Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
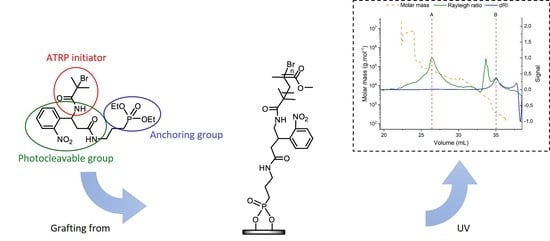
2.1.1. Photocleavable Initiator Synthesis
2.1.2. Model Polymer Synthesis
2.1.3. PMMA Grafting on Ti Plates and Photocleavage
- First, 40 mg of copper bromide (CuBr) (Sigma Aldrich, St. Louis, MO, USA), 18 mL of anisole (Acros Organics, Waltham, MA, USA), and 50 µL of pentamethylenediethyltriamine (PMDETA) (Acros Organics) were added to the Schlenck tube and agitated to achieve a homogenous solution.
- Third, 22 mL of MMA (Acros Organics) filtered from its stabilizer by flowing through a pad of basic aluminum oxide (Acros Organics) was added to the reactor to obtain a 5.3 M monomer solution [16].
3. Characterizations
4. Results and Discussion
4.1. Photocleavable Initiator
4.2. Model Polymer
4.3. Grafted Polymer Characteristics
4.4. PMMA in Solution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic-Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Hybrid materials from organophosphorus coupling molecules. J. Mater. Chem. 2005, 15, 3761–3768. [Google Scholar] [CrossRef]
- Pham, C.; Eck, M.; Krueger, M. Thiol functionalized reduced graphene oxide as a base material for novel graphene-nanoparticle hybrid composites. Chem. Eng. J. 2013, 231, 146–154. [Google Scholar] [CrossRef]
- Farkaš, B.; Terranova, U.; De Leeuw, N.H. Binding modes of carboxylic acids on cobalt nanoparticles. Phys. Chem. Chem. Phys. 2020, 22, 985–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Anchoring of Phosphonate and Phosphinate Coupling Molecules on Titania Particles. Chem. Mater. 2001, 13, 4367–4373. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [Green Version]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef]
- Barbey, R.; Kauffmann, E.; Ehrat, M.; Klok, H.-A. Protein Microarrays Based on Polymer Brushes Prepared via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2010, 11, 3467–3479. [Google Scholar] [CrossRef]
- Wu, L.; Glebe, U.; Böker, A. Surface-initiated controlled radical polymerizations from silica nanoparticles, gold nanocrystals, and bionanoparticles. Polym. Chem. 2015, 6, 5143–5184. [Google Scholar] [CrossRef] [Green Version]
- Bilgic, T.; Klok, H.-A. Surface-initiated controlled radical polymerization enhanced DNA biosensing. Eur. Polym. J. 2015, 62, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Xu, F.-J. Biomolecule-functionalized polymer brushes. Chem. Soc. Rev. 2013, 42, 3394–3426. [Google Scholar] [CrossRef] [PubMed]
- Khabibullin, A.; Mastan, E.; Matyjaszewski, K.; Zhu, S. Surface-Initiated Atom Transfer Radical Polymerization. In Controlled Radical Polymerization at and from Solid Surfaces; Vana, P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 270, pp. 29–76. [Google Scholar] [CrossRef]
- Vergnat, V.; Heinrich, B.; Rawiso, M.; Muller, R.; Pourroy, G.; Masson, P. Iron Oxide/Polymer Core–Shell Nanomaterials with Star-like Behavior. Nanomaterials 2021, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Koylu, D.; Carter, K.R. Stimuli-Responsive Surfaces Utilizing Cleavable Polymer Brush Layers. Macromolecules 2009, 42, 8655–8660. [Google Scholar] [CrossRef]
- Macchione, M.A.; Biglione, C.; Strumia, M. Design, Synthesis and Architectures of Hybrid Nanomaterials for Therapy and Diagnosis Applications. Polymers 2018, 10, 527. [Google Scholar] [CrossRef] [Green Version]
- Reggente, M.; Masson, P.; Dollinger, C.; Palkowski, H.; Zafeiratos, S.; Jacomine, L.; Passeri, D.; Rossi, M.; Vrana, N.E.; Pourroy, G.; et al. Novel Alkali Activation of Titanium Substrates to Grow Thick and Covalently Bound PMMA Layers. ACS Appl. Mater. Interfaces 2018, 10, 5967–5977. [Google Scholar] [CrossRef]
- Kang, C.; Crockett, R.M.; Spencer, N.D. Molecular-Weight Determination of Polymer Brushes Generated by SI-ATRP on Flat Surfaces. Macromolecules 2013, 47, 269–275. [Google Scholar] [CrossRef]
- Pelliccioli, A.P.; Wirz, J. Photoremovable protecting groups: Reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. [Google Scholar] [CrossRef]
- Romano, A.; Roppolo, I.; Giebler, M.; Dietliker, K.; Možina, Š.; Šket, P.; Mühlbacher, I.; Schlögl, S.; Sangermano, M. Stimuli-responsive thiol-epoxy networks with photo-switchable bulk and surface properties. RSC Adv. 2018, 8, 41904–41914. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Roppolo, I.; Rossegger, E.; Schlögl, S.; Sangermano, M. Recent Trends in Applying Ortho-Nitrobenzyl Esters for the Design of Photo-Responsive Polymer Networks. Materials 2020, 13, 2777. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Jiaming, Z.; Rui, L.; Jianying, H.; Jiayan, C.; Xurong, L.; Yutai, L.; Yousi, Z. A novel rate-accelerating additive for atom transfer radical polymerization of styrene. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4082–4090. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, G.; Pan, P. Rate acceleration for 4,4′-dimethoxydiphenyl nitroxide mediated polymerization of methyl methacrylate. RSC Adv. 2016, 6, 97995–98000. [Google Scholar] [CrossRef]
- Reggente, M.; Kriegel, S.; He, W.; Masson, P.; Pourroy, G.; Mura, F.; Faerber, J.; Passeri, D.; Rossi, M.; Palkowski, H.; et al. How alkali-activated Ti surfaces affect the growth of tethered PMMA chains: A close-up study on the PMMA thickness and surface morphology. Pure Appl. Chem. 2019, 91, 1687–1694. [Google Scholar] [CrossRef]
- De Gennes, P.G. Conformations of Polymers Attached to an Interface. Macromolecules 1980, 13, 1069–1075. [Google Scholar] [CrossRef]
- Patil, R.R.; Turgman-Cohen, S.; Šrogl, J.; Kiserow, D.; Genzer, J. On-Demand Degrafting and the Study of Molecular Weight and Grafting Density of Poly(methyl methacrylate) Brushes on Flat Silica Substrates. Langmuir 2015, 31, 2372–2381. [Google Scholar] [CrossRef]
- Fetters, L.J.; Lohsey, D.J.; Colby, R.H. Chain Dimensions and Entanglement Spacings. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Reggente, M.; Harhash, M.; Kriegel, S.; He, W.; Masson, P.; Faerber, J.; Pourroy, G.; Palkowski, H.; Carradò, A. Resin-free three-layered Ti/PMMA/Ti sandwich materials: Adhesion and formability study. Compos. Struct. 2019, 218, 107–119. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouillard, F.; Ferté, T.; Voirin, E.; Méry, S.; Masson, P.; Carradò, A. Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method. Polymers 2023, 15, 1265. https://doi.org/10.3390/polym15051265
Mouillard F, Ferté T, Voirin E, Méry S, Masson P, Carradò A. Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method. Polymers. 2023; 15(5):1265. https://doi.org/10.3390/polym15051265
Chicago/Turabian StyleMouillard, Flavien, Tom Ferté, Emilie Voirin, Stéphane Méry, Patrick Masson, and Adele Carradò. 2023. "Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method" Polymers 15, no. 5: 1265. https://doi.org/10.3390/polym15051265
APA StyleMouillard, F., Ferté, T., Voirin, E., Méry, S., Masson, P., & Carradò, A. (2023). Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method. Polymers, 15(5), 1265. https://doi.org/10.3390/polym15051265