Facile Synthesis of Reduced-Graphene-Oxide-Modified Ammonium Polyphosphate to Enhance the Flame Retardancy, Smoke Release Suppression, and Mechanical Properties of Epoxy Resin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of RGO-APP Flame Retardant
2.3. Preparation of EP and EP Composites
2.4. Characterization and Measurement
3. Results and Discussion
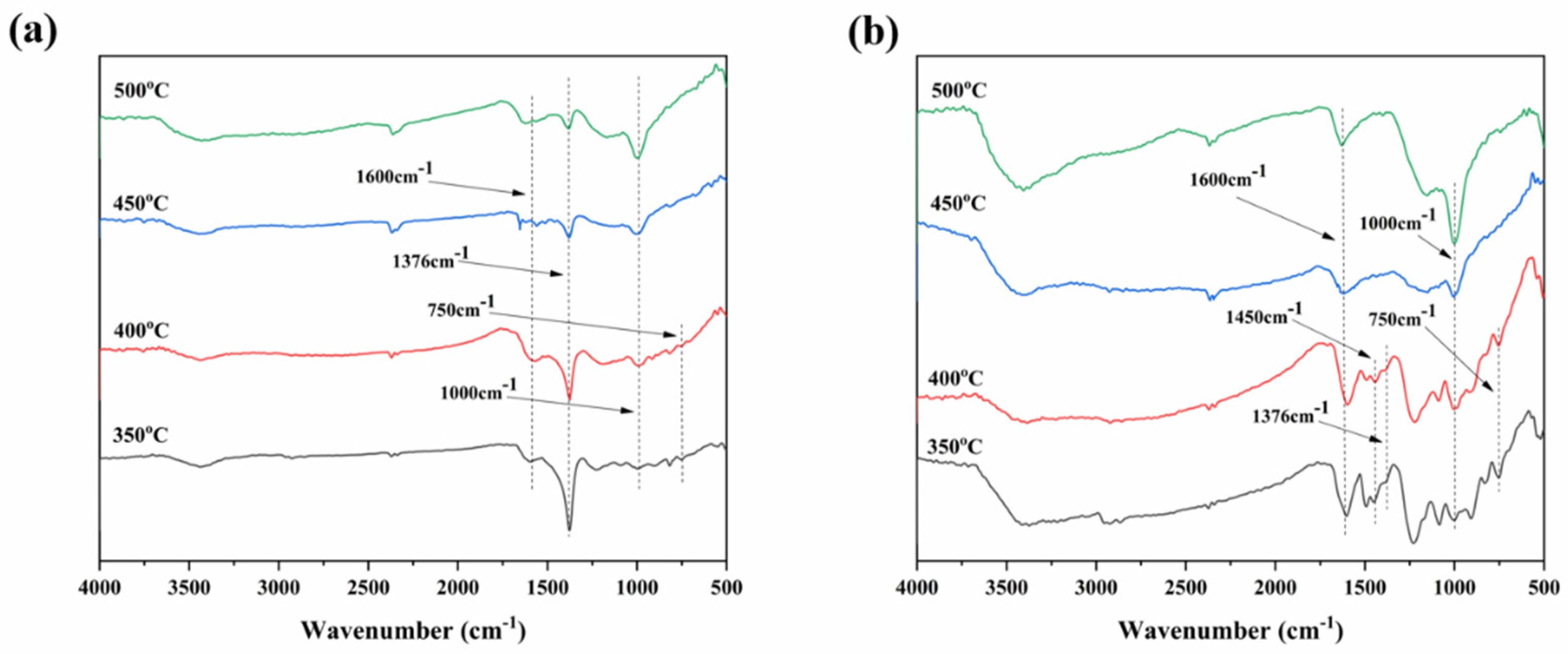
3.1. Characterization of RGO-APP Flame Retardant
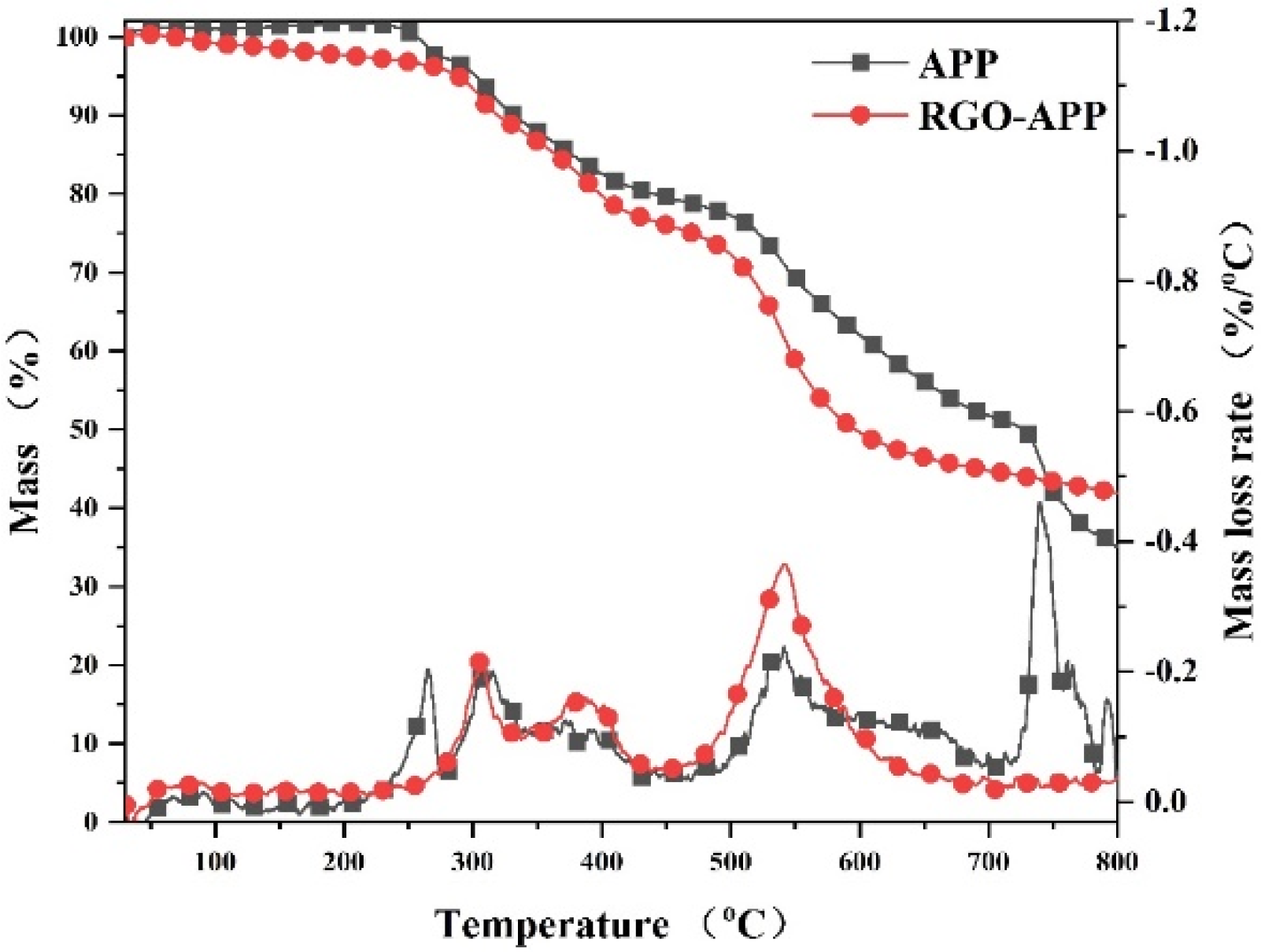
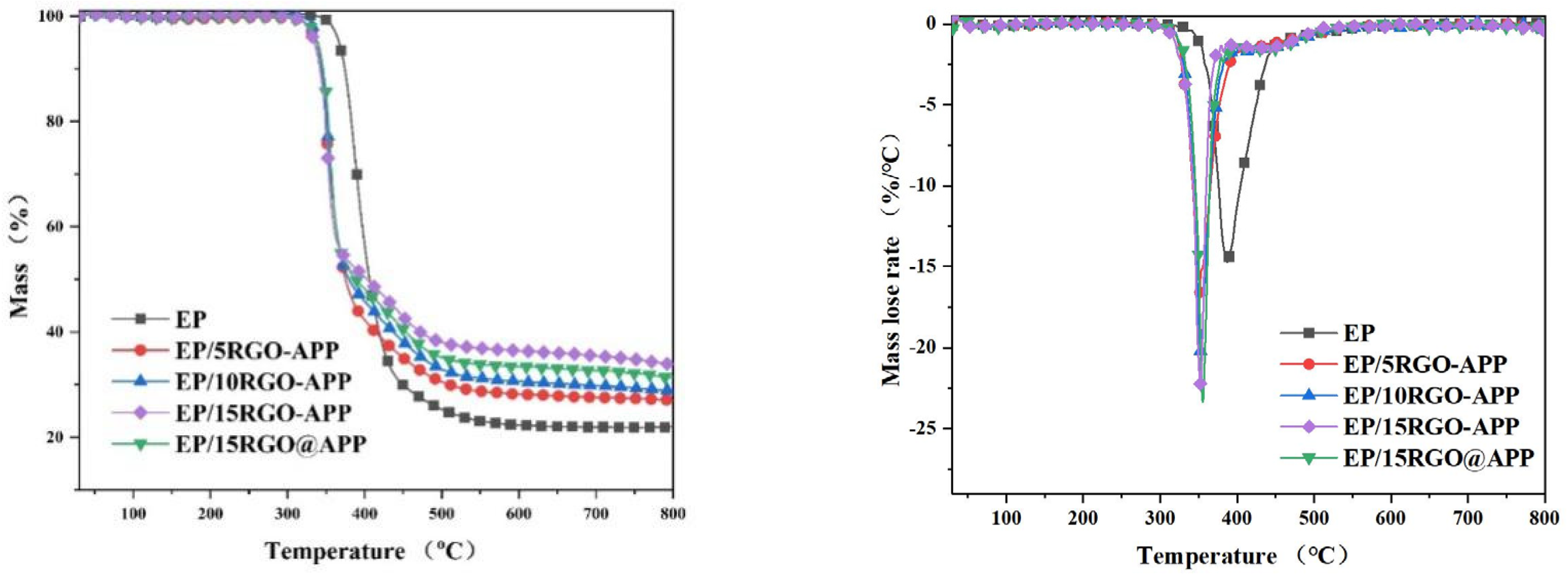
3.2. TG Analysis
3.3. DSC Test
3.4. LOI and UL94 Tests
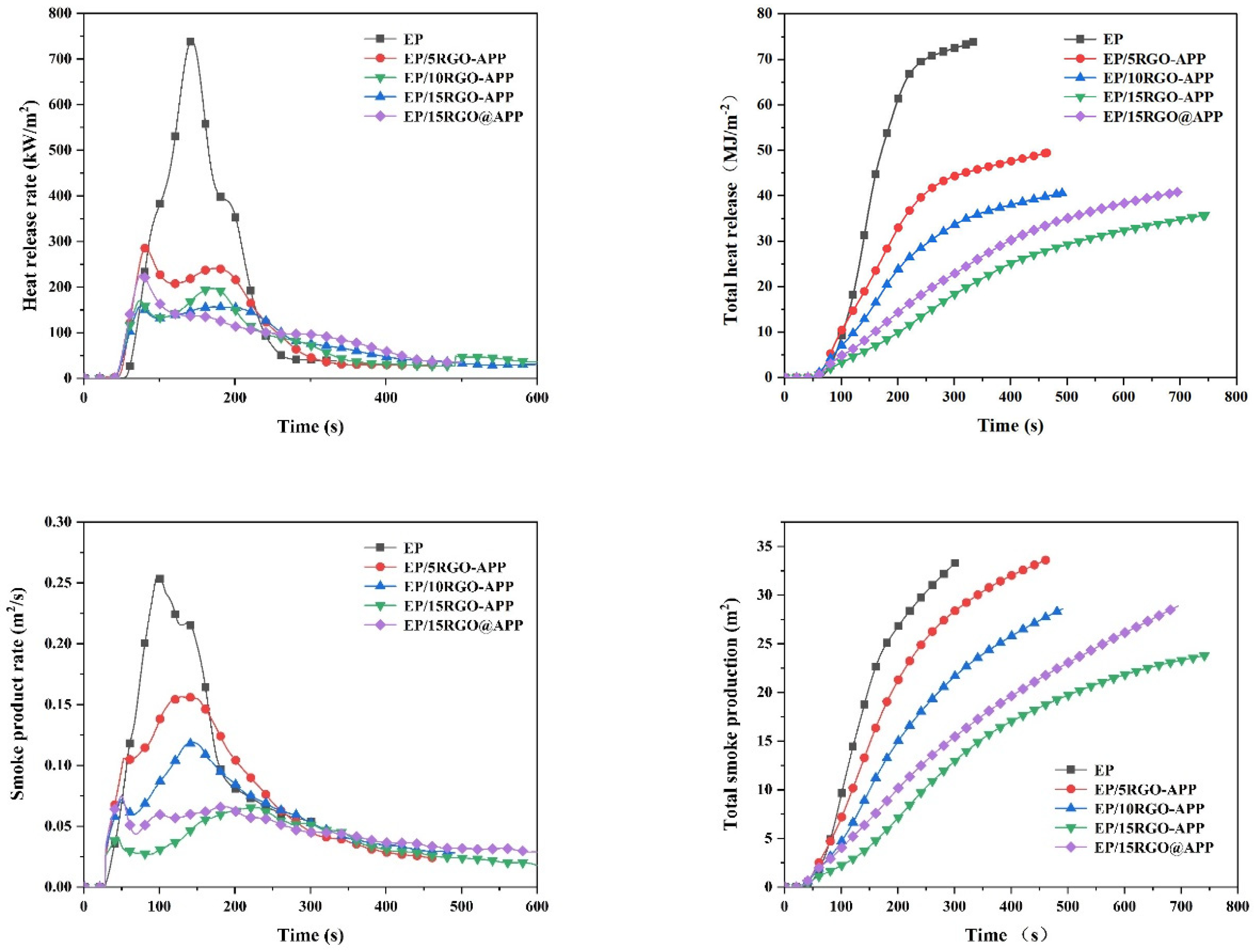
3.5. Cone Calorimeter Test
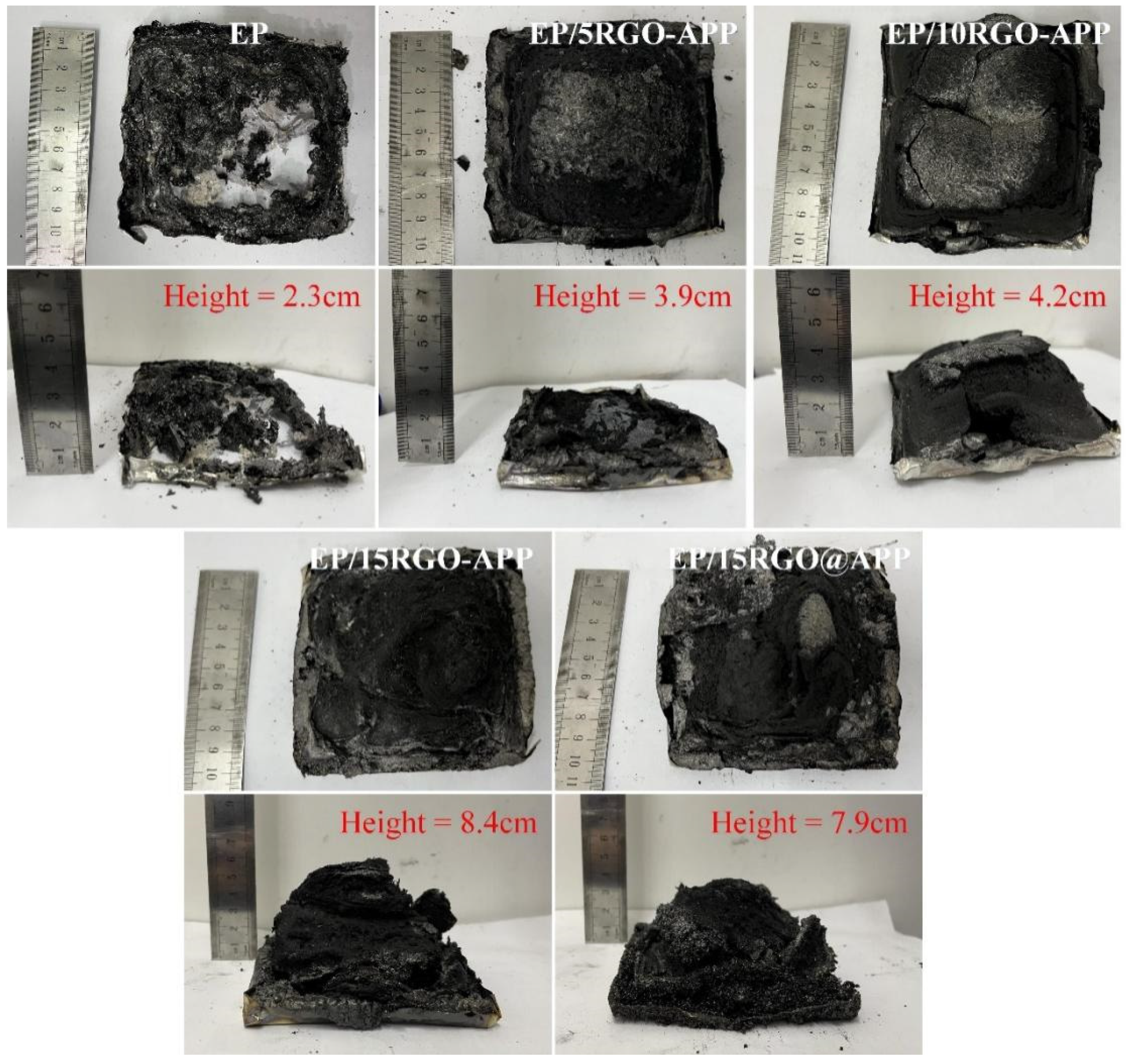
3.6. Char Residue Analysis
3.7. Mechanical Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, S.; Bauer, J.; Maryanski, M.; Heimann, P.; Barlow, J.; Gosau, J.; Allred, R. Characterization of epoxy functionalized graphite nanoparticles and the physical properties of epoxy matrix nanocomposites. Compos. Sci. Technol. 2010, 70, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In situ polymerization of graphene, graphite oxide, and functionalized graphite oxide into epoxy resin and comparison study of on-the-flame behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Chiang, C.; Hsu, S. Novel epoxy/expandable graphite halogen-free flame retardant composites–preparation, characterization, and properties. J. Polym. Res. 2009, 17, 315–323. [Google Scholar] [CrossRef]
- Kuan, C.; Yen, W.; Chen, C.; Yuen, S.; Kuan, H.; Chiang, C. Synthesis, characterization, flame retardance and thermal properties of halogen-free expandable graphite/PMMA composites prepared from sol–gel method. Polym. Degrad. Stab. 2008, 93, 1357–1363. [Google Scholar] [CrossRef]
- Cheng, C.; Lu, Y.; Cai, J.; Yan, J.; Li, S.; Du, S. Ammonium polyphosphate surface-modified with l-lysine as an intumescent flame retardant for epoxy resin. Polym. Adv. Technol. 2022, 33, 534–545. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, S.; Chen, L.; Liu, L.; Song, P.; Wei, L.; Yu, Y.; Lu, F.; Jun, Q.; Hao, W. Flame retardant and mechanically tough poly(lactic acid) biocomposites via combining ammonia polyphosphate and polyethylene glycol. Compos. Commun. 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Kandola, B.; Magnoni, F.; Ebdon, J. Flame retardants for epoxy resins: Application-related challenges and solutions. J. Vinyl. Addit. Technol. 2022, 28, 17–49. [Google Scholar] [CrossRef]
- Yi, L.; Long, M.; Yan, L.; Tang, X.; Liao, J. A facile strategy to construct multifunctional microencapsulated urea ammonium polyphosphate for epoxy resins towards satisfied fire safety, thermal stability and compatibility. J. Appl. Polym. Sci. 2023, e53675. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, H.; Zhang, Z.; Chen, X. Preparation and properties of an efficient smoke suppressant and flame-retardant agent for thermoplastic polyurethane. Polym. Adv. Technol. 2017, 28, 1690–1698. [Google Scholar] [CrossRef]
- Seefeldt, H.; Braun, U.; Wagner, M. Residue Stabilization in the Fire Retardancy of Wood–Plastic Composites: Combination of Ammonium Polyphosphate, Expandable Graphite, and Red Phosphorus. Macromol. Chem. Phys. 2012, 213, 2370–2377. [Google Scholar] [CrossRef]
- Potts, J.; Dreyer, D.; Bielawski, C.; Ruoff, R. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Abdala, A.; Macosko, C. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Xu, Y.; Bai, H.; Yao, Z.; Shi, G. Chemically converted graphene as substrate for immobilizing and enhancing the activity of a polymeric catalyst. Chem. Commun. 2010, 46, 4740–4742. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Zhang, A.; Wang, W.; Wang, Z.; Zhang, J.; Feng, J.; Huo, S.; Zeng, X.; Song, P. An iron phenylphosphinate@graphene oxide nanohybrid enabled flame-retardant, mechanically reinforced, and thermally conductive epoxy nanocomposites. Chem. Eng. J. 2023, 454, 140424. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Duan, R.; Zhang, K.; Meng, W.; Li, Y.; Qu, H. Graphene doped Sn flame retardant prepared by ball milling and synergistic with hexaphenoxy cyclotriphosphazene for epoxy resin. J. Mater. Res. Technol. 2022, 17, 774–788. [Google Scholar] [CrossRef]
- Huang, G.; Chen, W.; Wu, T.; Guo, H.; Fu, X.; Xue, Y.; Wang, K.; Song, P. Multifunctional graphene-based nano-additives toward high-performance polymer nanocomposites with enhanced mechanical, thermal, flame retardancy and smoke suppressive properties. Chem. Eng. J. 2021, 410, 127590. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, G.; Han, D.; Song, P.; Tang, W.; Bao, J.; Li, R.; Liu, Y. Functionalizing graphene decorated with phosphorus-nitrogen containing dendrimer for high-performance polymer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 86, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.; Gu, S.; Xiao, Y.; Wang, Y.; Chen, L. Novel organophosphonate-decorated WS2 nanosheets towards flame retardancy and mechanical enhancement of epoxy resin. Polym. Degrad. Stab. 2022, 206, 110170. [Google Scholar] [CrossRef]
- Xu, S.; Gu, S.; Pu, X.; Xiao, Y.; Lu, J.; Wang, Y.; Chen, L. Reaction-induced phase separation towards in situ network in epoxy resins for simultaneously improving thermal conductivity and fire safety. Compos. Part B-Eng. 2022, 247, 110326. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.; Li, X.; Yu, L.; Zhang, Z. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Xi, L.; Zhao, Z.; Huo, S.; Huang, G.; Fang, Z.; Song, P. Interface nanoengineering of a core-shell structured biobased fire retardant for fire-retarding polylactide with enhanced toughness and UV protection. J. Clean. Prod. 2022, 336, 130372. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Liao, S.; Liu, P.; Hsiao, M.; Teng, C.; Wang, C.; Ger, M.; Chiang, C. One-step reduction and functionalization of graphene oxide with phosphorus-based compound to produce flame-retardant epoxy nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Shi, X.; Pan, Y.; Wang, Y.; Jia, Z.; Chen, T.; Gong, J.; Jiang, J. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers 2021, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron-graphene and ammonium polyphosphate in flame-retardant thermoplastic polyurethane. J. Therm. Anal. Calorim. 2016, 126, 633–642. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Dong, X.; Wang, R.; Zhang, L.; Wang, J.; Zhong, J.; Wu, L.; Wang, X. A pre-constructed graphene-ammonium polyphosphate aerogel (GAPPA) for efficiently enhancing the mechanical and fire-safety performances of polymers. J. Mater. Chem. 2018, 6, 4449–4457. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Shen, M.; Huang, X.; Zhu, J.; Zhang, X. Preparation and properties of polystyrene nanocomposites with graphite oxide and graphene as flame retardants. J. Mater. Sci. 2013, 48, 4214–4222. [Google Scholar] [CrossRef]
- Yuan, B.; Song, L.; Liewc, K.; Hu, Y. Solid acid-reduced graphene oxide nanohybrid for enhancing thermal stability, mechanical property and flame retardancy of polypropylene. RSC Adv. 2015, 5, 41307–41316. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Ma, W.; Cheng, Y.; Tao, Y.; Wu, T.; Chen, W.; Zhou, Z.; Zhu, M. A novel reduced graphene oxide decorated with halloysite nanotubes (HNTs-d-rGO) hybrid composite and its flame-retardant application for polyamide 6. Express Polym. Lett. 2014, 8, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tian, Q.; Dong, L.; Guo, H.; Teng, C.; Liu, Z.; Lv, S.; Zhu, L. Microstructure and dielectric properties of bismaleimide composite synergistically modified by graphene oxide and polyetheretherketone. J. Mater. Sci. Mater. Electron. 2020, 31, 5368–5375. [Google Scholar] [CrossRef]
- Xin, W.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Wang, F.; Liao, J.; Yan, L.; Cai, M. Facile Construction of Polypyrrole Microencapsulated Melamine-Coated Ammonium Polyphosphate to Simultaneously Reduce Flammability and Smoke Release of Epoxy Resin. Polymers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wang, G.; Li, A.; Xu, B. A novel graphene for hybrid reducing fire hazard of epoxy resin. Polym. Adv. Technol. 2017, 29, 1194–1205. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Ma, Z.; Xu, X.; Seraji, S.; Yu, B.; Sun, Z.; Wang, H.; Song, P. A reactive copper-organophosphate-MXene heterostructure enabled antibacterial, self-extinguishing and mechanically robust polymer nanocomposites. Chem. Eng. J. 2021, 430, 132712. [Google Scholar] [CrossRef]
- Attia, N.; Elashery, S.; Zakria, A.; Eltaweil, A.; Oh, H. Recent advances in graphene sheets as new generation of flame retardant materials. Mater. Sci. Eng. B-Adv. 2021, 274, 115460. [Google Scholar] [CrossRef]
- Bi, X.; Di, H.; Liu, J.; Meng, Y.; Song, Y.; Meng, W.; Qu, H.; Feng, L.; Song, P.; Xu, J. A core–shell-structured APP@COFs hybrid for enhanced flame retardancy and mechanical property of epoxy resin (EP). Adv. Compos. Hybrid Mater. 2022, 5, 1743–1755. [Google Scholar] [CrossRef]
- Huo, S.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Hu, Y.; Ding, G.; Zhang, Q.; Song, P.; Wang, H. A Liquid Phosphaphenanthrene-Derived Imidazole for Improved Flame Retardancy and Smoke Suppression of Epoxy Resin. ACS. Appl. Polym. Mater. 2020, 2, 3566–3575. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Chen, L.; Chen, J.; He, Z.; Li, Z.; Li, S.; Wang, Y. Facile fabrication of intrinsically fire-safety epoxy resin cured with phosphorus-containing transition metal complexes for flame retardation, smoke suppression, and latent curing behavior. Chem. Eng. J. 2022, 442, 136079. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Luo, X.; Liu, B.; Guo, D.; Chen, L.; Wang, Y. Flame-Retardant multifunctional epoxy resin with high performances. Chem. Eng. J. 2022, 427, 132031. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How can graphene reduce the flammability of polymer nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Y.; Xu, J.; Fan, W.; Chen, Z.; Wang, Q. Preparation and combustion properties of flame retardant styrene-butyl acrylate copolymer/graphite oxide nanocomposites. Macromol. Mater. Eng. 2004, 289, 355–359. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L. Chemically modified graphene: Flame retardant or fuel for combustion. J. Mater. Chem. 2011, 21, 3277–3279. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. In situ polymerization of graphene nanosheets and poly-urethane with enhanced mechanical and thermal properties. J. Mater. Chem. 2011, 21, 4222–4227. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L. Enhancing the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings by introducing boric acid as synergistic agent. J. Anal. Calorim. 2018, 133, 1241–1252. [Google Scholar] [CrossRef]
- Wang, F.; Liao, J.; Yan, L.; Liu, H. Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins. Polymers 2021, 13, 3221. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, J. Thermal stabilities and flame retardancies of nitrogen phosphorus flame retardants based on bisphospho ramidates. Polym. Degrad. Stab. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Wang, R.; Wu, L.; Zhuo, D.; Wang, Z.; Tsai, Y. Fabrication of Fullerene Anchored Reduced Graphene Oxide Hybrids and Their Synergistic Reinforcement on the Flame Retardancy of Epoxy Resin. Nanoscale Res. Lett. 2018, 13, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Samples | EP (wt%) | DDM (wt%) | RGO-APP (wt%) | RGO (wt%) | APP (wt%) | LOI (%) | UL94 Rating |
|---|---|---|---|---|---|---|---|
| EP | 80 | 20 | 0 | 0 | 0 | 26.3 | No rating |
| EP/5RGO-APP | 76 | 19 | 5 | 0 | 0 | 26.4 | No rating |
| EP/7.5RGO-APP | 74 | 18.5 | 7.5 | 0 | 0 | 26.8 | No rating |
| EP/10RGO-APP | 72 | 18 | 10 | 0 | 0 | 28.3 | V-1 |
| EP/12.5RGO-APP | 70 | 17.5 | 12.5 | 0 | 0 | 32.1 | V-1 |
| EP/15RGO-APP | 68 | 17 | 15 | 0 | 0 | 35.8 | V-0 |
| EP/5RGO@APP | 76 | 19 | 0 | 4.29 | 0.71 | 26.2 | No rating |
| EP/7.5RGO@APP | 74 | 18.5 | 0 | 6.43 | 1.07 | 26.6 | No rating |
| EP/10RGO@APP | 72 | 18 | 0 | 8.57 | 1.43 | 27.4 | No rating |
| EP/12.5RGO@APP | 70 | 17.5 | 0 | 10.71 | 1.79 | 30.8 | V-1 |
| EP/15RGO@APP | 68 | 17 | 0 | 12.86 | 2.14 | 34.1 | V-0 |
| Samples | T5% (°C) | Tmax (°C) | PMLR (%/min) | Wexp (%) |
|---|---|---|---|---|
| EP | 366.9 | 387.2 | −14.7028 | 21.9 |
| EP/5RGO-APP | 335.6 | 349.3 | −18.37081 | 27.0 |
| EP/10RGO-APP | 338.9 | 352.7 | −20.191 | 28.8 |
| EP/15RGO-APP | 339.4 | 355.4 | −22.23751 | 33.8 |
| EP/15RGO@APP | 335.0 | 352.2 | −23.35312 | 31.4 |
| Samples | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | PSPR (m2/s) | TSP (m2/m2) | Residue (%) |
|---|---|---|---|---|---|---|
| EP | 30 ± 2 | 959.3 ± 25.2 | 95.8 ± 4.6 | 0.2536 ± 0.0025 | 33.4 ± 1.2 | 1.4 ± 0.2 |
| EP/5RGO-APP | 35 ± 2 | 285.1 ± 10.2 | 49.4 ± 3.8 | 0.1561 ± 0.0019 | 33.7 ± 1.5 | 10.5 ± 0.7 |
| EP/10RGO-APP | 37 ± 3 | 197.0 ± 8.9 | 40.4 ± 2.4 | 0.1186 ± 0.0016 | 28.6 ± 0.9 | 12.0 ± 0.6 |
| EP/15RGO-APP | 42 ± 3 | 157.7 ± 6.8 | 35.8 ± 2.3 | 0.0653 ± 0.0009 | 23.8 ± 1.0 | 13.1 ± 1.1 |
| EP/15GO@APP | 39 ± 2 | 227.7 ± 9.5 | 40.8 ± 2.5 | 0.0732 ± 0.0012 | 28.9 ± 1.1 | 10.1 ± 0.6 |
| Samples | Elasticity Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|
| EP | 5243.6231 | 4.341 |
| EP/5RGO-APP | 5832.0092 | 58.764 |
| EP/10RGO-APP | 6178.9101 | 62.344 |
| EP/15RGO-APP | 6595.3522 | 75.102 |
| EP/15RGO@APP | 6087.6518 | 64.337 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liao, J.; Long, M.; Yan, L.; Cai, M. Facile Synthesis of Reduced-Graphene-Oxide-Modified Ammonium Polyphosphate to Enhance the Flame Retardancy, Smoke Release Suppression, and Mechanical Properties of Epoxy Resin. Polymers 2023, 15, 1304. https://doi.org/10.3390/polym15051304
Wang F, Liao J, Long M, Yan L, Cai M. Facile Synthesis of Reduced-Graphene-Oxide-Modified Ammonium Polyphosphate to Enhance the Flame Retardancy, Smoke Release Suppression, and Mechanical Properties of Epoxy Resin. Polymers. 2023; 15(5):1304. https://doi.org/10.3390/polym15051304
Chicago/Turabian StyleWang, Feiyue, Jiahao Liao, Miaotian Long, Long Yan, and Mengtao Cai. 2023. "Facile Synthesis of Reduced-Graphene-Oxide-Modified Ammonium Polyphosphate to Enhance the Flame Retardancy, Smoke Release Suppression, and Mechanical Properties of Epoxy Resin" Polymers 15, no. 5: 1304. https://doi.org/10.3390/polym15051304




