Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaves Extract Production
2.3. Preparation of AgNPs
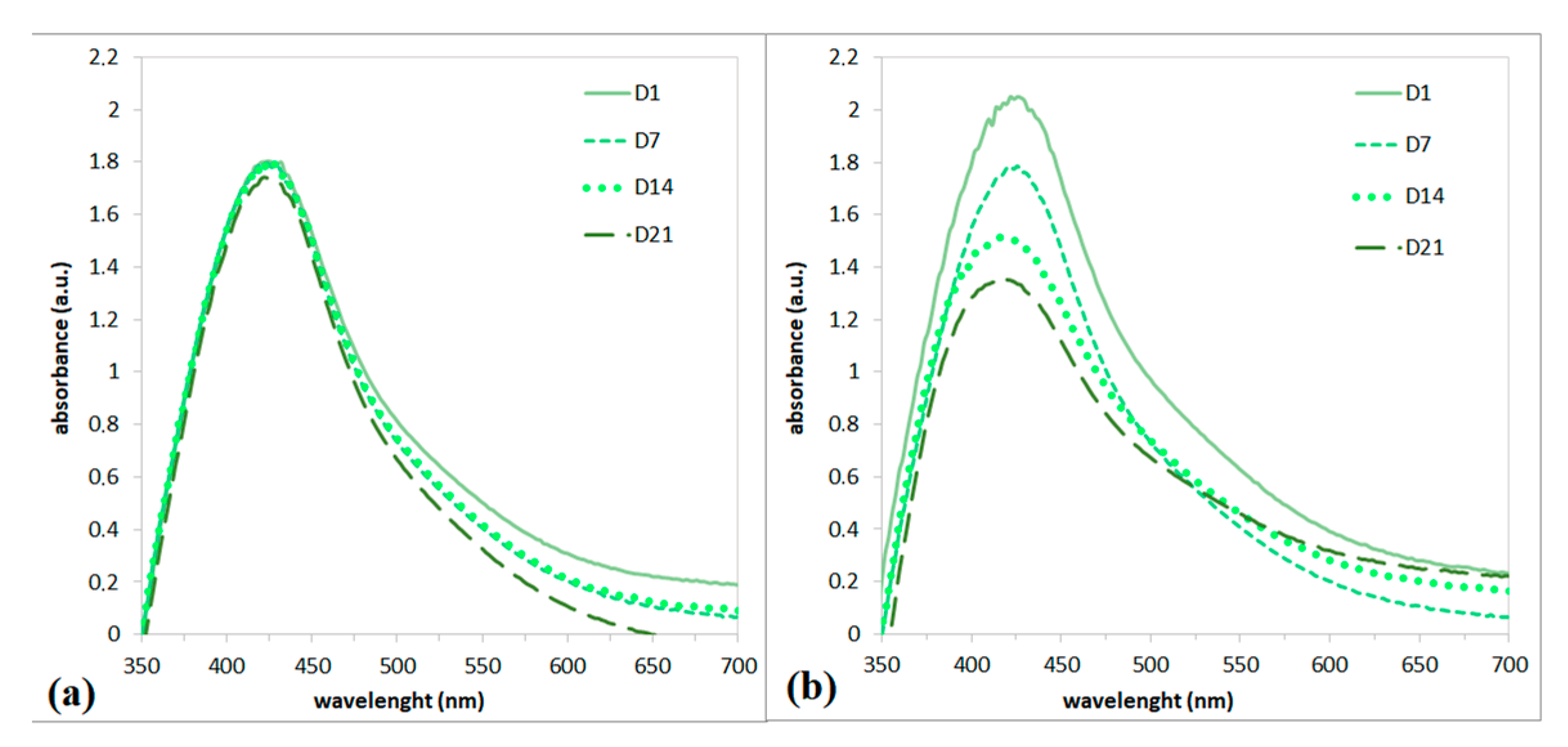
2.4. Long-Term Stability of AgNPs
- -
- L-Cold–AgNPs colloid stored in the refrigerator (~5 °C);
- -
- L-RT–AgNPs colloid stored at room temperature (~25 °C).
2.5. Preparation of Polymer Nanocomposite
2.6. Characterization of AgNPs and PVA-AgNPs Composites
2.7. The Anti-Biofilm Activity
3. Results and Discussion
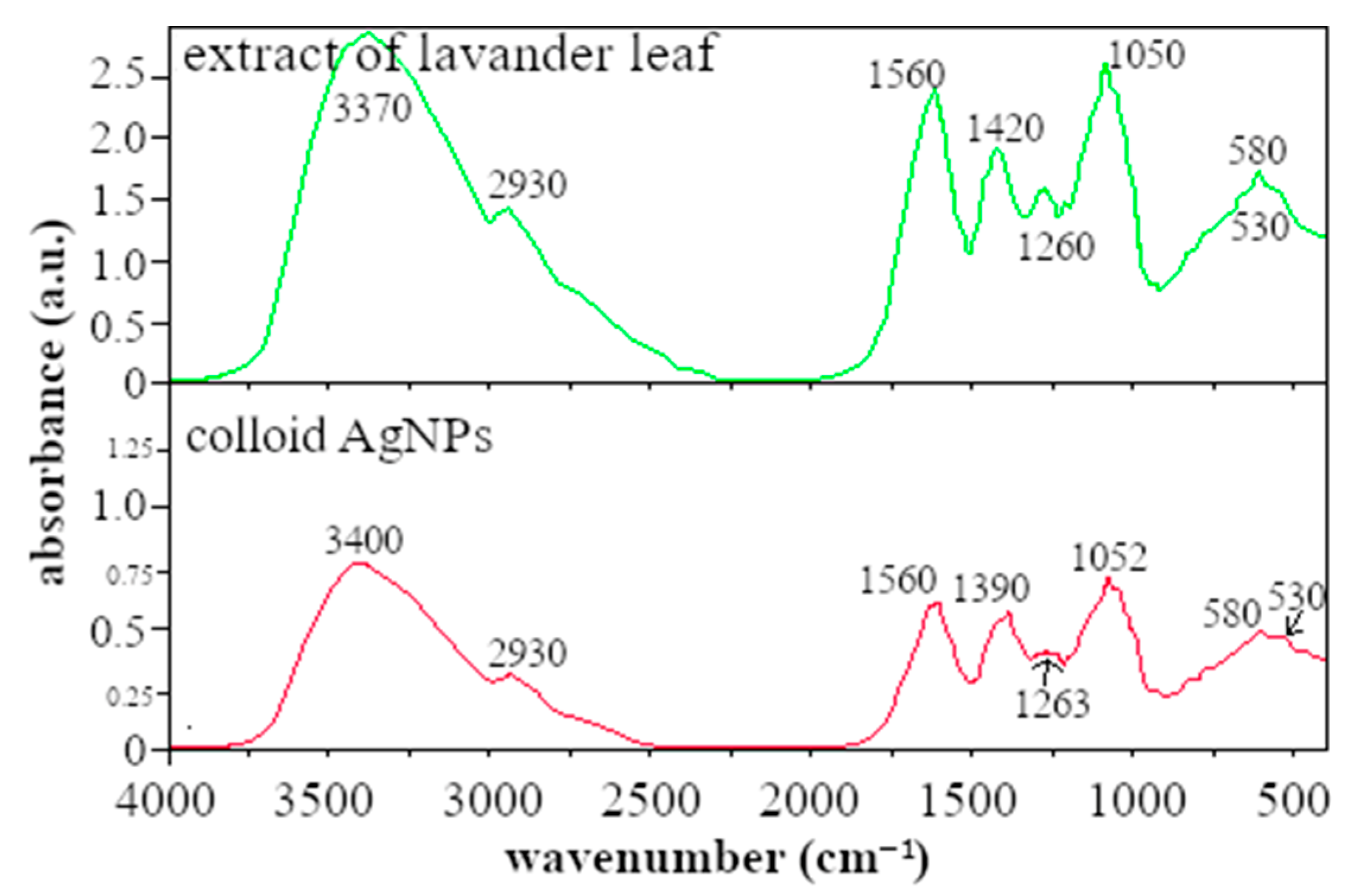
3.1. FTIR Analysis
3.2. UV-Vis Analysis
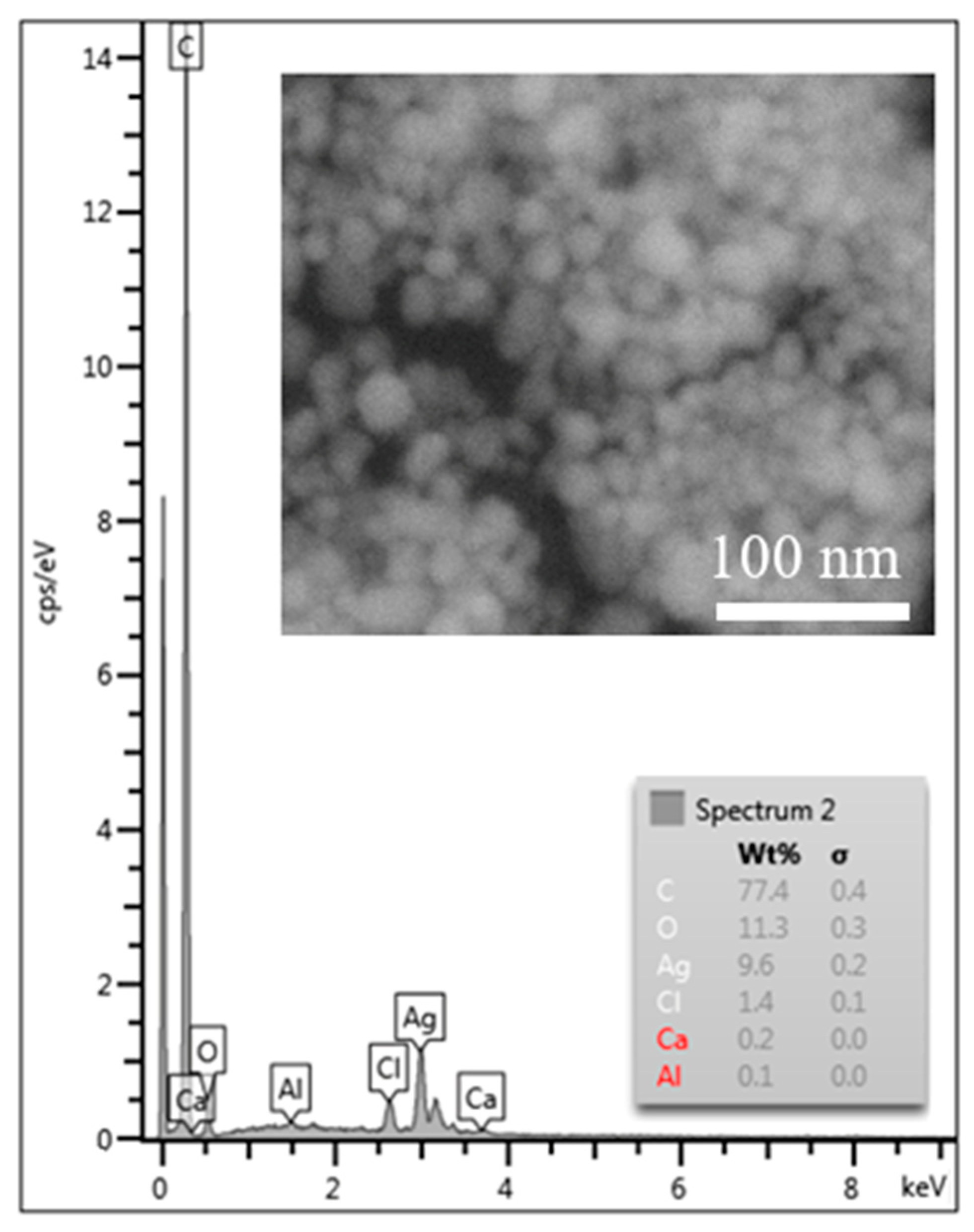
3.3. TEM and SEM Analyses
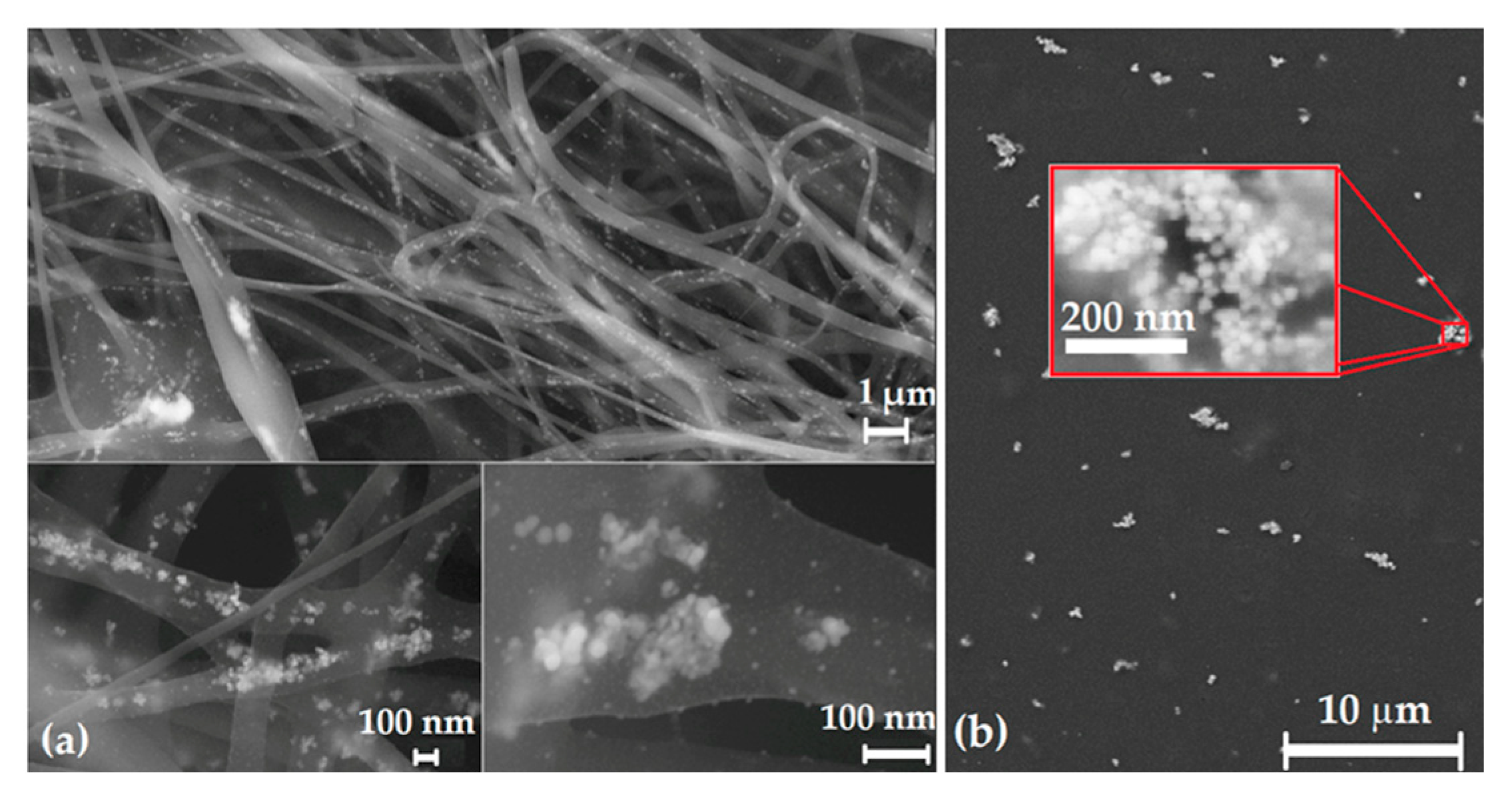
3.4. Polymer Matrix Nanocomposite (Nanofibers, Nanofilm)
3.5. Anti-Biofilm Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages. Adv. Colloid Interface Sci. 2021, 15, 102597. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-based green synthesis of metallic nanoparticles: Applications beyond fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. 2016, 62, 719–724. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, L.; Mao, J.; Huang, L.; Zhou, J. Transformers enhanced segmentation network for accurate nanoparticle size measurement of TEM images. Powder Technol. 2022, 407, 117673. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unnithan, A.R.; Arathyram, R.S.; Kim, C.S. Chapter 3—Electrospinning of polymers for tissue engineering. In Nanotechnology Applications for Tissue Engineering, 1st ed.; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 45–55. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L. Biosynthesis of silver nanoparticles using lavender leaf and their applications for catalytic, sensing, and antioxidant activities. Nanotechnol Rev. 2016, 5, 521–528. [Google Scholar] [CrossRef]
- Hassanin, M.S.; Emam, M.; Soliman, M.M.H.; Abdel Latif, R.R.; Salem, M.M.; El Raey, M.A.; Eisa, W.H. Green silver nanoparticles based on Lavandula coronopifolia aerial parts extract against mycotic mastitis in cattle. Biocatal. Agric. Biotechnol. 2022, 42, 102350. [Google Scholar] [CrossRef]
- Hassanin, H.A.; Taha, A.; Afkar, E. Novel bio-mediated Ag/Co3O4 nanocomposites of different weight ratios using aqueous neem leaf extract: Catalytic and microbial behaviour. Ceram. Int. 2021, 47, 3099–3107. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Shaik, J.P.; Ahamed, A.; Mahmood, A.; Khan, M.; et al. Plant-Extract-Assisted Green Synthesis of Silver Nanoparticles Using Origanum vulgare L. Extract and Their Microbicidal Activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; Bharath, K.N. Nanoparticles Filled Polymer Nanocomposites. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Greene, J.P. Polymer composite. Mater. Process. 2021, 191–222. [Google Scholar]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, C.H.; Tijing, L.D.; Lee, D.H.; Yu, M.H.; Pant, H.R.; Kim, Y.; Kim, C.S. Preparation and Characterization of (polyurethane/nylon-6) Nanofiber/(silicone) Film Composites via Electrospinning and Dip-coating. Fibers Polym. 2012, 13, 39–345. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Jha, B. 24-Branched 5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, V.; Melchias, G.; Kumaravel, P. Biogenic silver nanoparticles mediated by Broussonetia Papyrifera: Anticancer and antimicrobial activity against pathogenic organisms. Asian J. Pharm. Clin. Res. 2017, 10, 93–98. [Google Scholar]
- Kourmouli, A.; Valenti, M.; Rijn, E.; Beaumont, H.J.E.; Kalantzi, O.I.; Schmidt-Ott, A.; Biskos, G. Can disc diffusion susceptibility tests assess the antimicrobial activity of engineered nanoparticles? Nanopart Res. 2018, 20, 62. [Google Scholar] [CrossRef] [Green Version]
- Al Sufyani, N.M.; Hussein, N.A.; Hawsawi, Y.M. Characterization and Anticancer Potential of Silver Nanoparticles Biosynthesized from Olea chrysophylla and Lavandula dentata Leaf Extracts on HCT116 Colon Cancer Cells. J. Nanomater. 2019, 2019, 7361695. [Google Scholar] [CrossRef] [Green Version]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 23. [Google Scholar]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R. Properties of Basil and Lavender Essential Oils adsorbed on the Surface of Hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Mohandass, C.; Vijayaraj, A.S.; Rajasabapathy, R. Biosynthesis of Silver Nanoparticles from Marine Seaweed Sargassum cinereum and their Antibacterial Activity. Indian J. Pharm. Sci. 2013, 75, 606–610. [Google Scholar] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Katata-Seru, L. Biosynthesis, characterization, and antimicrobial effect of silver nanoparticles obtained using Lavandula x intermedia. Res. Chem. Intermed. 2017, 43, 1383–1394. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Kodam, K.M. Encapsulation of therapeutic lavender oil in an electrolyte assisted polyacrylonitrile nanofibres for antibacterial applications. RSC Adv. 2016, 79, 54892–54901. [Google Scholar] [CrossRef] [Green Version]
- Ekemini, I.; Lin, Y.; Chandrabhan, V.; Akram, A.; Onyewuchi, A.; Eno, E.E. Multifunctional silver nanocomposite: A potential material for antiscaling, antimicrobial and anticorrosive applications. JCIS Open. 2021, 3, 100012. [Google Scholar]
- Ahmed, S.W.; Anwar, H.; Shama, A.; Shah, M.R.; Ahmed, A.; Ali, S.A. Synthesis and chemosensing of nitrofurazone using olive oil based silver nanoparticles (O-AgNPs). Sens. Actuators 2019, 256, 429–439. [Google Scholar] [CrossRef]
- Baláž, M.; Bedlovičová, Z. Mechanochemistry as an Alternative Method of Green Synthesis of Silver Nanoparticles with Antibacterial Activity: A Comparative Study. Nanomaterials 2021, 11, 1139. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, M.N.; Chen, R.; Shar, A.H.; Chand, K.; Shah, A.H.; Ahmed, M.; Ali, I.; Ahmed, R.; Liu, J.; Takahashi, K.; et al. Eco-friendly green synthesis of clove buds extract functionalized silver nanoparticles and evaluation of antibacterial and antidiatom activity. J. Microbiol. Methods 2020, 173, 105934. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant extract mediated silver nanoparticles and their applications as antimicrobials and in sustainable food packaging: A state-of-the-art review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Mishra, D.P.; Azam, S. Experimental investigation on effects of particle size, dust concentration and dust-dispersion-air pressure on minimum ignition temperature and combustion process of coal dust clouds in a G-G furnace. Fuel 2018, 227, 424–433. [Google Scholar] [CrossRef]
- Gokce, Y.; Cengiz, B.; Yildiz, N.; Calimli, A.; Aktas, Z. Ultrasonication of chitosan nanoparticle suspension: Influence on particle size. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 75–81. [Google Scholar] [CrossRef]
- Sumitomo, S.; Koizumi, H.; Uddin, M.A.; Kato, Y. Comparison of dispersion behavior of agglomerated particles in liquid between ultrasonic irradiation and mechanical stirring. Ultrason. Sonochemistry 2018, 40, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Szerencsés, B.; Igaz, N.; Tóbiás, Á.; Prucsi, Z.; Rónavári, A.; Bélteky, P.; Madarász, D.; Papp, C.; Makra, I.; Vágvölgyi, C.; et al. Size-dependent activity of silver nanoparticles on the morphological switch and biofilm formation of opportunistic pathogenic yeasts. BMC Microbiol. 2020, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Z.F.; Li, C.T. Biosynthesis of Silver Nanoparticles Using Lavandula stoechas and an Enhancement of Its Antibacterial Activity with Antibiotics. Biotechnol. Bioprocess Eng. 2021, 26, 650–659. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mačák, L.; Velgosova, O.; Múdra, E.; Vojtko, M.; Dolinská, S. Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix. Polymers 2023, 15, 1238. https://doi.org/10.3390/polym15051238
Mačák L, Velgosova O, Múdra E, Vojtko M, Dolinská S. Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix. Polymers. 2023; 15(5):1238. https://doi.org/10.3390/polym15051238
Chicago/Turabian StyleMačák, Lívia, Oksana Velgosova, Erika Múdra, Marek Vojtko, and Silvia Dolinská. 2023. "Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix" Polymers 15, no. 5: 1238. https://doi.org/10.3390/polym15051238
APA StyleMačák, L., Velgosova, O., Múdra, E., Vojtko, M., & Dolinská, S. (2023). Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix. Polymers, 15(5), 1238. https://doi.org/10.3390/polym15051238







