Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
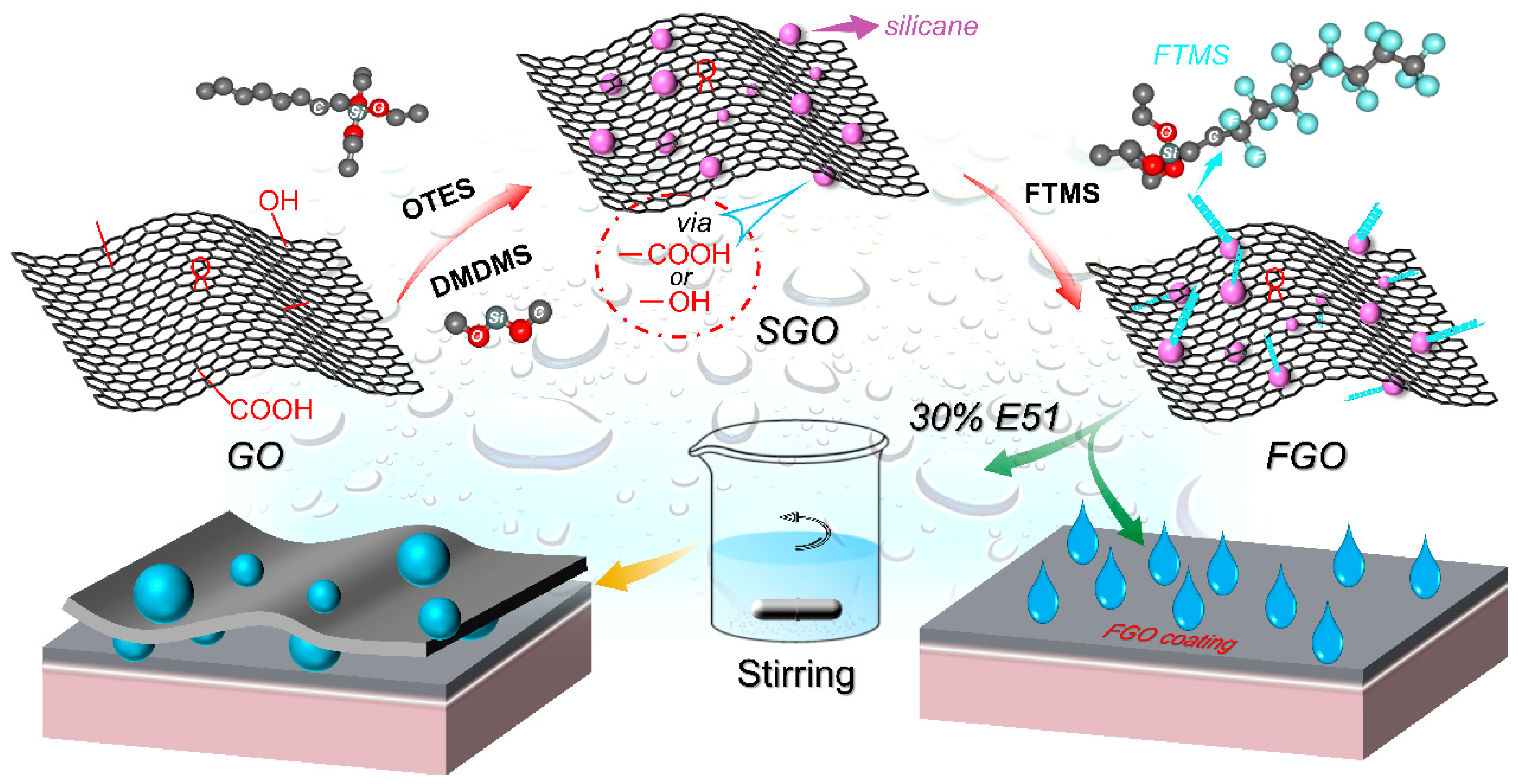
2.2. Preparation of Silane-Modified Graphene Oxide (SGO)
2.3. Preparation of Fluorosilane-Modified Graphene Oxide FGO and E-FGO Composite Coating
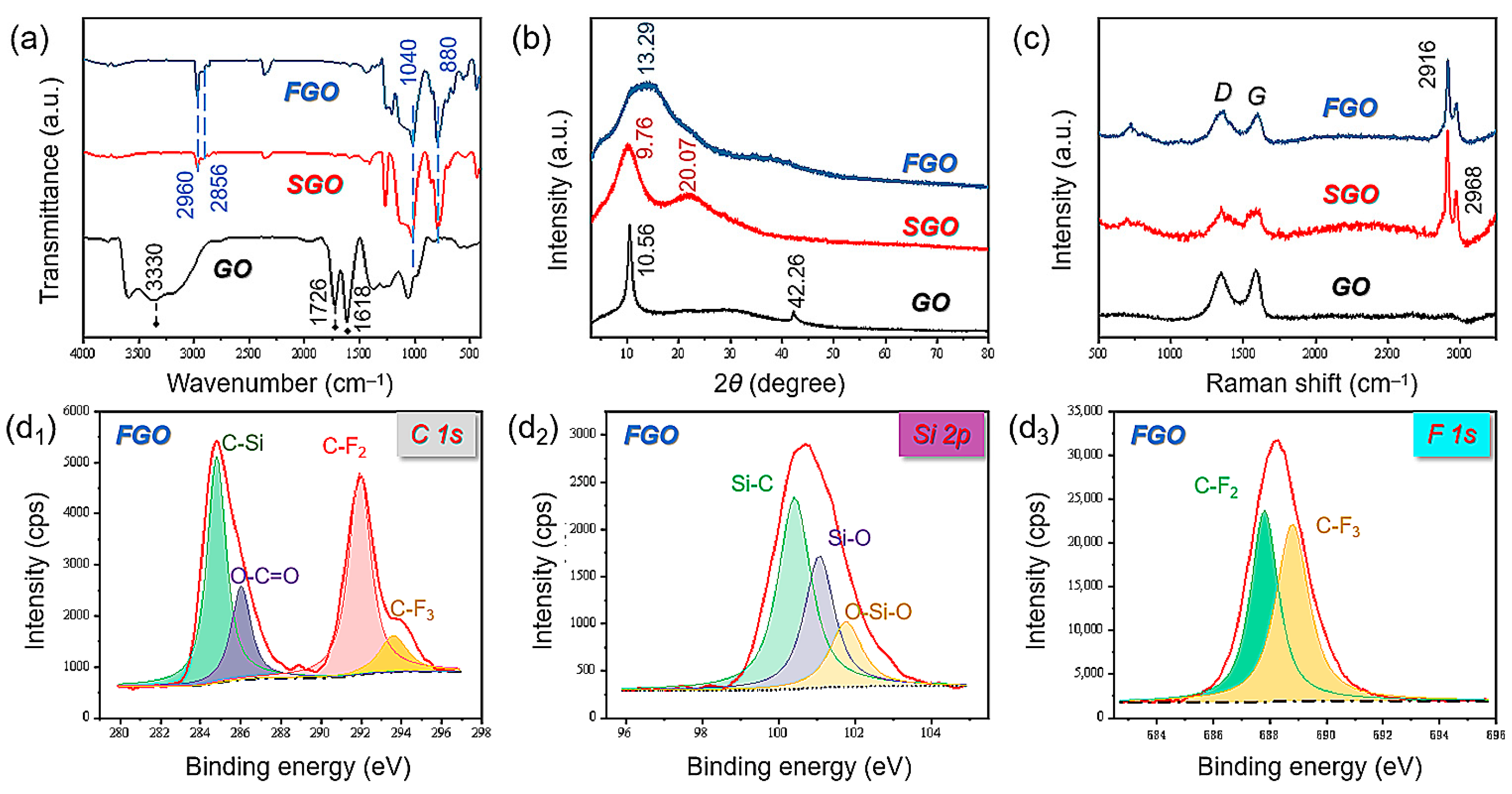
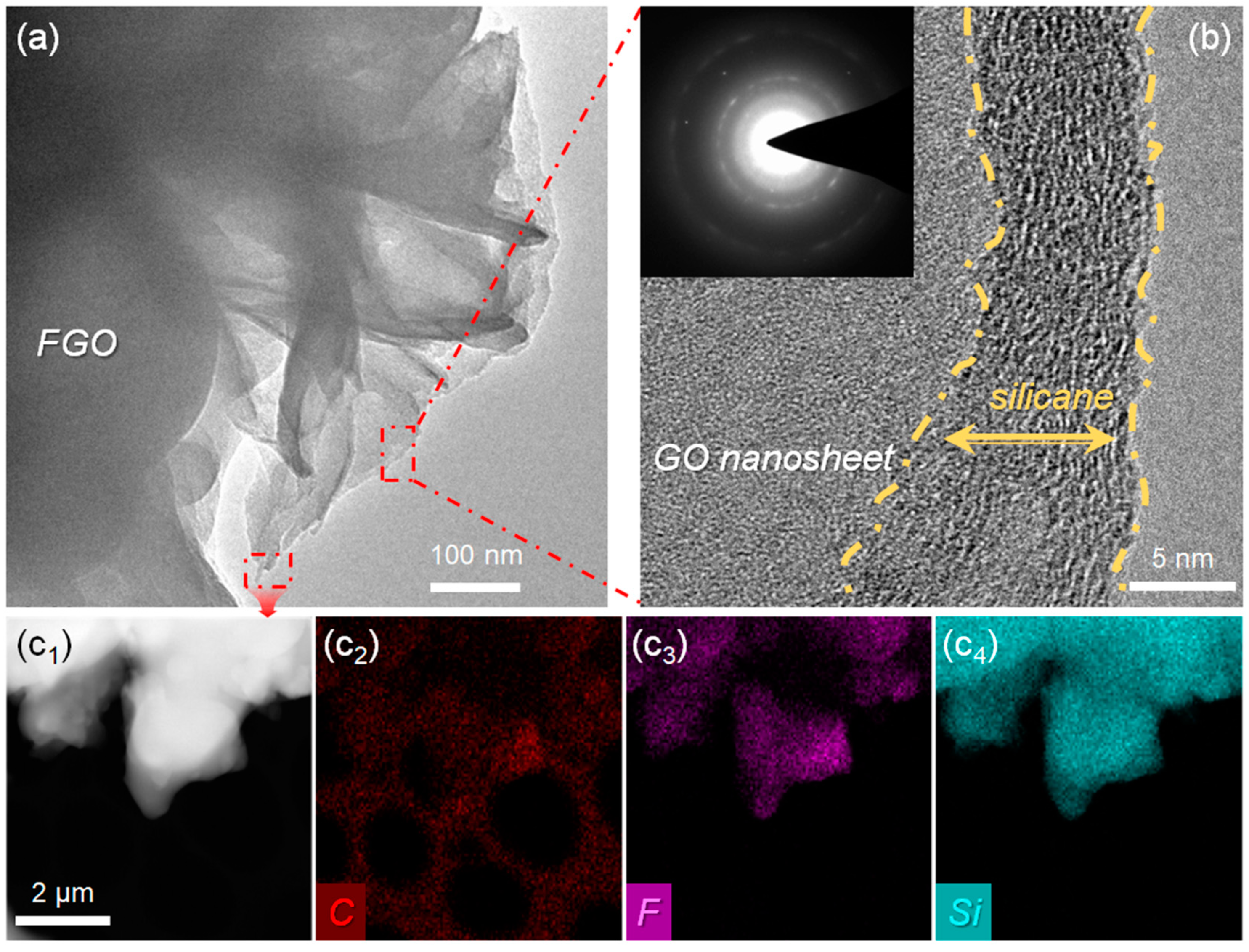
2.4. Characterization
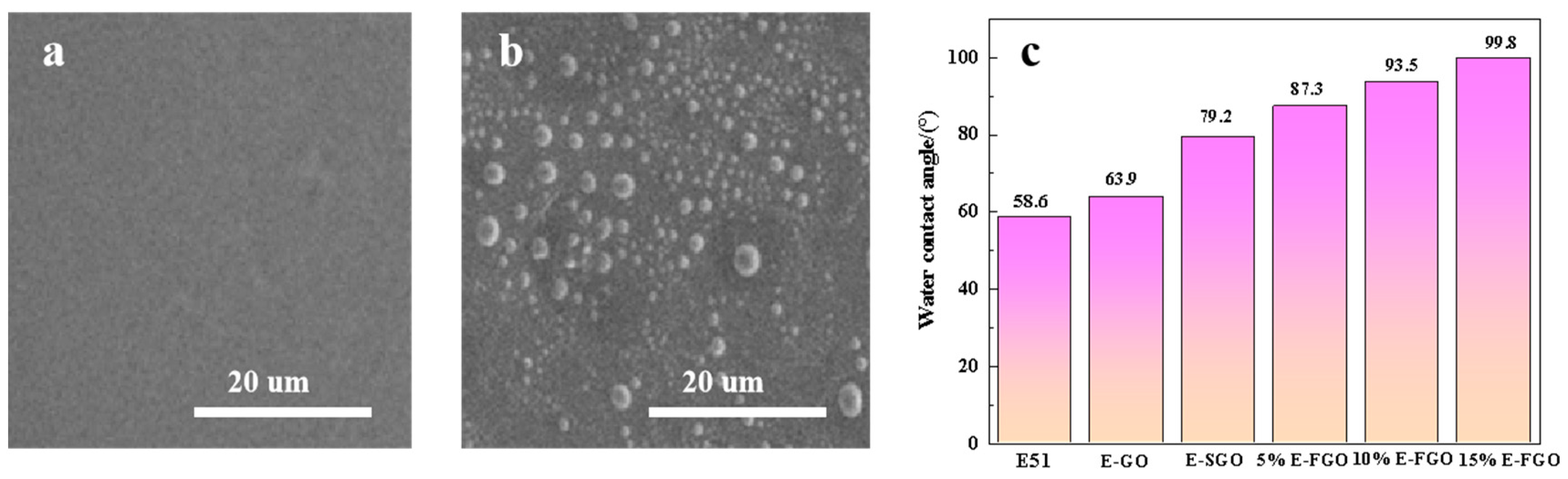
3. Results and Discussions
4. Performance Analysis of E-FGO Composite Coating
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Idumah, C.I.; Obele, C.M.; Emmanuel, E.O.; Hassan, A. Recently emerging nanotechnological advancements in polymer nanocomposite coatings for anti-corrosion, anti-fouling and self-healing. Surf. Interfaces 2020, 21, 100734–100782. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- Liu, C.; Yin, Q.; Zhang, W.B.; Bao, Y.; Li, P.P.; Hao, L.F. Tribological properties of graphene-modified with ionic liquids and carbon quantum dots/bismaleimide composites. Carbon 2021, 183, 504–514. [Google Scholar] [CrossRef]
- Sun, M.; Ma, Z.D.; Li, A.H.; Zhu, G.Y.; Zhang, Y. Anticorrosive performance of polyaniline/waterborne epoxy/poly (methylhydrosiloxane) composite coatings. Prog. Org. Coat. 2020, 139, 105462–105473. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Ma, I.A.W.; Farah, Z.; Vengadaesvaran, B.; Ramesh, S.; Aro, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Figueira R, B. Hybrid sol–gel coatings for corrosion mitigation: A critical review. Polymers 2020, 12, 689–722. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.Y.; Zhang, J.; Qin, Y.; Wang, D.Y. Phytic acid intercalated graphene oxide for anticorrosive reinforcement of waterborne epoxy resin coating. Polymers 2019, 11, 1950–1964. [Google Scholar] [CrossRef]
- Zhang, E.L.; Zhao, X.T.; Hu, J.L.; Wang, R.X.; Fu, S.; Qin, G.W. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Abadchi, M.R.; Mirzaee, M.; Tabar, F.A.; Ramezanzadeh, B. Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf. Interfaces 2021, 23, 100994–101029. [Google Scholar] [CrossRef]
- Gao, F.J.; Mu, J.; Bi, Z.X.; Wang, S.; Li, Z.L. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071–106091. [Google Scholar] [CrossRef]
- Gillam, T.A.; Goh, C.K.; Ninan, N.; Bilimoria, K.; Shirazi, H.S.; Saboohi, S.; Al-Bataineh, S.; Whittle, J.; Blencowe, A. Iodine complexed poly (vinyl pyrrolidone) plasma polymers as broad-spectrum antiseptic coatings. Appl. Surf. Sci. 2021, 537, 147866–147875. [Google Scholar] [CrossRef]
- De, S.; Lutkenhaus, J.L. Corrosion behaviour of eco-friendly airbrushed reduced graphene oxide-poly (vinyl alcohol) coatings. Green Chem. 2018, 20, 506–514. [Google Scholar] [CrossRef]
- Ammar, A.; Shahid, M.; Ahmed, M.; Khan, M.; Khalid, A.; Khan, Z. Electrochemical study of polymer and ceramic-based nanocomposite coatings for corrosion protection of cast iron pipeline. Materials 2018, 11, 332–342. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Li, S.J.; Liu, X. Enhancement of mechanical properties and bonding properties of flake-zinc-powder-modified epoxy resin composites. Polymers 2022, 14, 5323–5332. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.H.; Jiang, S.Y.; Zhou, Y.L.; Gu, X.H.; Zhang, Y.; Kong, X.Z. Preparation and curing mechanism of modified corn straw by 3-Glycidyl ether oxypropyl trimethoxysilane/epoxy resin composites. Polymers 2022, 14, 5233. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.X.; Gao, Y.; Wang, J.; Daniel, J.B.; Serena, T. Freeze-dried graphene oxide modified with trimethylhexamethylene in the mix solvent for improved anti-corrosion property of epoxy. J. Appl. Polym. Sci. 2020, 137, 49139–49152. [Google Scholar] [CrossRef]
- Marra, F.; D’Aloia, A.G.; Tamburrano, A.; Ochando, I.M.; Bellis, G.D.; Ellis, G.; Sarto, M.S. Electromagnetic and dynamic mechanical properties of epoxy and vinylester-based composites filled with graphene nanoplatelets. Polymers 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. A novel coating material that uses nano-sized SiO2 particles to intensify hydrophobicity and corrosion protection properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Ding, J.H.; Zhao, H.R.; Ji, D.; Xu, B.Y.; Zhao, X.P.; Wang, Z.; Wang, D.L.; Zhou, Q.B.; Yu, H.B. Achieving long-term anticorrosion via the inhibition of graphene’s electrical Activity. J. Mater. Chem. 2019, 7, 2864–2874. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.D.; Liu, J.; Wang, X.; Gui, T.J.; Li, B.J.; Tang, Y.Z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Kulyk, B.; Freitas, M.A.; Santos, N.F.; Mohseni, F.; Carvalho, A.F.; Yasakau, K.; Fernandes, A.J.S.; Bernardes, A.; Figueiredo, B.; Silva, R.; et al. A critical review on the production and application of graphene and graphene-based materials in anti-corrosion coatings. Crit. Rev. Solid State Mater. Sci. 2022, 47, 309–355. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.J.; Zhao, W.J.; Zhang, S.T. 2-Mercaptobenzimidazole-inbuilt metal-organic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating. Corros. Sci. 2021, 191, 109715. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Guo, H.F.; Chao, B.; Zhao, Z.Q.; Nan, D. Preparation of aniline trimer modifified graphene oxide new composite coating and study on anticorrosion performance. Mater. Res. Express 2020, 7, 125601–125613. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.Q.; Shi, H.Y.; Zhang, F.Y.; Liu, C.H.; Liang, Y.Y.; Pi, K. Co-modification of nano-silica and lysine on graphene oxide nanosheets to enhance the corrosion resistance of waterborne epoxy coatings in 3.5% NaCl solution. Polymer 2021, 222, 123665. [Google Scholar] [CrossRef]
- Wang, H.Y.; Di, D.Y.; Zhao, Y.M.; Yuan, R.H.; Zhu, Y.J. A multifunctional polymer composite coating assisted with pore-forming agent: Preparation, superhydrophobicity and corrosion resistance. Prog. Org. Coat. 2019, 132, 370–378. [Google Scholar] [CrossRef]
- Du, X.Q.; Liu, Y.W.; Chen, Y. Enhancing the corrosion resistance of aluminum by superhydrophobic silane/graphene oxide coating. Appl. Phys. A 2021, 127, 580. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Guo, H.; Zhang, H.H.; Zhang, N.; Hayat, T.; Sun, Y.B. Decontamination of U (VI) on graphene oxide/Al2O3 composites investigated by XRD, FT-IR and XPS techniques. Environ. Pollut. 2019, 248, 332–338. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, B.; Zhu, X.; Meng, L.; Huang, Y. Investigation of thermostability of modified graphene oxide/methylsilicone resin nanocomposites. Eng. Sci. 2018, 5, 73–78. [Google Scholar] [CrossRef]
- Shu, S.S.; Wu, L.; Liu, J.M.; Liu, Y.; Hu, Y.; Xu, J.H.; Wang, L. Synthesis and corrosion Resistance of silane coupling agent modified graphene oxide/waterborne polyurethane. IOP Conf. Ser. Mater. Sci. Eng. 2019, 631, 22085. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H. Fabrication of silane-grafted graphene oxide and its effect on the structural, thermal, mechanical, and hysteretic behavior of polyurethane. Sci. Rep. 2020, 10, 19152. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.S.; Rees, G.J.; Kelly, N.L.; Dancer, C.E.J.; Hanna, J.V.; McNally, T. Facile silane functionalization of graphene oxide. Nanoscale 2018, 10, 16231–16242. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Yari, H.; Sari, M.G.; Azizi, A. Fabrication of a highly hard yet tough epoxy nanocomposite coating by incorporating graphene oxide nanosheets dually modified with amino silane coupling agent and hyperbranched polyester-amide. Prog. Org. Coat. 2022, 162, 106570. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Li, H.J.; Zhao, Z.X.; Wang, Y.Z.; Wang, X.M. The use of in-situ Raman spectroscopy in investigating carbon materials as anodes of alkali metal-ion batteries. New Carbon Mater. 2021, 36, 93–105. [Google Scholar] [CrossRef]
- Okuda, H.; Young, R.J.; Wolverson, D.; Tanaka, F.; Yamamoto, G.; Okabe, T. Investigating nanostructures in carbon fibres using Raman spectroscopy. Carbon 2018, 130, 178–184. [Google Scholar] [CrossRef]
- Yao, J.; Wang, M.; Bai, Y. Automobile active tilt based on active suspension with H∞ robust control. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2021, 235, 1320–1329. [Google Scholar] [CrossRef]
- Chu, Z.M.; Jiao, W.C.; Huang, Y.F.; Yan, M.L.; Zheng, Y.T.; Wang, R.G.; He, X.D. Smart superhydrophobic films with self-sensing and anti-icing properties based on silica nanoparticles and graphene. Adv. Mater. Interfaces 2020, 7, 2000492. [Google Scholar] [CrossRef]
- Li, Z.J.; He, Y.; Yan, S.M.; Li, H.J.; Chen, J.; Zhang, C.; Li, C.H.; Zhao, Y.; Fan, Y.; Guo, C.H. A novel silk fibroin-graphene oxide hybrid for reinforcing corrosion protection performance of waterborne epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127959. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Li, M.; Zhang, M.; Xiao, L.; Shang, L.; Ao, Y. Improving interfacial properties of hierarchical reinforcement carbon fibers modified by graphene oxide with different bonding types. Compos. Part A Appl. Sci. Manuf. 2018, 107, 616–625. [Google Scholar] [CrossRef]
- An, Q.F.; Wang, K.F.; Jia, Y. Film morphology, orientation and performance of dodecyl/carboxyl functional polysiloxane on cotton substrates. Appl. Surf. Sci. 2011, 257, 4569–4574. [Google Scholar] [CrossRef]
- Yang, B.W.; Lu, P.; An, Q.F.; Zhu, C. Protective effect of silicone-modified epoxy resin coating on iron-based materials against sulfate-reducing bacteria-induced corrosion. Prog. Org. Coat. 2023, 174, 107241–107251. [Google Scholar] [CrossRef]
- Sam, E.K.; Sam, D.K.; Lv, X.M.; Liu, B.T.; Xiao, X.X.; Gong, S.H.; Yu, W.T.; Chen, J.; Liu, J. Recent development in the fabrication of self-healing superhydrophobic surfaces. Chem. Eng. J. 2019, 373, 531–546. [Google Scholar]
- Zhang, D.; Zhang, H.Q.; Zhao, S.; Li, Z.G.; Hou, S.X. Electrochemical impedance spectroscopy evaluation of corrosion protection of X65 carbon steel by halloysite nanotube-filled epoxy composite coatings in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2019, 14, 4659–4667. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior corrosion protection and adhesion strength of epoxy coating applied on AZ31 magnesium alloy pre-treated by PEO/Silane with inorganic and organic corrosion inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym. Plast. Technol. Mater. 2020, 59, 658–673. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Lin, Y.; Xue, X.; Yuan, Q.; Zhang, W.; Bao, Y.; Ma, J. Tribological properties of bismaleimide-based self-lubricating composite enhanced by MoS2 quantum dots/graphene hybrid. Compos. Commun. 2021, 28, 100922. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, Y.; Dai, F.; Huang, H.; Zhong, L.; Zhang, X. Ammonium-grafted graphene oxide for enhanced corrosion resistance of waterborne epoxy coatings. Surf. Coat. Technol. 2020, 383, 125227. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, C.; Zhang, X.; Lin, D.; Xu, F.; Zhu, Y.; Wang, H.; Gong, J.; Wang, T. Highly orientated graphene/epoxy coating with exceptional anti-corrosion performance for harsh oxygen environments. Corros. Sci. 2020, 176, 109049. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, F.; Qiang, Y.; Zhao, W. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021, 176, 39–51. [Google Scholar] [CrossRef]

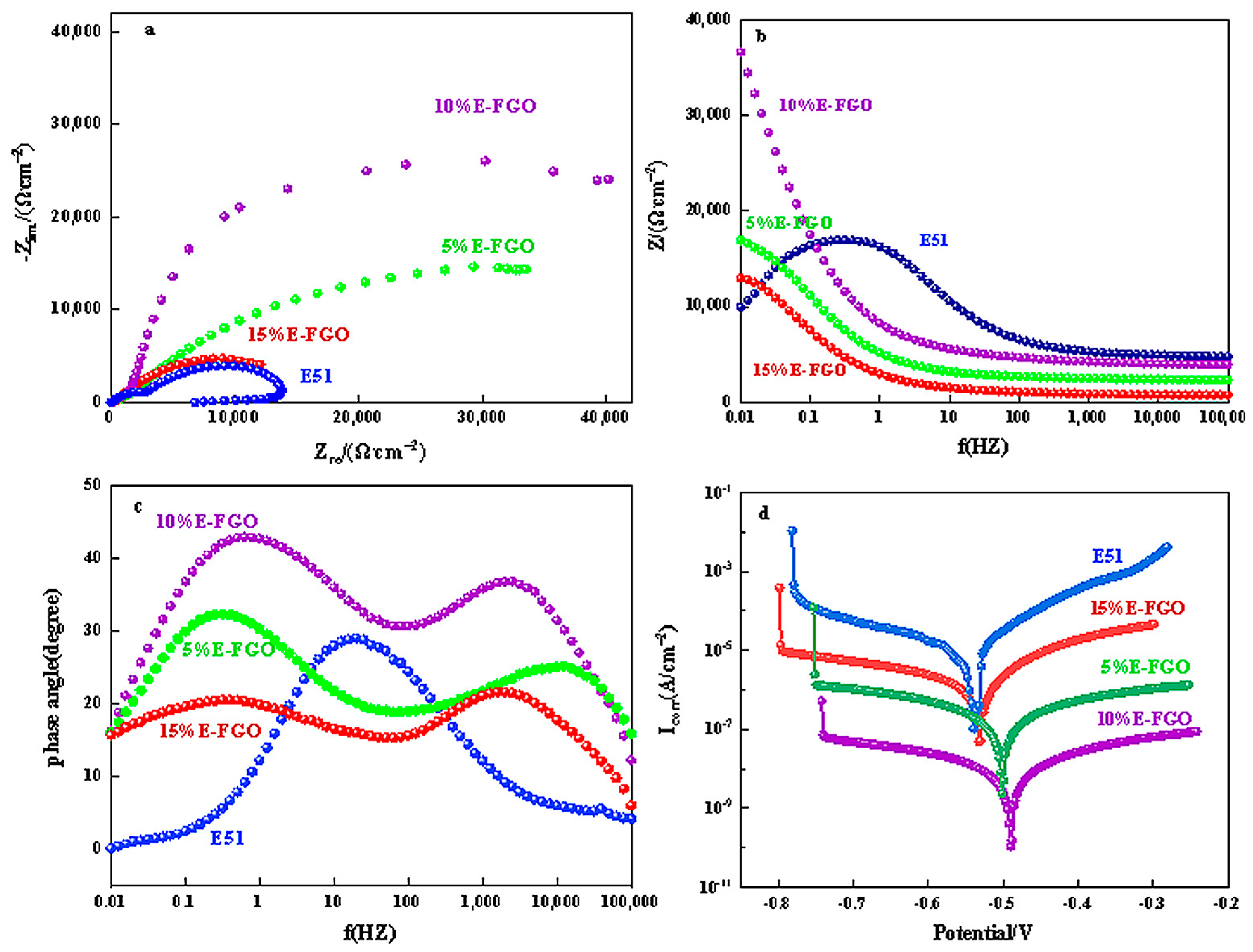
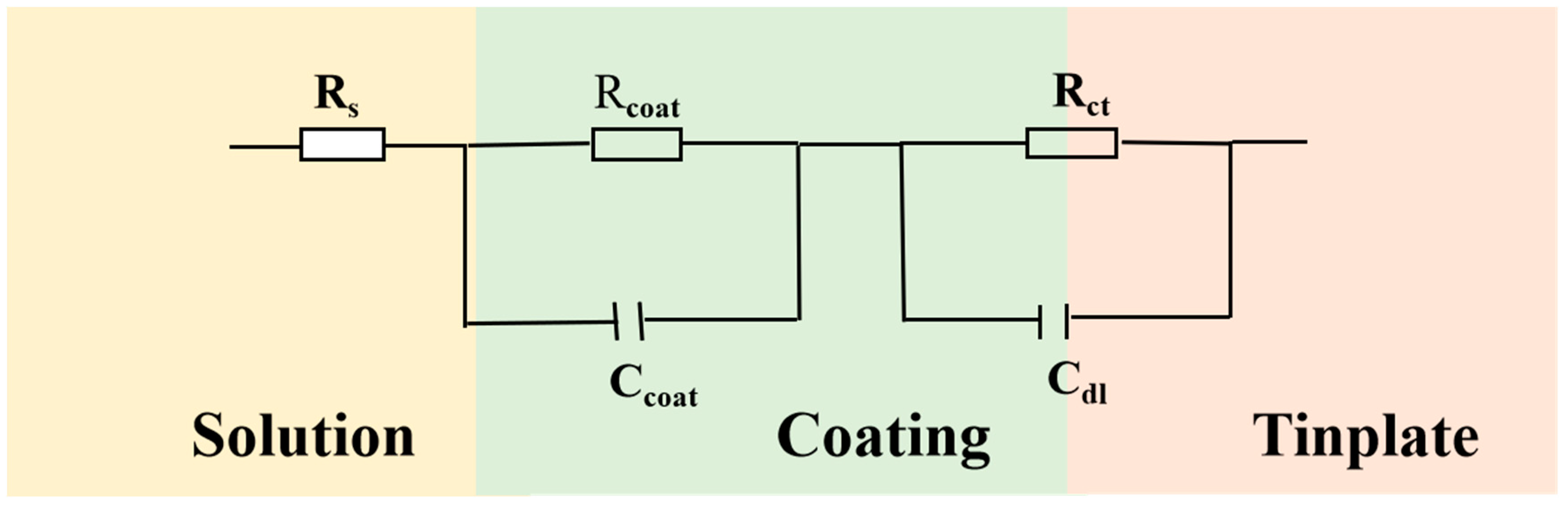

| Sample | CPEc | Rc | CPEdl | Rct |
|---|---|---|---|---|
| E51 | 7.42 × 10−4 | 1.10 × 104 | 1.90 × 10−4 | 15.91 |
| 5% E-FGO | 5.05 × 10−5 | 6.83 × 104 | 3.42 × 10−5 | 25.85 |
| 10% E-FGO | 2.61 × 10−5 | 8.27 × 104 | 2.99 × 10−5 | 169.10 |
| 15% E-FGO | 1.22 × 10−4 | 1.89 × 104 | 2.73 × 10−5 | 23.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; An, Q.; Li, J.; Ge, P.; Wu, Y.; Li, Y. Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide. Polymers 2023, 15, 1280. https://doi.org/10.3390/polym15051280
Gu J, An Q, Li J, Ge P, Wu Y, Li Y. Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide. Polymers. 2023; 15(5):1280. https://doi.org/10.3390/polym15051280
Chicago/Turabian StyleGu, Jiangdong, Qiufeng An, Jialong Li, Ping Ge, Yanyan Wu, and Yihan Li. 2023. "Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide" Polymers 15, no. 5: 1280. https://doi.org/10.3390/polym15051280
APA StyleGu, J., An, Q., Li, J., Ge, P., Wu, Y., & Li, Y. (2023). Preparation of Superhydrophobic Materials and Establishment of Anticorrosive Coatings on the Tinplate Substrate by Alkylation of Graphene Oxide. Polymers, 15(5), 1280. https://doi.org/10.3390/polym15051280





