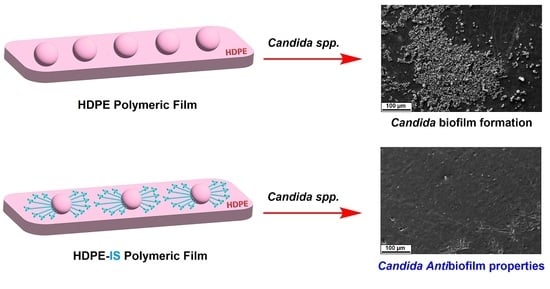
Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, A.S.; Miranda, I.M.; Branco, J.; Soares, M.M.; Vaz, C.P.; Rodrigues, A.G. Adhesion, Biofilm Formation, Cell Surface hydrophobicity, and Antifungal Planktonic Susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 1–8. [Google Scholar]
- Fox, E.P.; Bui, C.K.; Nett, J.E.; Hartooni, N.; Mui, M.C.; Andes, D.R.; Nobile, C.J.; Johnson, A.D. An Expanded Regulatory Network Temporally Controls Candida albicans Biofilm Formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Oncu, S. Optimal Dosage and Dwell Time of Ethanol Lock Therapy on Catheters Infected with Candida species. Clin. Nutr. 2014, 33, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Kidd, S.E.; Ellis, D.H.; Griesser, H.J. Biomaterials Surfaces Capable of Resisting Fungal Attachment and Biofilm Formation. Biotechnol. Adv. 2014, 32, 296–307. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Falk, S.P.; Masters, K.S.; Weisblum, B.; Gellman, S.H. Nylon-3 Polymers Active Against Drug-Resistant Candida albicans Biofilms. J. Am. Chem. Soc. 2015, 137, 2183–2186. [Google Scholar] [CrossRef] [Green Version]
- Ramage, G.; Robertson, S.N.; Williams, C. Strength in Numbers: Antifungal Strategies Against Fungal Biofilms. Int. J. Antimicrob. Agents 2014, 43, 114–120. [Google Scholar] [CrossRef]
- Pannanusorn, S.; Fernandez, V.; Römling, U. Prevalence of Biofilm Formation in Clinical Isolates of Candida species Causing Bloodstream Infection. Mycoses 2013, 56, 264–272. [Google Scholar] [CrossRef]
- Costa, A.C.B.P.; Pereira, C.A.; Freire, F.; Junqueira, J.C.; Jorge, A.O.C. Methods for Obtaining Reliable and Reproducible Results in Studies of Candida Biofilms Formed in vitro. Mycoses 2013, 56, 614–622. [Google Scholar] [CrossRef]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal Drug Resistance in Candida species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef]
- Seddiki, S.M.L.; Boucherit-Otmani, Z.; Boucherit, K.; KunKel, D. Infectivités Fongiques des Cathéters Implantes dues à Candida sp. Formation des biofilms et Résistance. J. De Mycol. Médicale 2015, 25, 130–135. [Google Scholar] [CrossRef]
- Azizi, M.; Farag, N.; Khardori, N. Antifungal Activity of Amphotericin B and Voriconazole Against the Biofilms and Biofilm-Dispersed Cells of Candida albicans Employing a Newly Developed in vitro Pharmacokinetic Model. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourdanesh, F.; Jebali, A.; Hekmatimoghaddam, S.; Allaveisie, A. In vitro and in vivo Evaluation of a New Nanocomposite, containing High Density Polyethylene, Tricalcium Phosphate, Hydroxyapatite, and Magnesium Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 40, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Niechajev, I. Facial Reconstruction Using Porous High-Density Polyethylene (Medpor): Long-Term Results Aesthetic. Plast. Surg. 2012, 36, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Kook, M.S.; Jung, S.; Park, H.J.; Ohk, S.H.; Oh, H.K. Plasma Treated High-Density Polyethylene (HDPE) Medpor Implant Immobilized With rhBMP-2 for Improving the Bone Regeneration. J. Nanomater. 2014, 2014, 810404. [Google Scholar] [CrossRef] [Green Version]
- Reznickova, A.; Novotna, Z.; Kolska, Z.; Kasalkova, N.S.; Rimpelova, S.; Svorcik, V. Enhanced Adherence of Mouse Fibroblast and Vascular Cells to Plasma Modified Polyethylene. Mater. Sci. Eng. C 2015, 52, 259–266. [Google Scholar] [CrossRef]
- Durbec, M.; Mayer, N.; Ciolino, D.V.; Disant, F.; Gerin, F.M.; Groult, E.P. Reconstruction du Cartilage Nasal par Ingénierie Tissulaire à Base de Polyéthylène de Haute Densité et d’un Hydrogel. Pathol. Biol. 2014, 62, 137–145. [Google Scholar] [CrossRef]
- Sobczac, M.; Debek, C.; Oledzka, E.; Kozlowski, R. Polymeric Systems of Antimicrobial Peptides- Strategies and Potential. Appl. Mol. 2013, 18, 14122–14137. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Borowiecki, P.; Krawczyk, M.M.; Plenkiewicz, J. Chemoenzymatic Synthesis and Biological Evaluation of Enantiomerically Enriched 1-(β-hydroxypropyl)Imidazolium- and Triazolium-Based Ionic Liquids Beilstein. J. Org. Chem. 2013, 9, 516–525. [Google Scholar]
- Pilz-Junior, H.L.; de Lemos, A.B.; de Almeida, K.N.; Corção, G.; Schrekker, H.S.; Silva, C.E.; da Silva, O.S. Microbiota potentialized larvicidal action of imidazolium salts against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 16164–16172. [Google Scholar] [CrossRef] [Green Version]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium salts as antifungal agents: Activity against emerging yeast pathogens, without human leukocyte toxicity. MedChemComm 2013, 4, 1457–1460. [Google Scholar] [CrossRef]
- Riduan, S.T.; Zhang, Y. Imidazolium Salts and Their Polymeric Materials for Biological Applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gilmore, B.F. The Antimicrobial Potential of Ionic Liquids: A Source of Chemical Diversity for Infection and Biofilm Control International. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic Liquids for Energy, Materials, and Medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, H.; Riduan, S.N.; Ying, J.Y.; Zhang, Y. Short Imidazolium Chains Effectively Clear Fungal Biofilm in Keratitis Treatment. Biomaterials 2013, 34, 1018–1023. [Google Scholar] [CrossRef]
- McCann, M.; Curran, R.; Shoshan, M.B.; Mckee, V.; Devereux, M.; Kevin, K.; Kellett, A. Synthesis, Sctructure and Biological Activity of Silver(I) Complexes of Substituted Imidazoles. Polyhedron 2013, 56, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Zhou, C.H.; Rao, X.C. Synthesis and Biological Activities of Novel Amine-Derived bis-Azoles as Potencial Antibacterial and Antifungal Agents European. J. Med. Chem. 2010, 45, 4388–4398. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2015, 60, 66–71. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Balbueno, E.A.; Hatwig, C.; Pippi, B.; Dalla Lana, D.F.; Donato, R.K.; Schrekker, H.S.; Fuentefria, A.M. 1-n-Hexadecyl-3-methylimidazolium methanesulfonate and chloride salts with effective activities against Candida tropicalis biofilms. Lett. Appl. Microbiol. 2015, 61, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Navale, G.R.; Dharne, M.S.; Shinde, S. Antibiofilm Activity of tert-BuOH Functionalized Ionic Liquids With Methylsulfonate Counter Anions. R. Soc. Chem. Adv. 2015, 5, 68136–68142. [Google Scholar]
- Schrekker, C.M.L.; Sokolovicz, Y.C.A.; Raucci, M.G.; Selukar, B.S.; Klitzke, J.S.; Lopes, W.; Leal, C.A.M.; de Souza, I.O.P.; Galland, G.B.; dos Santos, J.H.Z.; et al. Multitask Imidazolium Salt Additives for Innovative Poly(L-lactide) Biomaterials: Morphology Control, Candida spp. Biofilm Inhibition, Human Mesenchymal Stem Cell Biocompatibility, and Skin Tolerance. ACS Appl. Mater. Interfaces 2016, 8, 21163–21176. [Google Scholar] [CrossRef] [PubMed]
- Trafny, E.A.; Lewandowski, R.; Marciniak, I.Z.; Stepinska, M. Use of MTT Assay for Determination of the Biofilm Formation Capacity of Microorganisms in Metalworking Fluids Word. J. Microbiol. Biotechnol. 2013, 29, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, Q.; Zhao, Y. Insights into the Structure and Dynamics of Imidazolium Ionic Liquid and Tetraethylene Glycol Dimethyl Ether Cosolvent Mixtures: A Molecular Dynamics Approach. Nanomaterials 2021, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Benabid, F.Z.; Kharchi, N.; Zouai, F.; Mourad, A.-H.; Benachour, D. Impact of co-mixing technique and surface modification of ZnO nanoparticles using stearic acid on their dispersion into HDPE to produce HDPE/ZnO nanocomposites. Polym. Polym. Compos. 2019, 27, 389–399. [Google Scholar] [CrossRef]
- Seddiki, S.M.L.; Boucherit-Otmani, Z.; Boucherit, K.; Kunkel, D. Fungal Infectivities of Implanted Catheters due to Candida sp. Biofilms Formation and Resistance. J. Med. Mycol. 2015, 25, 130–135. [Google Scholar] [CrossRef]
- Dühring, S.; Germerodt, S.; Skerka, C.; Zipfel, P.F.; Dandekar, T.; Schuster, S. Host-Pathogen Interactions between the Human Innate Immune System and Candida albicans-Understanding and Modeling Defense and Evasion Strategies. Front. Microbiol. 2015, 6, 625. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Le, P.H.; Nguyen, D.H.K.; Aburto-Medina, A.; Linklater, D.P.; Crawford, R.J.; MacLaughlin, S.; Ivanova, E.P. Nanoscale Surface Roughness Influences Candida albicans Biofilm Formation. ACS Appl. Bio Mater. 2020, 3, 8581–8591. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Guarino, V.; Ambrosio, L. Biomimetic strategies for bone repair and regeneration. J. Funct. Biomater. 2012, 3, 688–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Sannino, A.; Ambrosio, L. Proliferation and osteoblastic differentiation of hMSCs on celulose-based hydrogels. J. Appl. Biomater. Funct. Mater. 2011, 10, 302–307. [Google Scholar]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 105, 110046. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Alvarez-Perez, M.A.; Meikle, S.; Ambrosio, L.; Santin, M. Poly(epsilon-lysine) dendrons tethered with phosphoserine increase mesenchymal stem cell differentiation potential of calcium phosphate gels. Tissue Eng. Part A 2014, 20, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2022, 110, 266–272. [Google Scholar] [CrossRef] [PubMed]










| Sample | Tm 1 [°C] | Tc 2 [°C] | ΔHm 3 [J/g] | ΔHc 4 [J/g] | Xc 5 [%] | T5% 6 [°C] | T10% 7 [°C] | T50% 8 [°C] | Residue 9 [%] |
|---|---|---|---|---|---|---|---|---|---|
| HDPE | 132.3 | 116.8 | 209.8 | 221.4 | 71.6 | 429.6 | 443.5 | 483.3 | 0.1 |
| HDPE.Cl.0125 HDPE.MeS.0125 | 131.6 | 117.5 | 212.9 | 216.0 | 72.7 | 416.7 | 441.6 | 482.5 | 0.2 |
| 132.0 | 117.2 | 212.8 | 207.9 | 72.7 | 431.0 | 445.1 | 483.0 | 0 | |
| HDPE.Cl.0250 HDPE.MeS.0250 | 131.7 | 117.6 | 205.7 | 223.9 | 70.4 | 400.8 | 429.3 | 473.1 | 0.7 |
| 131.6 | 117.4 | 207.2 | 211.1 | 70.9 | 415.4 | 434.6 | 474.1 | 0 | |
| HDPE.Cl.0500 HDPE.MeS.0500 | 131.5 | 117.8 | 220.3 | 231.6 | 75.6 | 414.8 | 441.9 | 480.4 | 0.4 |
| 131.8 | 117.3 | 206.1 | 210.1 | 70.7 | 412.2 | 432.6 | 481.4 | 0.7 |
| Sample | G’-40 1 [GPa] | G’40 2 [GPa] | G’’-40 3 [GPa] | G’’40 4 [GPa] | S-40 5 [kN/m] | S40 6 [kN/m] | S90 7 [kN/m] |
|---|---|---|---|---|---|---|---|
| HDPE | 3.33 | 1.48 | 0.07 | 0.17 | 315.45 | 140.50 | 26.71 |
| HDPE.Cl.0125 HDPE.MeS.0125 | 3.14 | 1.44 | 0.05 | 0.16 | 271.63 | 124.96 | 22.05 |
| 3.17 | 1.40 | 0.06 | 0.16 | 349.31 | 154.66 | 28.11 | |
| HDPE.Cl.0250 HDPE.MeS.0250 | 3.28 | 1.52 | 0.07 | 0.16 | 346.19 | 160.34 | 29.83 |
| 3.09 | 1.38 | 0.05 | 0.15 | 288.79 | 129.50 | 23.64 | |
| HDPE.Cl.0500 | 2.44 | 1.12 | 0.04 | 0.12 | 230.62 | 106.03 | 19.45 |
| HDPE.MeS.0500 | 3.18 | 1.45 | 0.05 | 0.16 | 416.42 | 190.24 | 32.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins Leal Schrekker, C.; Sokolovicz, Y.C.A.; Raucci, M.G.; Leal, C.A.M.; Ambrosio, L.; Lettieri Teixeira, M.; Meneghello Fuentefria, A.; Schrekker, H.S. Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials. Polymers 2023, 15, 1259. https://doi.org/10.3390/polym15051259
Martins Leal Schrekker C, Sokolovicz YCA, Raucci MG, Leal CAM, Ambrosio L, Lettieri Teixeira M, Meneghello Fuentefria A, Schrekker HS. Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials. Polymers. 2023; 15(5):1259. https://doi.org/10.3390/polym15051259
Chicago/Turabian StyleMartins Leal Schrekker, Clarissa, Yuri Clemente Andrade Sokolovicz, Maria Grazia Raucci, Claudio Alberto Martins Leal, Luigi Ambrosio, Mário Lettieri Teixeira, Alexandre Meneghello Fuentefria, and Henri Stephan Schrekker. 2023. "Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials" Polymers 15, no. 5: 1259. https://doi.org/10.3390/polym15051259
APA StyleMartins Leal Schrekker, C., Sokolovicz, Y. C. A., Raucci, M. G., Leal, C. A. M., Ambrosio, L., Lettieri Teixeira, M., Meneghello Fuentefria, A., & Schrekker, H. S. (2023). Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials. Polymers, 15(5), 1259. https://doi.org/10.3390/polym15051259