Thermal Lens Measurements of Thermal Expansivity in Thermosensitive Polymer Solutions
Abstract
:1. Introduction
2. Materials and Methods
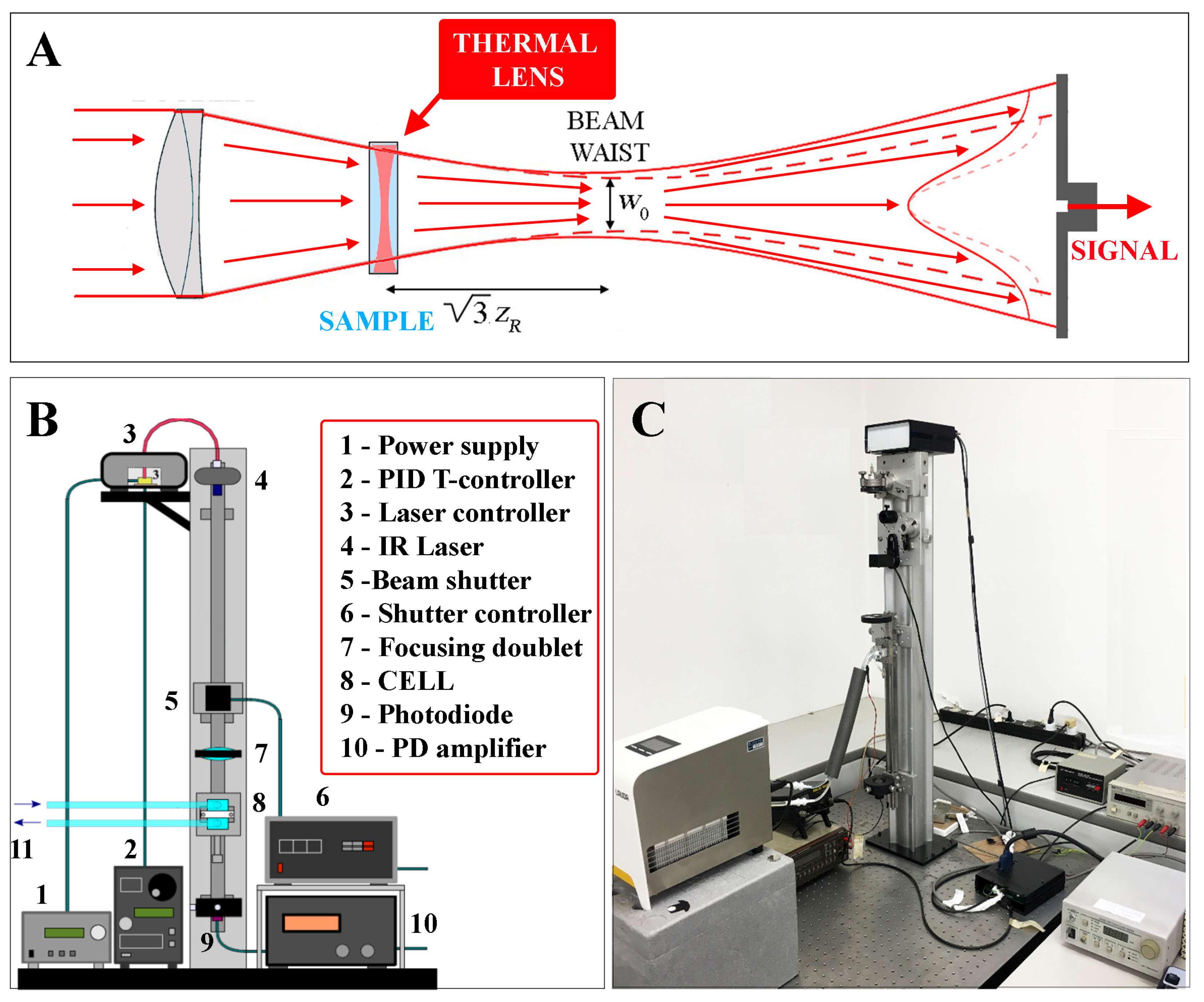
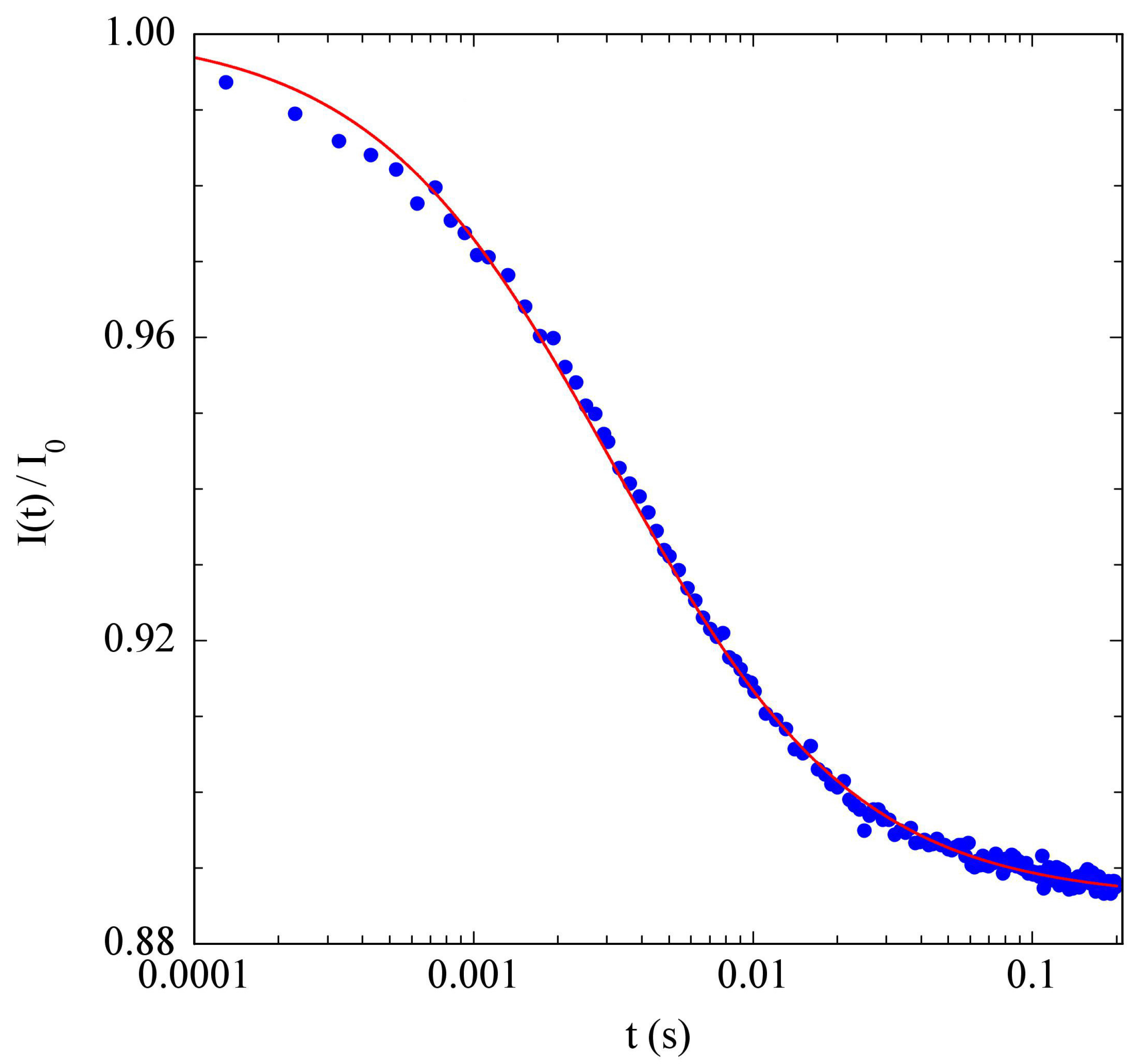
2.1. Thermal Lens Principle and Thermal Expansivity Measurements
2.2. Samples
3. Results
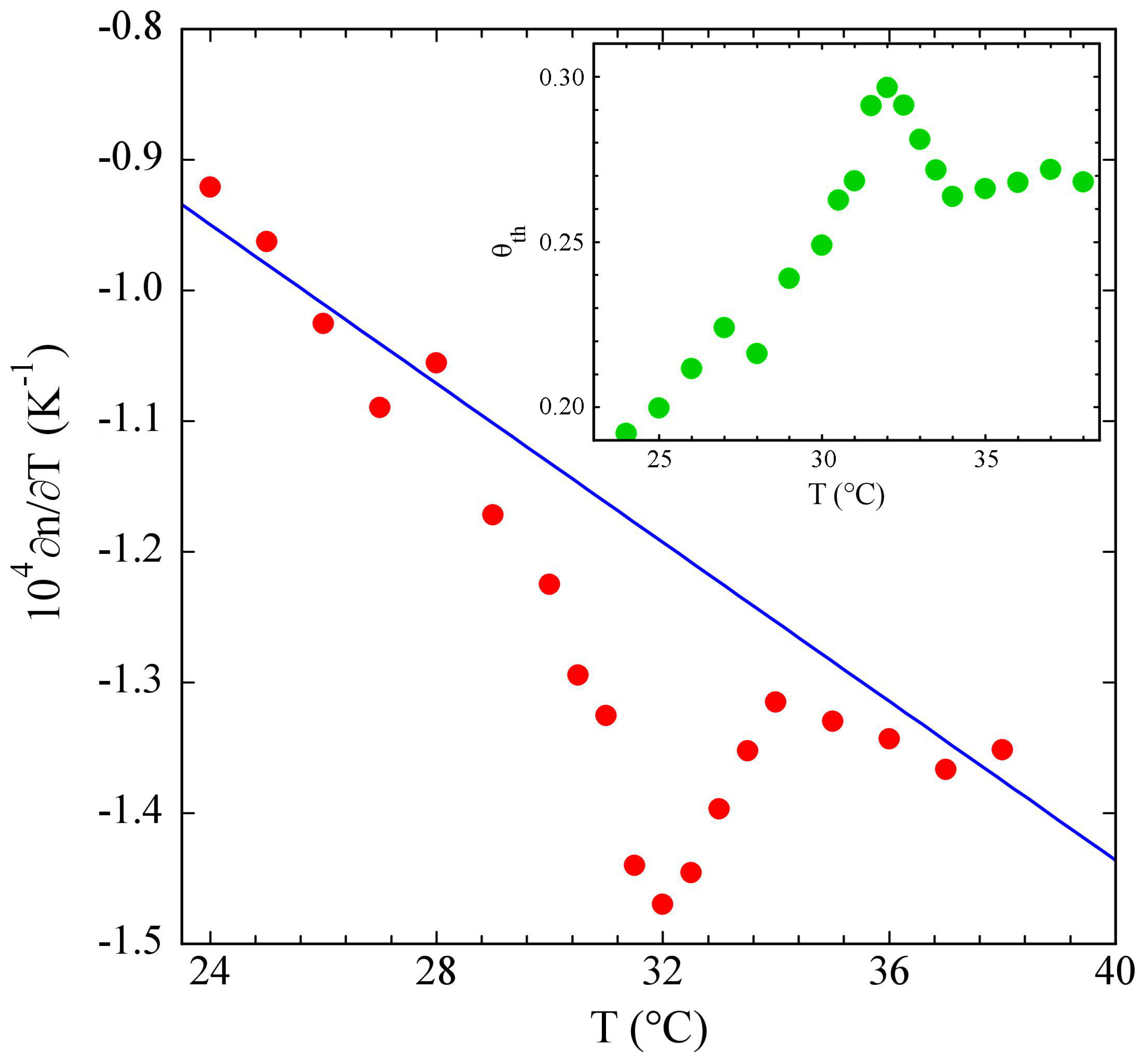
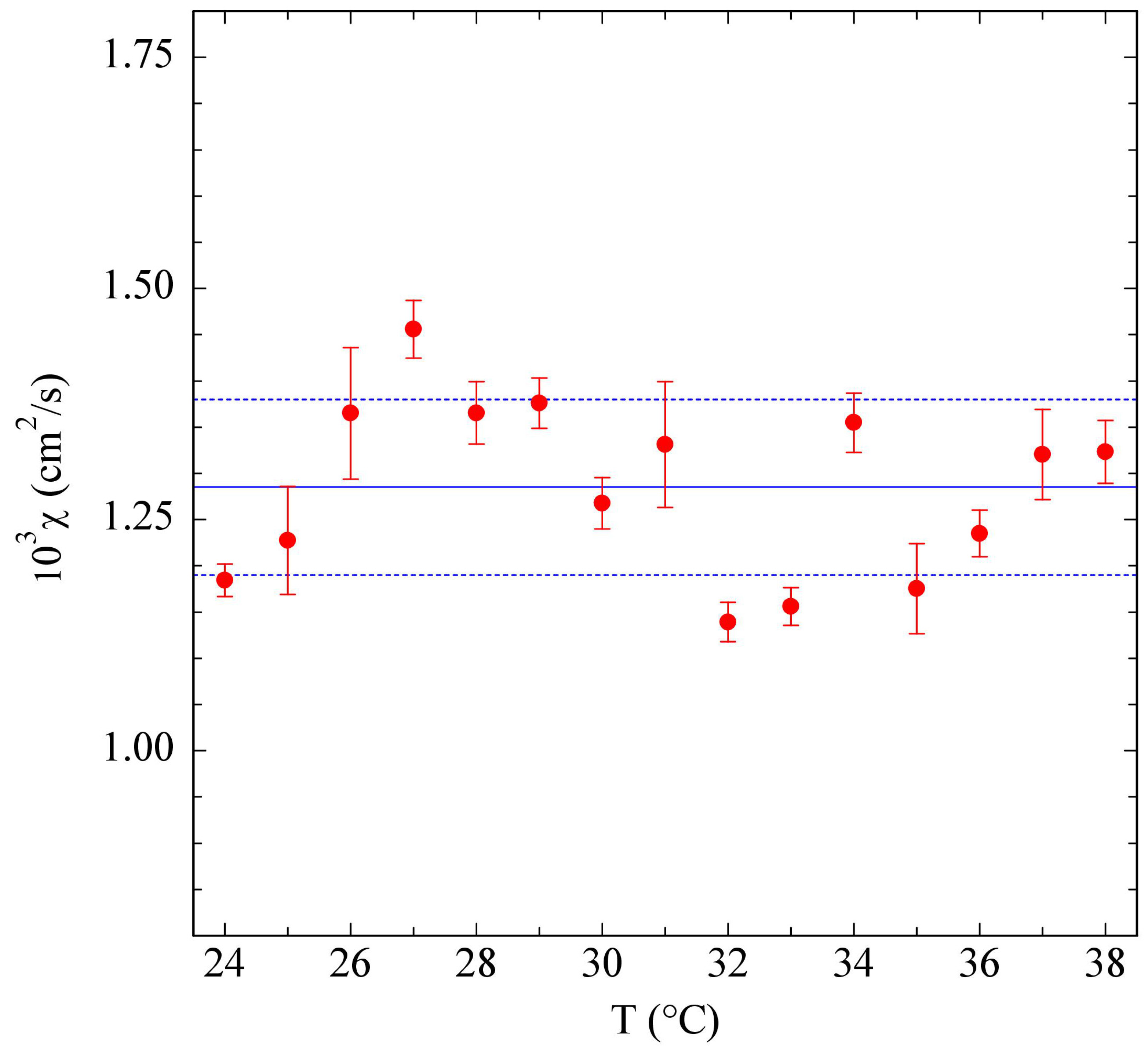
3.1. PniPAM Microgels
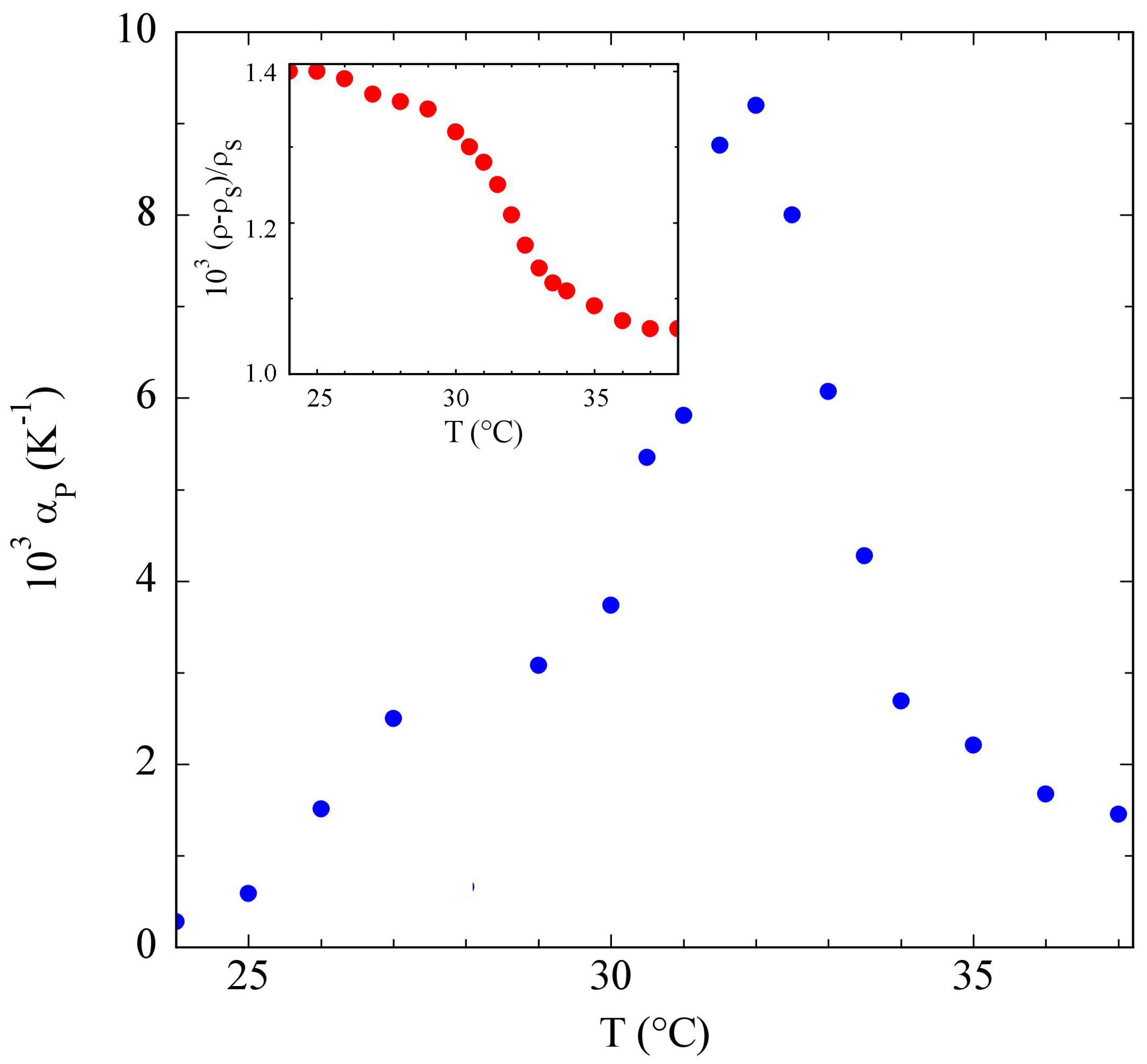
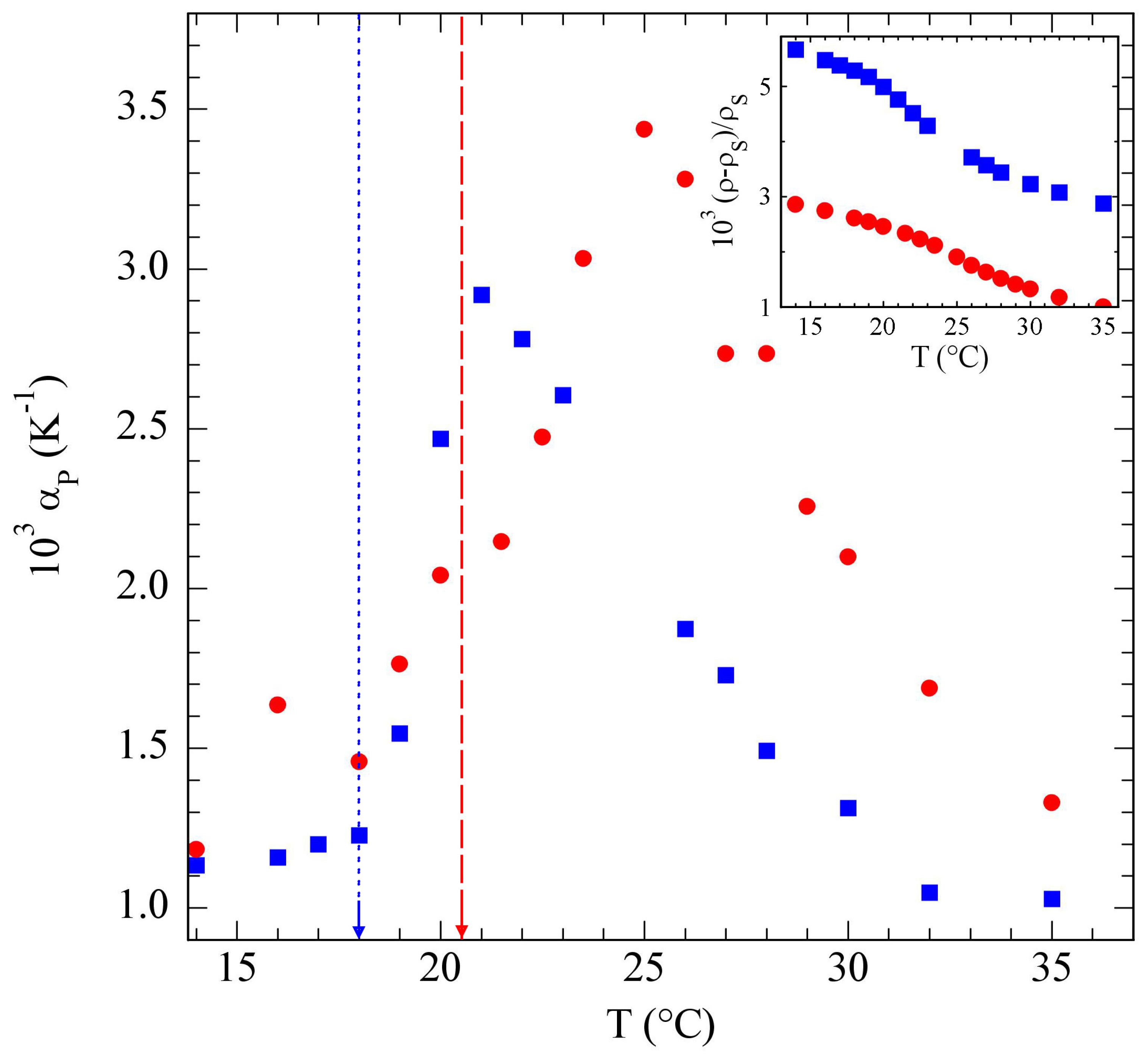
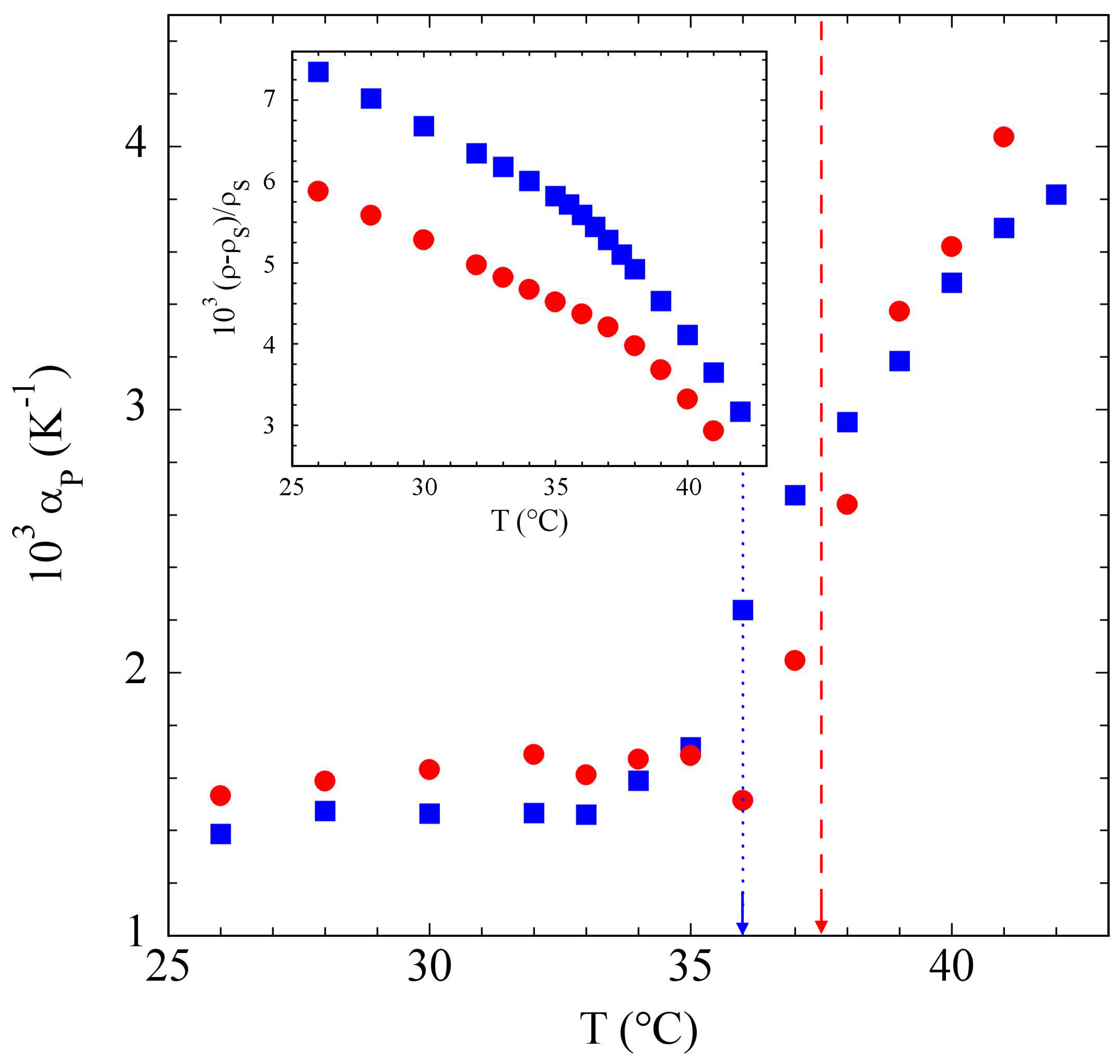
3.2. Poloxamers
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TL | Thermal Lensing |
| PniPAM | poly-N-isopyilacrylamyde |
| VPTT | Volume Phase Transition Temperature |
| PPO | PolyPropyleneOxide |
| PEO | PolyEthyleneOxide |
| CMT | Critical Micellization Temperature |
| DLS | Dynamic Light Scattering |
| SANS | Small-Angle Neutron Scattering |
References
- Majhi, P.R.; Blume, A. Thermodynamic characterization of temperature-induced micellization and demicellization of detergents studied by differential scanning calorimetry. Langmuir 2001, 17, 3844–3851. [Google Scholar] [CrossRef]
- Nazari, M.; Fan, H.Y.; Heerklotz, H. Effect of hydrophobic interactions on volume and thermal expansivity as derived from micelle formation. Langmuir 2012, 28, 14129–14136. [Google Scholar] [CrossRef]
- Chalikian, T.V. Volumetric properties of proteins. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 207–235. [Google Scholar] [CrossRef]
- Brovchenko, I.; Burri, R.; Krukau, A.; Oleinikova, A.; Winter, R. Intrinsic thermal expansivity and hydrational properties of amyloid peptide A β 42 in liquid water. J. Chem. Phys. 2008, 129, 11B614. [Google Scholar] [CrossRef]
- Dellarole, M.; Kobayashi, K.; Rouget, J.B.; Caro, J.A.; Roche, J.; Islam, M.M.; Garcia-Moreno E, B.; Kuroda, Y.; Royer, C.A. Probing the physical determinants of thermal expansion of folded proteins. J. Phys. Chem. B 2013, 117, 12742–12749. [Google Scholar] [CrossRef]
- de la Rosa, V.R.; Woisel, P.; Hoogenboom, R. Supramolecular control over thermoresponsive polymers. Mater. Today 2016, 19, 44–55. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Tian, Z. Thermal switching of thermoresponsive polymer aqueous solutions. ACS Macro Lett. 2018, 7, 53–58. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Flemming, P.; Münch, A.S.; Fery, A.; Uhlmann, P. Constrained thermoresponsive polymers–new insights into fundamentals and applications. Beilstein J. Org. Chem. 2021, 17, 2123–2163. [Google Scholar] [CrossRef]
- Greiner, R.; Schwarzl, F.R. Thermal contraction and volume relaxation of amorphous polymers. Rheol. Acta 1984, 23, 378–395. [Google Scholar] [CrossRef]
- Bauer, C.; Richert, R.; Böhmer, R.; Christensen, T. Dynamic thermal expansivity near the glass transition. J. Non-Cryst. Solids 2000, 262, 276–281. [Google Scholar] [CrossRef]
- Niss, K.; Gundermann, D.; Christensen, T.; Dyre, J.C. Dynamic thermal expansivity of liquids near the glass transition. Phys. Rev. E 2012, 85, 041501. [Google Scholar] [CrossRef] [Green Version]
- Reading, M.; Ludegt, A.; Wilson, R. Modulated differential scanning calorimetry. Thermochim. Acta 1994, 238, 295–307. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. JBT 2010, 21, 167–193. [Google Scholar]
- Müller, U.; Philipp, M.; Thomassey, M.; Sanctuary, R.; Krüger, J. Temperature modulated optical refractometry: A quasi-isothermal method to determine the dynamic volume expansion coefficient. Thermochim. Acta 2013, 555, 17–22. [Google Scholar] [CrossRef]
- Philipp, M.; Aleksandrova, R.; Müller, U.; Ostermeyer, M.; Sanctuary, R.; Müller-Buschbaum, P.; Krüger, J.K. Molecular versus macroscopic perspective on the demixing transition of aqueous PNIPAM solutions by studying the dual character of the refractive index. Soft Matter 2014, 10, 7297–7305. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, P.; Winnik, F.M. Volumetric studies of aqueous polymer solutions using pressure perturbation calorimetry: A new look at the temperature-induced phase transition of poly (N-isopropylacrylamide) in water and D2O. Macromolecules 2001, 34, 4130–4135. [Google Scholar] [CrossRef]
- Lin, L.N.; Brandts, J.F.; Brandts, J.; Plotnikov, V. Determination of the Volumetric Properties of Proteins and Other Solutes Using Pressure Perturbation Calorimetry. Anal. Biochem. 2002, 302, 144–160. [Google Scholar] [CrossRef]
- Falconer, R.J. Pressure Perturbation Calorimetry. In Encyclopedia of Biophysics; Roberts, G., Watts, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–12. [Google Scholar]
- Hepler, L.G. Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 1969, 47, 4613–4617. [Google Scholar] [CrossRef]
- Gordon, J.P.; Leite, R.C.C.; Moore, R.S.; Porto, S.P.S.; Whinnery, J.R. Long-Transient Effects in Lasers with Inserted Liquid Samples. J. Appl. Phys. 1965, 36, 3–8. [Google Scholar] [CrossRef]
- Whinnery, J.; Miller, D.; Dabby, F. Thermal convention and spherical aberration distortion of laser beams in low-loss liquids. IEEE J. Quantum Electron. 1967, 3, 382–383. [Google Scholar] [CrossRef]
- Wu, S.; Dovichi, N.J. Fresnel diffraction theory for steady-state thermal lens measurements in thin films. J. Appl. Phys. 1990, 67, 1170–1182. [Google Scholar] [CrossRef]
- Giglio, M.; Vendramini, A. Thermal lens effect in a binary liquid mixture: A new effect. Appl. Phys. Lett. 1974, 25, 555–557. [Google Scholar] [CrossRef]
- Rusconi, R.; Isa, L.; Piazza, R. Thermal-lensing measurement of particle thermophoresis in aqueous dispersions. J. Opt. Soc. Am. B 2004, 21, 605–616. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Volkov, D.S.; Gor’kova, T.A.; Bendrysheva, S.N.; Smirnova, A.P.; Nedosekin, D.A. Advances in thermal lens spectrometry. J. Anal. Chem. 2015, 70, 249–276. [Google Scholar] [CrossRef]
- Liu, M.; Franko, M. Thermal Lens Spectrometry: Still a Technique on the Horizon? Int. J. Thermophys. 2016, 37, 1–16. [Google Scholar] [CrossRef]
- Cassano, C.L.; Mawatari, K.; Kitamori, T.; Fan, Z.H. Thermal lens microscopy as a detector in microdevices. Electrophoresis 2014, 35, 2279–2291. [Google Scholar] [CrossRef]
- Dada, O.O.; Feist, P.E.; Dovichi, N.J. Thermal diffusivity imaging with the thermal lens microscope. Appl. Opt. 2011, 50, 6336–6342. [Google Scholar] [CrossRef]
- Rusconi, R.; Rodari, E.; Piazza, R. Optical measurements of the thermal properties of nanofluids. Appl. Phys. Lett. 2006, 89, 261916. [Google Scholar] [CrossRef]
- Parola, A.; Piazza, R.; Degiorgio, V. Optical extinction, refractive index, and multiple scattering for suspensions of interacting colloidal particles. J. Chem. Phys. 2014, 141, 124902. [Google Scholar] [CrossRef] [Green Version]
- Lattuada, E.; Buzzaccaro, S.; Piazza, R. Thermophoresis in self-associating systems: Probing poloxamer micellization by opto-thermal excitation. Soft Matter 2019, 15, 2140–2151. [Google Scholar] [CrossRef]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Gil, E.; Hudson, S. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Conley, G.M.; Nöjd, S.; Braibanti, M.; Schurtenberger, P.; Scheffold, F. Superresolution microscopy of the volume phase transition of pNIPAM microgels. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Z.; Gooding, E.A.; Pimenov, K.V.; Wang, L.; Asher, S.A. UV Resonance Raman Determination of Molecular Mechanism of Poly(N-isopropylacrylamide) Volume Phase Transition. J. Phys. Chem. B 2009, 113, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. On the molecular origin of the cooperative coil-to-globule transition of poly(N-isopropylacrylamide) in water. Phys. Chem. Chem. Phys. 2018, 20, 9997–10010. [Google Scholar] [CrossRef] [Green Version]
- Del Monte, G.; Truzzolillo, D.; Camerin, F.; Ninarello, A.; Chauveau, E.; Tavagnacco, L.; Gnan, N.; Rovigatti, L.; Sennato, S.; Zaccarelli, E. Two-step deswelling in the Volume Phase Transition of thermoresponsive microgels. Proc. Natl. Acad. Sci. USA 2021, 118, e2109560118. [Google Scholar] [CrossRef]
- Wongsuwarn, S.; Vigolo, D.; Cerbino, R.; Howe, A.M.; Vailati, A.; Piazza, R.; Cicuta, P. Giant thermophoresis of poly(N-isopropylacrylamide) microgel particles. Soft Matter 2012, 8, 5857. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase Diagrams and Aggregation Behavior of Poly(oxyethylene)-Poly(oxypropylene)-Poly(oxyethylene) Triblock Copolymers in Aqueous Solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Prud’homme, R.K.; Wu, G.; Schneider, D.K. Structure and Rheology Studies of Poly(oxyethylene-oxypropylene-oxyethylene) Aqueous Solution. Langmuir 1996, 12, 4651–4659. [Google Scholar] [CrossRef]
- Wu, C.; Liu, T.; Chu, B.; Schneider, D.K.; Graziano, V. Characterization of the PEO-PPO-PEO Triblock Copolymer and Its Application as a Separation Medium in Capillary Electrophoresis. Macromolecules 1997, 30, 4574–4583. [Google Scholar] [CrossRef]
- Huff, A.; Patton, K.; Odhner, H.; Jacobs, D.T.; Clover, B.C.; Greer, S.C. Micellization and Phase Separation for Triblock Copolymer 17R4 in H2O and in D2O. Langmuir 2011, 27, 1707–1712. [Google Scholar] [CrossRef]
- Balsara, N.P.; Tirrell, M.; Lodge, T.P. Micelle formation of BAB triblock copolymers in solvents that preferentially dissolve the A block. Macromolecules 1991, 24, 1975–1986. [Google Scholar] [CrossRef]
- Nguyen-Misra, M.; Mattice, W.L. Micellization and Gelation of Symmetric Triblock Copolymers with Insoluble End Blocks. Macromolecules 1995, 28, 1444–1457. [Google Scholar] [CrossRef]
- Zhou, Z.; Chu, B. Phase Behavior and Association Properties of Poly(oxypropylene)-Poly(oxyethylene)-Poly(oxypropylene) Triblock Copolymer in Aqueous Solution. Macromolecules 1994, 27, 2025–2033. [Google Scholar] [CrossRef]
- Clover Kumi, B.; Hammouda, B.; Greer, S. Self-assembly of the triblock copolymer 17R4 poly(propylene oxide)14–poly(ethylene oxide)24–poly(propylene oxide)14 in D2O. J. Colloid Interface Sci. 2014, 434, 201–207. [Google Scholar] [CrossRef]
- Woodward, N.C.; Chowdhry, B.Z.; Snowden, M.J.; Leharne, S.A.; Griffiths, P.C.; Winnington, A.L. Calorimetric Investigation of the Influence of Cross-Linker Concentration on the Volume Phase Transition of Poly(N-isopropylacrylamide) Colloidal Microgels. Langmuir 2003, 19, 3202–3211. [Google Scholar] [CrossRef]
- Zanatta, M.; Tavagnacco, L.; Buratti, E.; Chiessi, E.; Natali, F.; Bertoldo, M.; Orecchini, A.; Zaccarelli, E. Atomic scale investigation of the volume phase transition in concentrated PNIPAM microgels. J. Chem. Phys. 2020, 152, 204904. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. The aggregation behavior of poly-(oxyethylene)-poly-(oxypropylene)-poly-(oxyethylene)- block-copolymers in aqueous solution. Colloid Polym. Sci. 1990, 268, 101–117. [Google Scholar] [CrossRef]
- Dumancas, L.V.; Simpson, D.E.; Jacobs, D.T. Heat capacity anomaly in a self-aggregating system: Triblock copolymer 17R4 in water. J. Chem. Phys. 2015, 142, 174902. [Google Scholar] [CrossRef] [Green Version]
- Suladze, S.; Kahse, M.; Erwin, N.; Tomazic, D.; Winter, R. Probing volumetric properties of biomolecular systems by pressure perturbation calorimetry (PPC)—The effects of hydration, cosolvents and crowding. Methods 2015, 76, 67–77. [Google Scholar] [CrossRef]
- Piazza, R.; Campello, M.; Buzzaccaro, S.; Sciortino, F. Phase Behavior and Microscopic Dynamics of a Thermosensitive Gel-Forming Polymer. Macromolecules 2021, 54, 3897–3906. [Google Scholar] [CrossRef]
- Buzzaccaro, S.; Ruzzi, V.; Faleo, T.; Piazza, R. Microrheology of a thermosensitive gelling polymer for cell culture. J. Chem. Phys. 2022, 157, 174901. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzzi, V.; Buzzaccaro, S.; Piazza, R. Thermal Lens Measurements of Thermal Expansivity in Thermosensitive Polymer Solutions. Polymers 2023, 15, 1283. https://doi.org/10.3390/polym15051283
Ruzzi V, Buzzaccaro S, Piazza R. Thermal Lens Measurements of Thermal Expansivity in Thermosensitive Polymer Solutions. Polymers. 2023; 15(5):1283. https://doi.org/10.3390/polym15051283
Chicago/Turabian StyleRuzzi, Vincenzo, Stefano Buzzaccaro, and Roberto Piazza. 2023. "Thermal Lens Measurements of Thermal Expansivity in Thermosensitive Polymer Solutions" Polymers 15, no. 5: 1283. https://doi.org/10.3390/polym15051283






