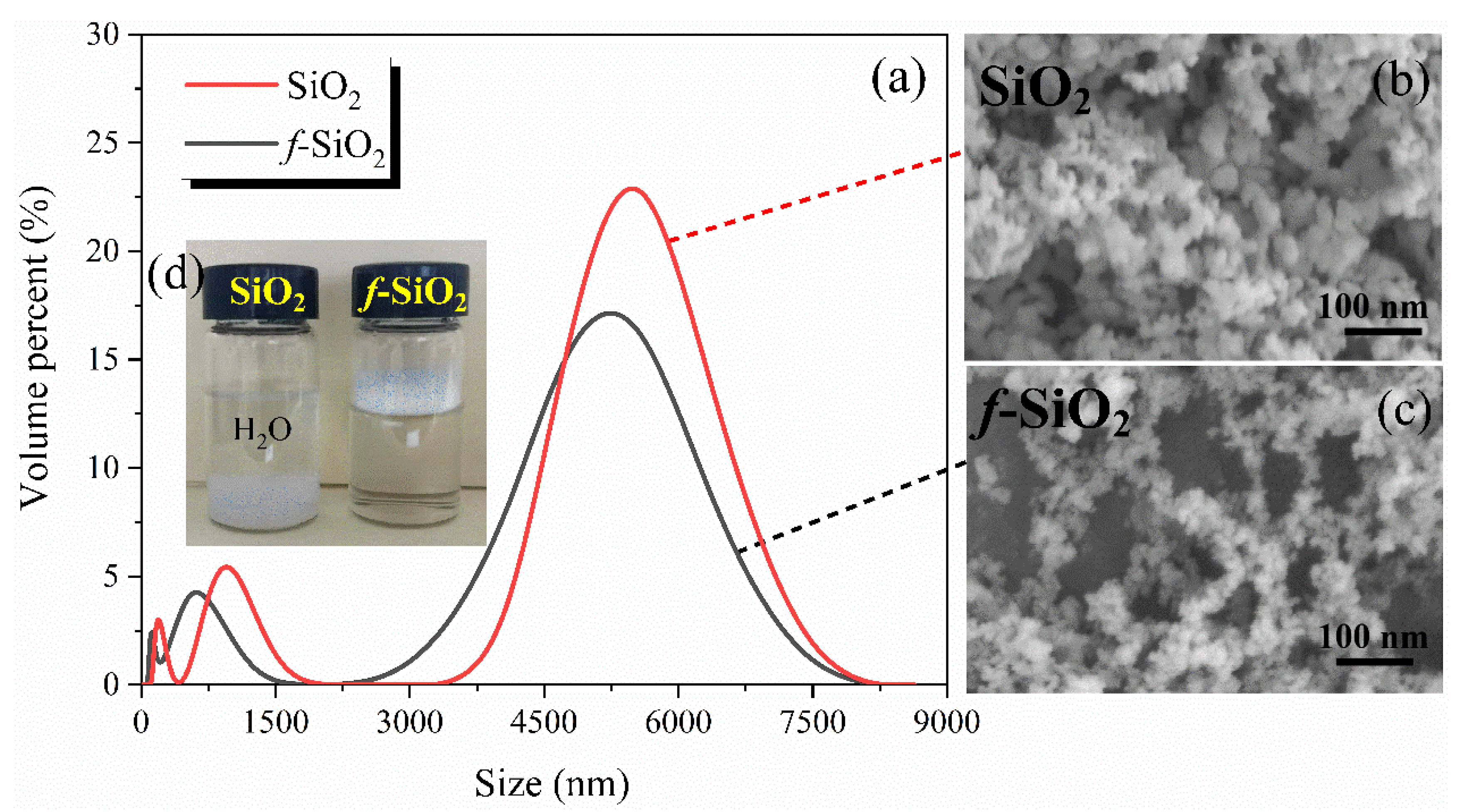
3.2. Particle Size and BET Analysis of f-SiO2
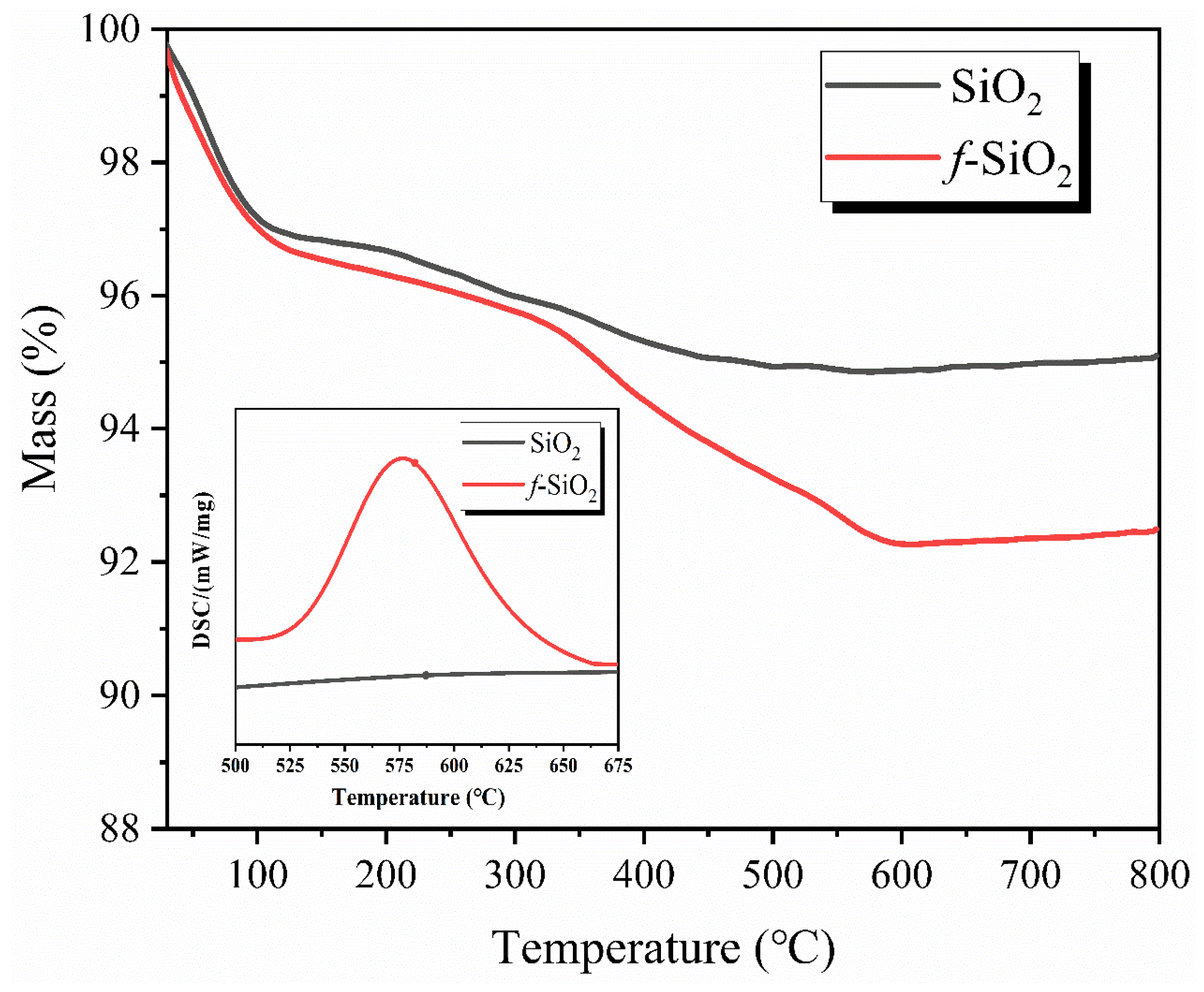
3.3. TG-DSC Analysis of f-SiO2
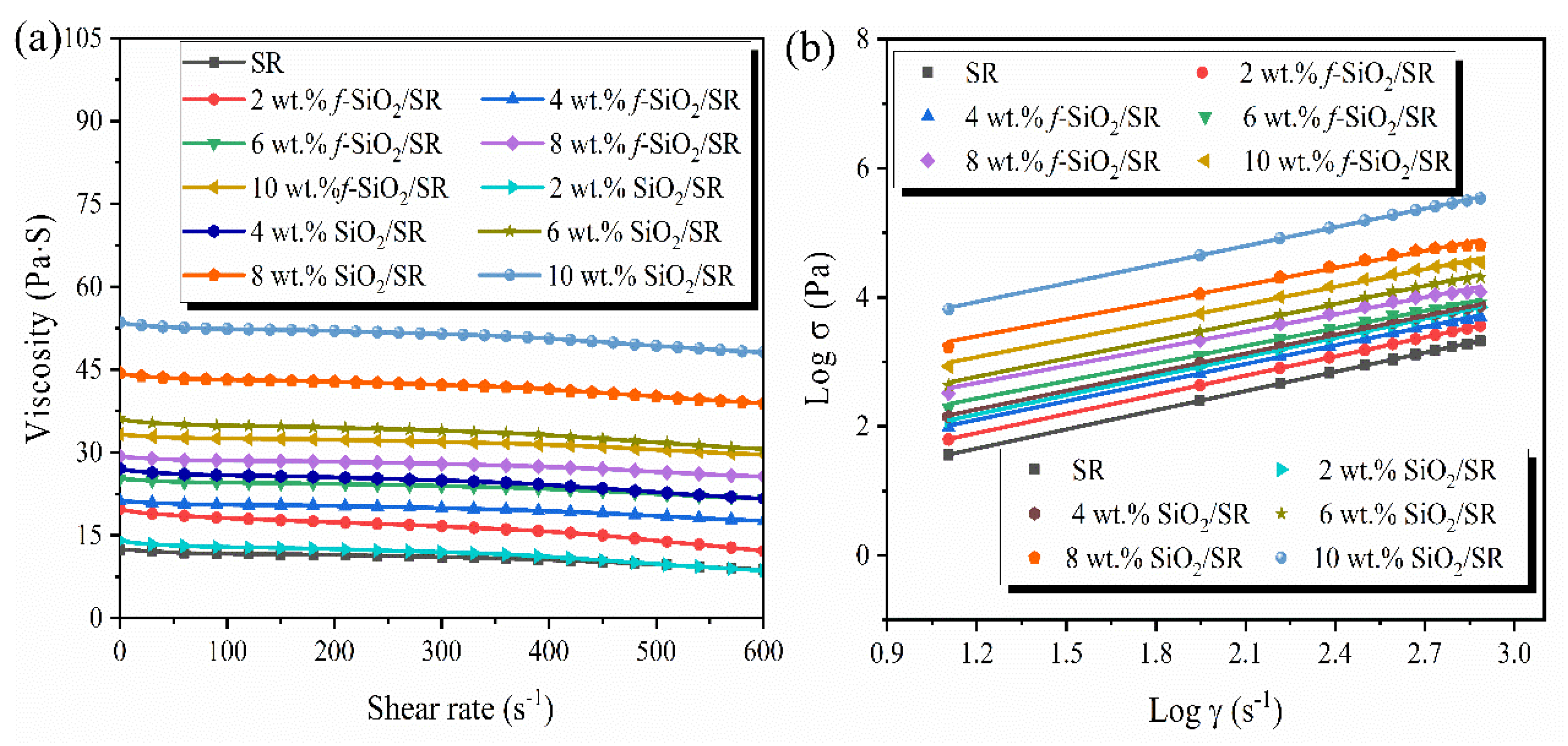
3.4. Rheological Properties
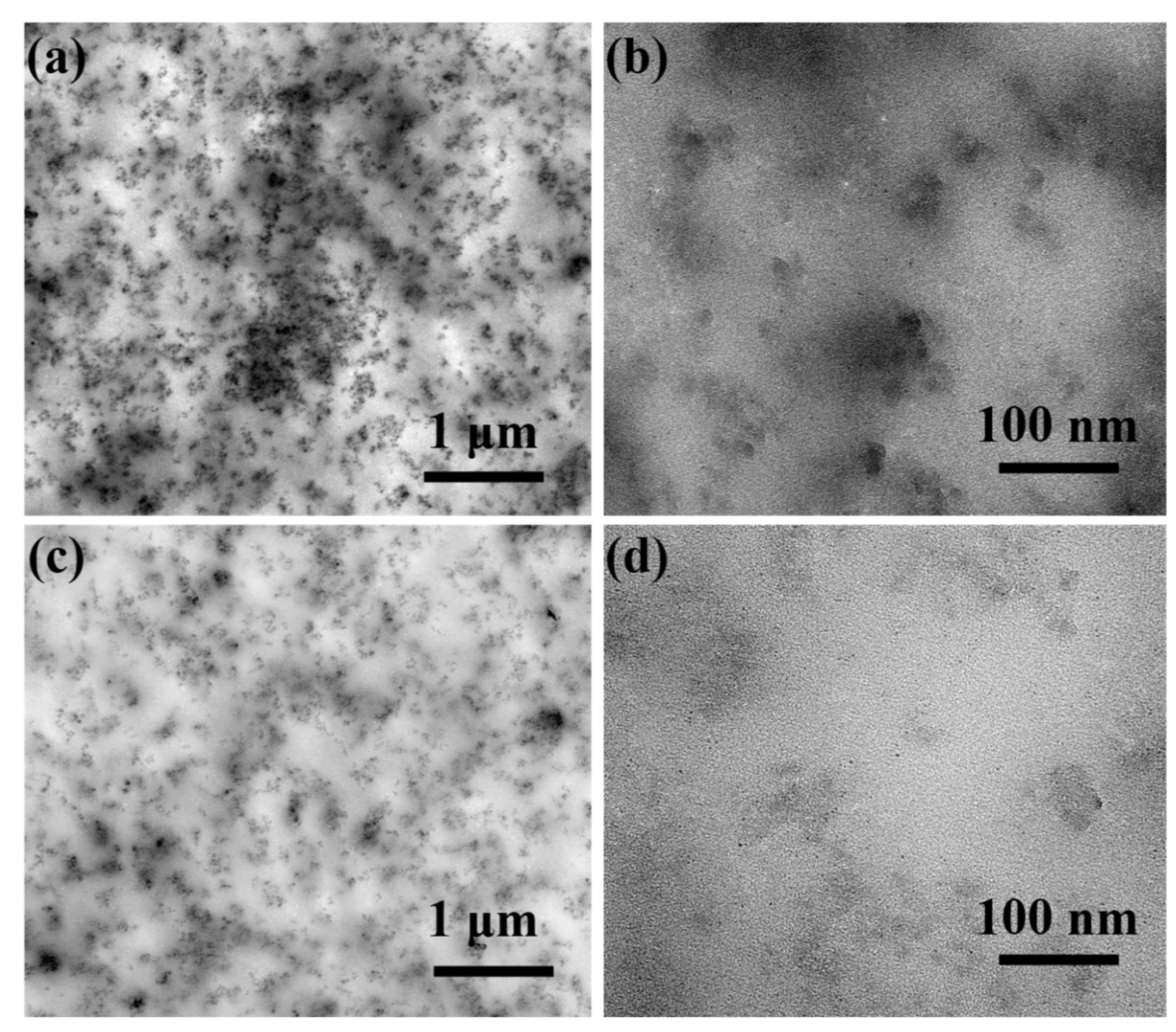
3.5. Micrographs Analysis
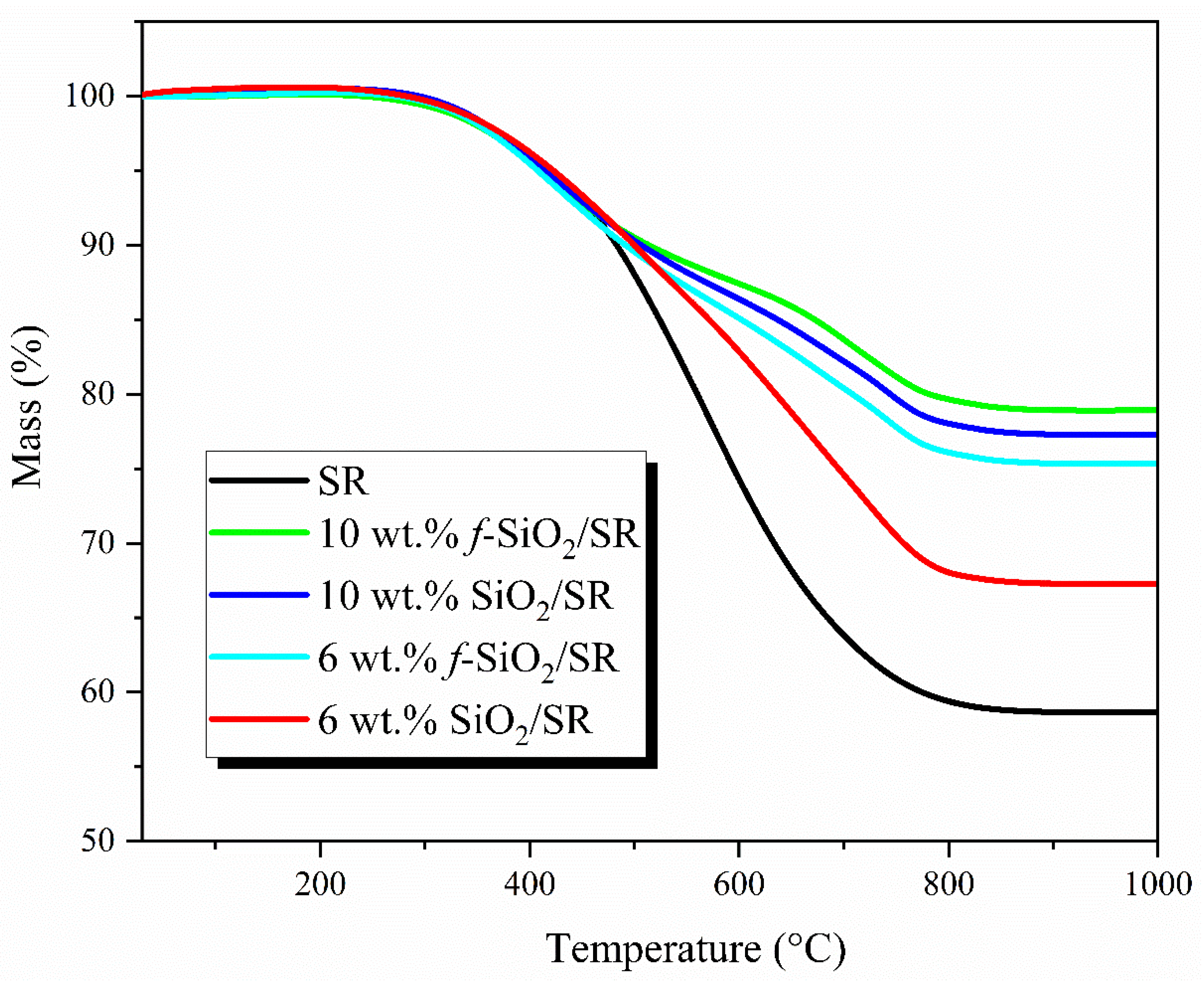
3.6. Thermal Stability Analysis of SiO2/SR Composites
3.7. Thermal Conductive Property
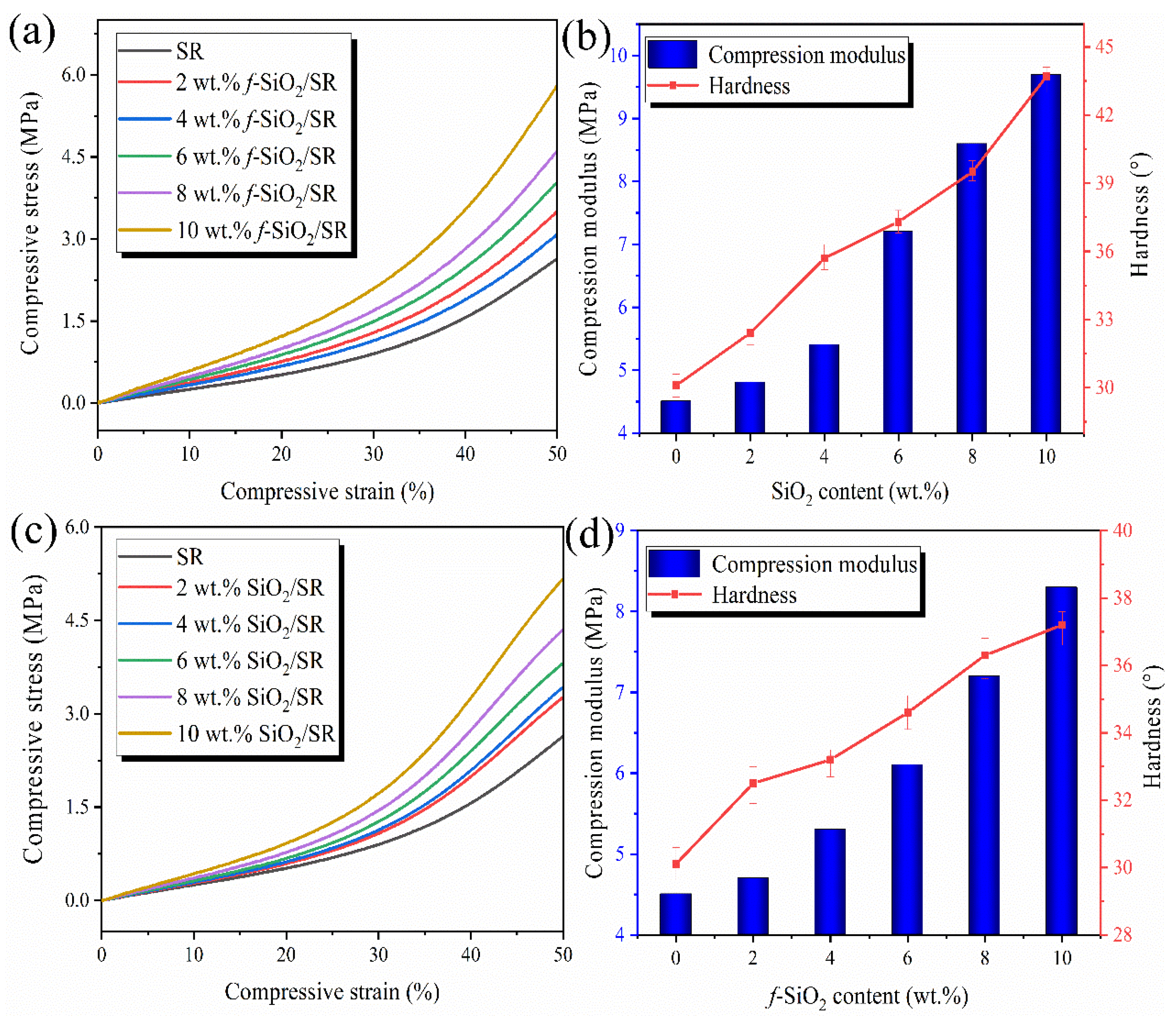
3.8. Mechanical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koushki, P.; Kwok, T.-H.; Hof, L.; Wuthrich, R. Reinforcing silicone with hemp fiber for additive manufacturing. Compos. Sci. Technol. 2020, 194, 108139. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Q.; Xu, H.; Bei, Y.; Feng, S. Preparation and characterization of room temperature vulcanized silicone rubber using α-amine ketoximesilanes as auto-catalyzed cross-linkers. RSC Adv. 2016, 6, 38447–38453. [Google Scholar] [CrossRef]
- Ragheb, A.M.; Brook, M.A.; Hrynyk, M. Highly active, lipase silicone elastomers. Biomaterials 2005, 26, 1653–1664. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, G.; Wu, J.; Xia, H. Preparation of microporous silicone rubber membrane with tunable pore size via solvent evaporation-induced phase separation. ACS Appl. Mater. Interfaces 2013, 5, 2040–2046. [Google Scholar] [CrossRef]
- Pan, G.; Mark, J.E.; Schaefer, D.W. Synthesis and characterization of fillers of controlled structure based on polyhedral oligomeric silsesquioxane cages and their use in reinforcing siloxane elastomers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 3314–3323. [Google Scholar] [CrossRef]
- Baumann, T.F.; Jones, T.V.; Wilson, T.; Saab, A.P.; Maxwell, R.S. Synthesis and characterization of novel PDMS nanocomposites using POSS derivatives as cross-linking filler. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2589–2596. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Huang, C. Synergistic effect between POSS and fumed silica on thermal stabilities and mechanical properties of room temperature vulcanized (RTV) silicone rubbers. Polym. Degrad. Stab. 2012, 97, 308–315. [Google Scholar] [CrossRef]
- Dong, X.; Niu, C.; Qi, M. Enhancement of electrorheological performance of electrorheological elastomers by improving TiO2 particles/silicon rubber interface. J. Mater. Chem. C 2016, 4, 6806–6815. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Fereidoon, A.; Memarian, S.; Albooyeh, A.; Tarahomi, S. Influence of mesoporous silica and hydroxyapatite nanoparticles on the mechanical and morphological properties of polypropylene. Mater. Des. 2014, 57, 201–210. [Google Scholar] [CrossRef]
- Rostamiyan, Y.; Mashhadzadeh, A.H.; SalmanKhani, A. Optimization of mechanical properties of epoxy-based hybrid nanocomposite: Effect of using nano silica and high-impact polystyrene by mixture design approach. Mater. Des. (1980–2015) 2014, 56, 1068–1077. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, L.; Wang, Y.; Liu, Z. Vinyl Functionalized Porous Silica Nanospheres Prepared by Poly (1-vinyl-2-pyrrolidone) Grafted Surface-Protected Selective Etching Strategy. J. Nanosci. Nanotechnol. 2018, 18, 2914–2920. [Google Scholar] [CrossRef]
- Nik Ismail, N.; Ansarifar, A.; Song, M. Effect of hybrid reinforcement based on precipitated silica and montmorillonite nanofillers on the mechanical properties of a silicone rubber. Polym. Eng. Sci. 2014, 54, 1909–1921. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-k.; Lee, N.-H.; Oh, H.-J.; Kim, J.-W.; Rhee, C.-K.; Park, K.-S.; Kim, S.-J. Surface modification and characterization of highly dispersed silica nanoparticles by a cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 172–176. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Li, X.; Zeng, H. A new surface modification method to improve the dispersity of nano-silica in organic solvents. J. Sol-Gel Sci. Technol. 2011, 58, 290–295. [Google Scholar] [CrossRef]
- Zhao, F.; Zeng, X.; Li, H.; Guo, J. Preparation of fluorinated polyacrylate composite latex with in situ generated nano-silica dispersion and film durability. Iran. Polym. J. 2013, 22, 775–784. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Zou, M.; Zheng, X.; Cai, Z. Surface modification of silica and its compounding with polydimethylsiloxane matrix: Interaction of modified silica filler with PDMS. Iran. Polym. J. 2012, 21, 583–589. [Google Scholar] [CrossRef]
- Rangsunvigit, P.; Imsawatgul, P.; Na-ranong, N.; O’Haver, J.H.; Chavadej, S. Mixed surfactants for silica surface modification by admicellar polymerization using a continuous stirred tank reactor. Chem. Eng. J. 2008, 136, 288–294. [Google Scholar] [CrossRef]
- An, D.; Wang, Z.; Zhao, X.; Liu, Y.; Guo, Y.; Ren, S. A new route to synthesis of surface hydrophobic silica with long-chain alcohols in water phase. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 218–222. [Google Scholar] [CrossRef]
- Li-Li, X.; Xiao-Guang, L.; Xing-Yuan, N.; Jun, S. Micro-structure and surface modification of porous silica coating and their effects on hydrophobicity. Chin. J. Inorg. Chem. 2013, 29, 449. [Google Scholar]
- Kartal, A.M.; Erkey, C. Surface modification of silica aerogels by hexamethyldisilazane–carbon dioxide mixtures and their phase behavior. J. Supercrit. Fluids 2010, 53, 115–120. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Hong, Y. Effects of vinylsilane-modified nanosilica particles on electrical and mechanical properties of silicone rubber nanocomposites. Polymer 2020, 197, 122493. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties enhancement of room temperature vulcanized silicone rubber by rosin modified aminopropyltriethoxysilane as a cross-linking agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Zhao, X.-W.; Song, L.-Y.; Zhu, X.-D.; Liu, K.-G.; Zang, C.-G.; Wen, Y.-Q.; Jiao, Q.-J. One-step enrichment of silica nanoparticles on milled carbon fibers and their effects on thermal, electrical, and mechanical properties of polymethyl-vinyl siloxane rubber composites. Compos. Part A Appl. Sci. Manuf. 2018, 113, 287–297. [Google Scholar] [CrossRef]
- Soraru, G.D.; Dallabona, N.; Gervais, C.; Babonneau, F. Organically modified SiO2− B2O3 gels displaying a high content of borosiloxane (B−O−Si⋮) bonds. Chem. Mater. 1999, 11, 910–919. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.-K.; Tang, B.Z. Functionalization of carbon nanotubes using a silane coupling agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Li, Q.; Zhao, Y.; Wang, J.; Kang, M. Effect of surface silanization of carbon fiber on mechanical properties of carbon fiber reinforced polyurethane composites. Compos. Sci. Technol. 2015, 110, 87–94. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, H.; Zhang, Z.; Chen, Y. Polymer–filler interaction of fumed silica filled polydimethylsiloxane investigated by bound rubber. Compos. Sci. Technol. 2013, 86, 1–8. [Google Scholar] [CrossRef]
- Miao, J.; Qian, J.; Wang, X.; Zhang, Y.; Yang, H.; He, P. Synthesis and characterization of ordered mesoporous silica by using polystyrene microemulsion as templates. Mater. Lett. 2009, 63, 989–990. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Liu, C.; Sun, Z.; Zhao, D. A simple approach to the synthesis of hollow microspheres with magnetite/silica hybrid walls. J. Colloid Interface Sci. 2009, 333, 329–334. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Huang, Y.-W.; Yuen, S.-M.; Weng, C.-C.; Chen, C.-H.; Su, S.-F. Effect of MWCNT content on rheological and dynamic mechanical properties of multiwalled carbon nanotube/polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1869–1875. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Ye, Y.S.; Xue, Z.; Xue, Y.; Xie, X.; Mai, Y.-W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Huang, L.P.; Zhou, X.P.; Cui, W.; Xie, X.L.; Tong, S.Y. Maleic anhydride-grafted linear low-density polyethylene with low gel content. Polym. Eng. Sci. 2009, 49, 673–679. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zang, C.G.; Sun, Y.L.; Zhang, Y.L.; Wen, Y.-Q.; Jiao, Q.J. Effect of hybrid hollow microspheres on thermal insulation performance and mechanical properties of silicone rubber composites. J. Appl. Polym. Sci. 2018, 135, 46025. [Google Scholar] [CrossRef]
- Louis, M.; Joshi, S.; Brockmann, W. An experimental investigation of through-thickness electrical resistivity of CFRP laminates. Compos. Sci. Technol. 2001, 61, 911–919. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zang, C.G.; Jiao, Q.J. High electrical conductivity of nylon 6 composites obtained with hybrid multiwalled carbon nanotube/carbon fiber fillers. J. Appl. Polym. Sci. 2014, 131, 40923. [Google Scholar] [CrossRef]
- Gulgunje, P.V.; Newcomb, B.A.; Gupta, K.; Chae, H.G.; Tsotsis, T.K.; Kumar, S. Low-density and high-modulus carbon fibers from polyacrylonitrile with honeycomb structure. Carbon 2015, 95, 710–714. [Google Scholar] [CrossRef]
- García-Valdez, O.; Ledezma-Rodríguez, R.; Saldívar-Guerra, E.; Yate, L.; Moya, S.; Ziolo, R.F. Graphene oxide modification with graft polymers via nitroxide mediated radical polymerization. Polymer 2014, 55, 2347–2355. [Google Scholar] [CrossRef]
- Jiajun, W.; Xiao-Su, Y. Effects of interfacial thermal barrier resistance and particle shape and size on the thermal conductivity of AlN/PI composites. Compos. Sci. Technol. 2004, 64, 1623–1628. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Dang, Z.-M.; Xu, H.-P. Enhanced electrical conductivity in chemically modified carbon nanotube/methylvinyl silicone rubber nanocomposite. Eur. Polym. J. 2007, 43, 4924–4930. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Yao, Y.; Chen, S. Effect of microstructural damage on the mechanical properties of silica nanoparticle-reinforced silicone rubber composites. Eng. Fract. Mech. 2020, 235, 107195. [Google Scholar] [CrossRef]
- Zare, Y. The roles of nanoparticles accumulation and interphase properties in properties of polymer particulate nanocomposites by a multi-step methodology. Compos. Part A Appl. Sci. Manuf. 2016, 91, 127–132. [Google Scholar] [CrossRef]
- Ziraki, S.; Zebarjad, S.M.; Hadianfard, M.J. A study on the role of polypropylene fibers and silica nanoparticles on the compression properties of silicone rubber composites as a material of finger joint implant. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 48–52. [Google Scholar] [CrossRef]
- Gogoi, R.; Sethi, S.K.; Manik, G. Surface functionalization and CNT coating induced improved interfacial interactions of carbon fiber with polypropylene matrix: A molecular dynamics study. Appl. Surf. Sci. 2021, 539, 148162. [Google Scholar] [CrossRef]
- Li, Y.-f.S.; Zang, C.-g.; Zhang, Y.-l. Effect of the structure of hydrogen-containing silicone oil on the properties of silicone rubber. Mater. Chem. Phys. 2020, 248, 122734. [Google Scholar] [CrossRef]
- Xu, H.; Ding, L.; Song, Y.; Wang, W. Rheology of end-linking polydimethylsiloxane networks filled with silica. J. Rheol. 2020, 64, 1425–1438. [Google Scholar] [CrossRef]
- Goswami, M.; Ghosh, M.M.; Dalmiya, M.S.; Sharma, S.; Ghorai, S.K.; Chattopadhyay, S. A finite element method based comparative fracture assessment of carbon black and silica filled elastomers: Reinforcing efficacy of carbonaceous fillers in flexible composites. Polym. Test. 2020, 91, 106856. [Google Scholar] [CrossRef]




| BET Surface Area (m2/g) | Mass Fraction of TMDVS Relative to SiO2 (wt.%) | |||||
|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | 100 | |
| f-SiO2 | 258.65 | 192.76 | 213.13 | 218.27 | 221.46 | 227.29 |
| Samples | n | Samples | n | ||
|---|---|---|---|---|---|
| SR | 0.978 | 2.017 | / | / | / |
| 2 wt.% f-SiO2/SR | 0.975 | 2.841 | 2 wt.% SiO2/SR | 0.967 | 3.887 |
| 4 wt.% f-SiO2/SR | 0.953 | 8.763 | 4 wt.% SiO2/SR | 0.921 | 12.365 |
| 6 wt.% f-SiO2/SR | 0.924 | 20.136 | 6 wt.% SiO2/SR | 0.887 | 28.760 |
| 8 wt.% f-SiO2/SR | 0.887 | 38.456 | 8 wt.% SiO2/SR | 0.842 | 43.566 |
| 10 wt.% f-SiO2/SR | 0.853 | 49.453 | 10 wt.% SiO2/SR | 0.803 | 61.391 |
| Samples | T10 (°C) | Tmax (°C) | R1000 (%) |
|---|---|---|---|
| SR | 483.4 | 554.3 | 58.6 |
| 10 wt.% f-SiO2/SR | 518.3 | 603.2 | 78.9 |
| 10 wt.% SiO2/SR | 507.9 | 593.5 | 77.2 |
| 6 wt.% f-SiO2/SR | 492.1 | 587.7 | 75.3 |
| 6 wt.% SiO2/SR | 500.3 | 571.3 | 67.2 |
| Samples | MC (g/mol) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 wt.% | 4 wt.% | 6 wt.% | 8 wt.% | 10 wt.% | |
| f-SiO2/SR | 2159 | 2278 | 2267 | 2372 | 2451 | 2512 |
| SiO2/SR | 2159 | 2367 | 2416 | 2563 | 2598 | 2725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, W.; Zhou, Q.; Meng, Y.; Zhong, Y.; Xu, J.; Xiao, C.; Zhang, G.; Zhang, Y. Effects of Vinyl Functionalized Silica Particles on Thermal and Mechanical Properties of Liquid Silicone Rubber Nanocomposites. Polymers 2023, 15, 1224. https://doi.org/10.3390/polym15051224
Zhang Y, Liu W, Zhou Q, Meng Y, Zhong Y, Xu J, Xiao C, Zhang G, Zhang Y. Effects of Vinyl Functionalized Silica Particles on Thermal and Mechanical Properties of Liquid Silicone Rubber Nanocomposites. Polymers. 2023; 15(5):1224. https://doi.org/10.3390/polym15051224
Chicago/Turabian StyleZhang, Yulong, Wei Liu, Qiang Zhou, Yiting Meng, Ye Zhong, Jing Xu, Chuan Xiao, Guangpu Zhang, and Yanan Zhang. 2023. "Effects of Vinyl Functionalized Silica Particles on Thermal and Mechanical Properties of Liquid Silicone Rubber Nanocomposites" Polymers 15, no. 5: 1224. https://doi.org/10.3390/polym15051224





