Pyrolysis of Waste Tires: A Review
Abstract
1. Introduction
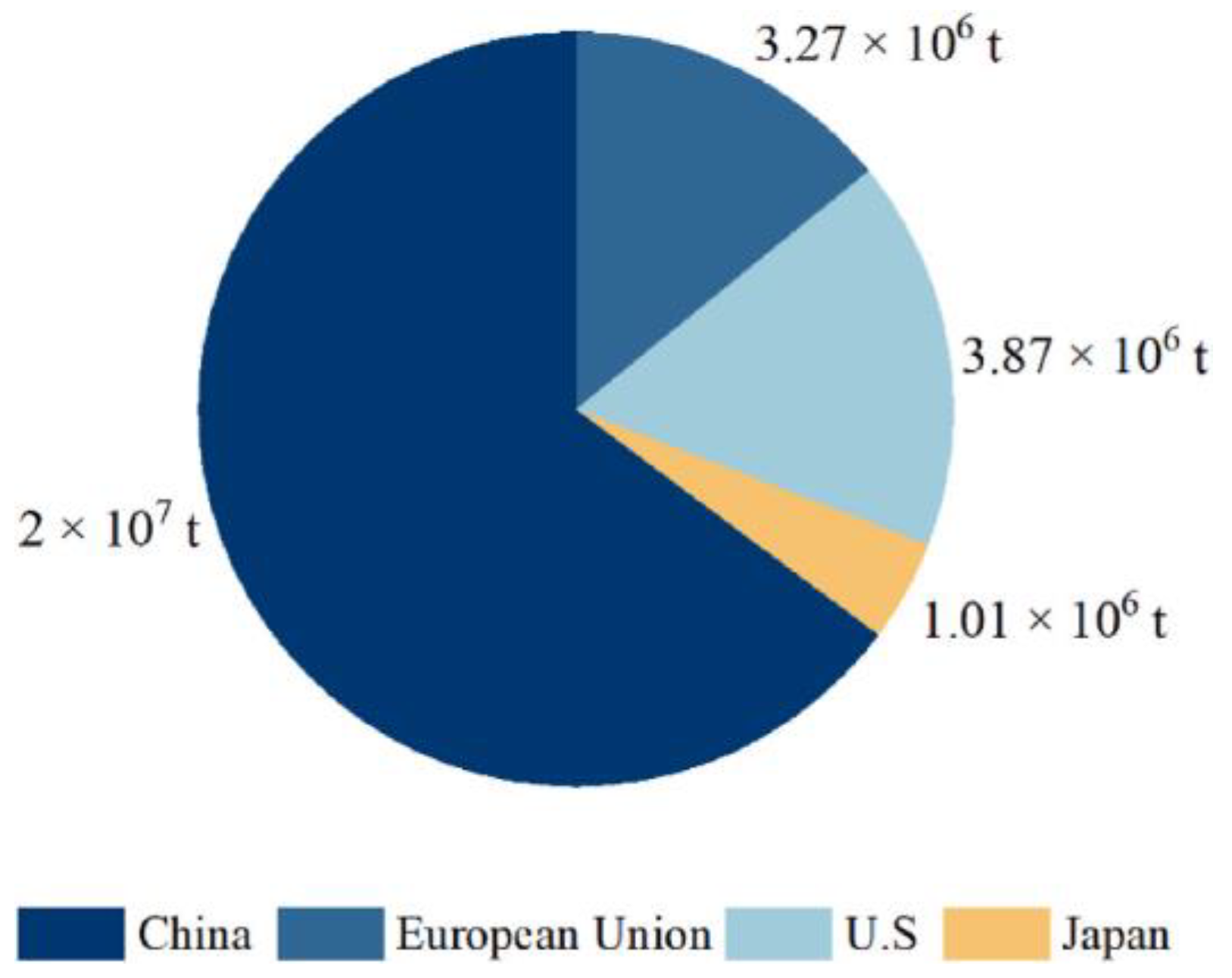
1.1. Sources of Large Quantities of WTs
1.2. Composition of Tires
1.3. Disposal of WTs
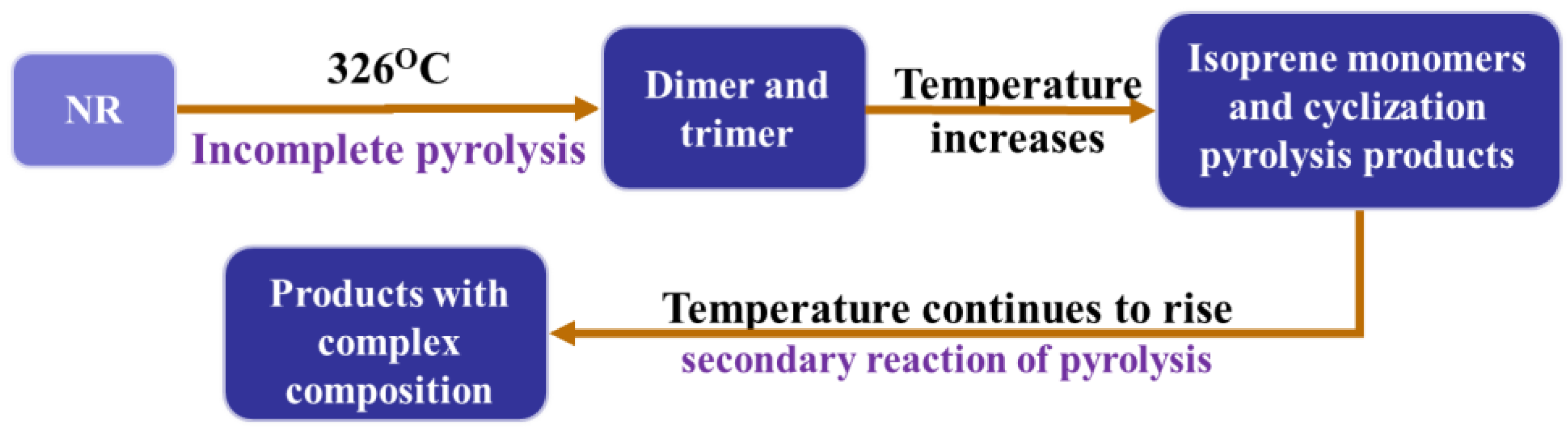
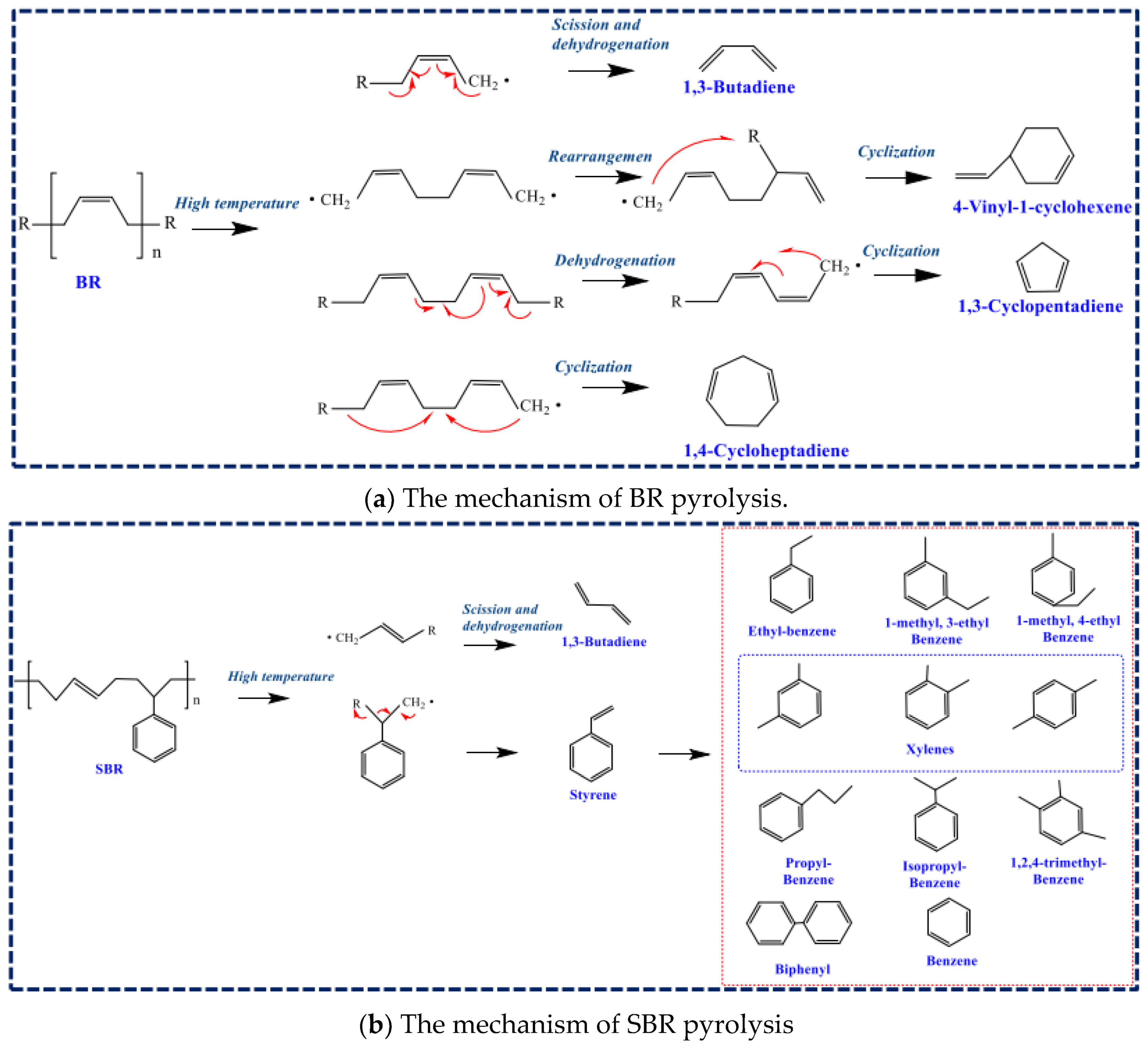
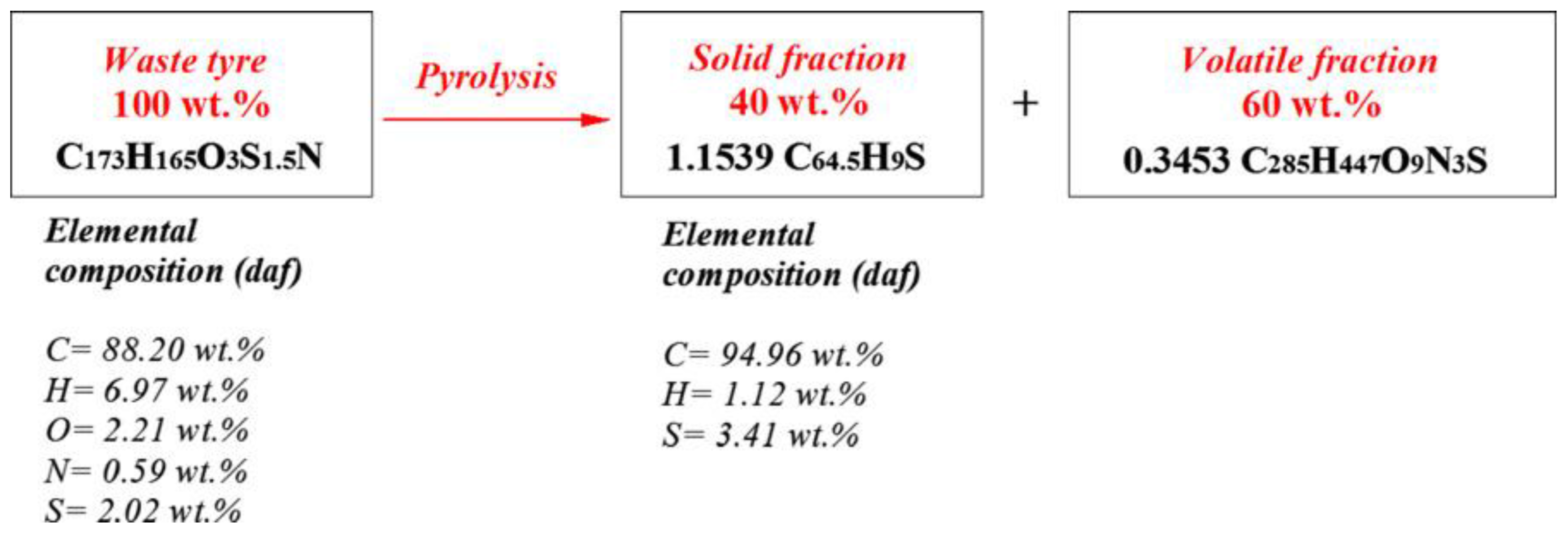
2. The Pyrolysis Mechanism of WTs
3. The Important Influencing Factors of WT Pyrolysis Process
3.1. Temperature
3.1.1. Pyrolysis Process of WTs at Different Temperatures
3.1.2. Effect of Temperature on Pyrolysis Products
3.2. Catalysts
3.2.1. Zeolite Catalysts
3.2.2. Other Catalysts
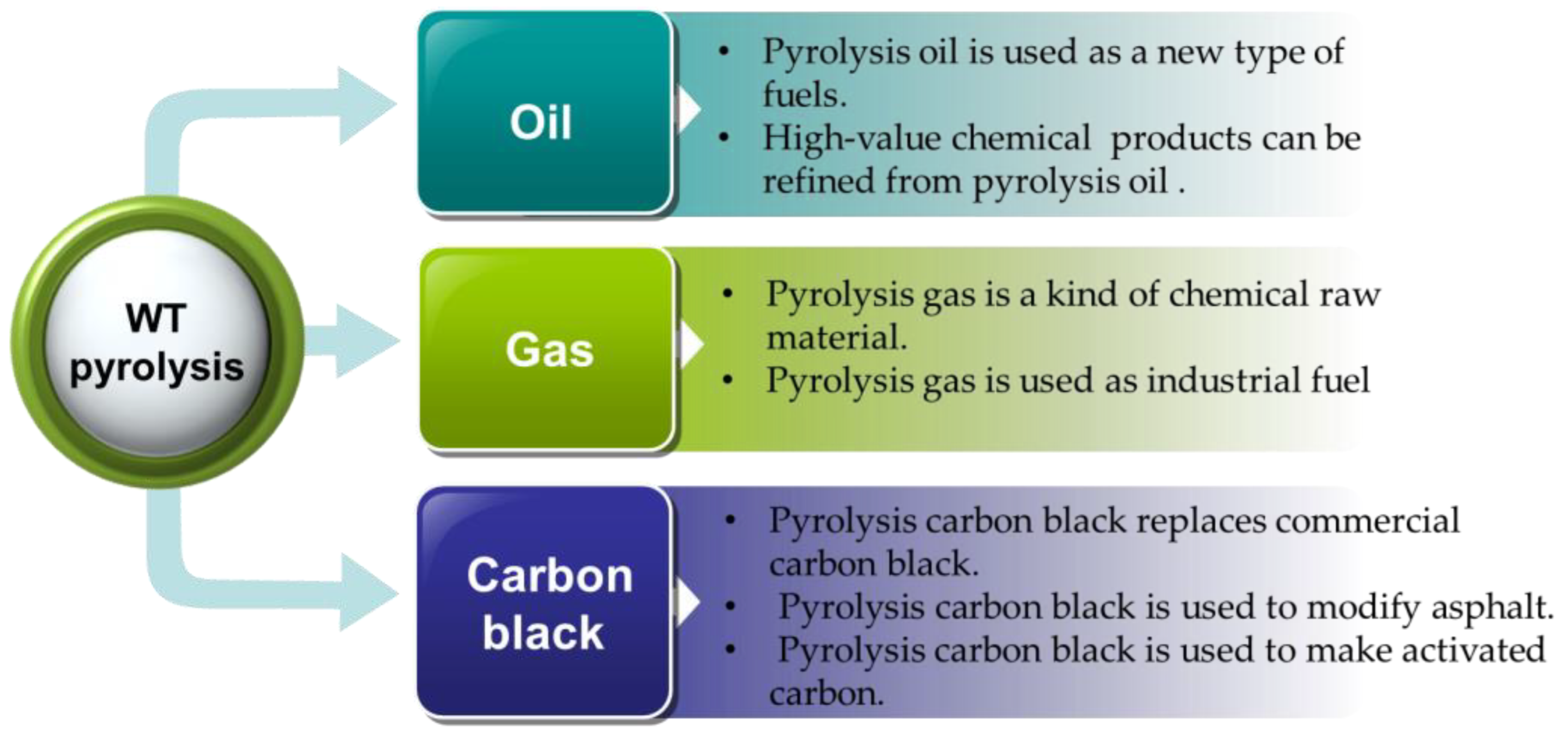
4. Properties and Applications of Pyrolysis Products
4.1. High Value Pyrolysis Oil
4.1.1. Pyrolysis Oil Is Used as a New Type of Fuel
4.1.2. Refining High-Value Chemical Products from Pyrolysis Oil
4.2. Properties and Applications of Pyrolysis Gas
4.3. Properties and Applications of Pyrolysis Carbon Black
4.3.1. Pyrolysis Carbon Black Replaces Commercial Carbon Black
4.3.2. Pyrolysis Carbon Is Used to Modify Asphalt
4.3.3. Pyrolysis Carbon Is Used to Make Activated Carbon
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WT | waste tire |
| NR | natural rubber |
| SR | synthetic rubber |
| SBR | styrene butadiene rubber |
| BR | butyl rubber |
| MS | mass spectrometric |
| MRI | magnetic resonance imaging |
| TG-FTIR | thermogravimetric analyzer coupled with Fourier-transform infrared spectrometry |
| RMD | reactive molecular dynamics |
| GC-MS | gas chromatography-mass spectrometry |
| TG-MS | thermogravimetric–mass spectrometric |
| TGA | thermogravimetric analysis |
| TG | thermogravimetric |
| COx | carbon oxides |
| N2 | nitrogen |
| PAH | polycyclic aromatic hydrocarbons |
| FCC | fluid catalytic cracking |
| Ca(OH)2 | calcium hydroxide |
| Na2CO3 | sodium bicarbonate |
| USY | ultra-stable Y zeolite |
| Ru | ruthenium |
| LFF | light fuel fraction |
| NP | normal pressure |
| VP | vacuum pressure |
| HHV | high heat value |
| Mo | molybdenum |
| NiMo | nickel molybdenum |
| AL2O3 | alumina oxide |
| FBR | fixed-bed reactor |
| FLBR | fluidized bed reactor |
| MBR | moving bed reactor |
| n.r. | not reported |
| H2 | hydrogen |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| CH4 | methane |
| C2H6 | ethane |
| C2H4 | ethylene |
| C3H6 | propylene |
| C3H8 | propane |
| C2H2 | acetylene |
| C4H10 | butane |
| C4H6 | 1,3-butadiene |
| C5H12 | pentane |
| C6H6 | benzene |
| C7H8 | toluene |
| C8H10 | xylene |
| N2 | nitrogen |
| H2S | hydrogen sulfide |
| ADSD | atomization dispersion and high temperature sputtering drying |
References
- Soares, F.A.; Steinbüchel, A. Natural rubber degradation products: Fine chemicals and reuse of rubber waste. Eur. Polym. J. 2022, 165, 111001. [Google Scholar] [CrossRef]
- Kumar, A.; Ahmad, D.; Patra, K.; Hossain, M. Enhancement of electromechanical properties of natural rubber by adding barium titanate filler: An electro-mechanical study. J. Appl. Polym. Sci. 2021, 138, 50991. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. Optimization of fuel recovery through the stepwise co-pyrolysis of palm shell and scrap tire. Energy Convers. Manag. 2015, 99, 334–345. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Hossain, M.; Umer, R. Recycling of wind turbine blades through modern recycling technologies: A road to zero waste. Renew. Energy Focus 2023, 44, 373–389. [Google Scholar] [CrossRef]
- Han, W.; Jiang, C.; Wang, J.; Chen, H. Enhancement of heat transfer during rubber pyrolysis process. J. Clean. Prod. 2022, 348, 131363. [Google Scholar] [CrossRef]
- Martínez, J.; Puy, N.; Murillo, R.; Garcia, T.; Navarro, M.; Mastral, A. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste tyre valorization by catalytic pyrolysis—A review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. Mathematical modeling of rubber tire pyrolysis. J. Anal. Appl. Pyrolysis 2012, 95, 79–86. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C.; Panicker, V.J. Recycling of waste tire rubber as aggregate in concrete: Durability-related performance. J. Clean. Prod. 2016, 112, 504–513. [Google Scholar] [CrossRef]
- Kumar Singh, R.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Kumar Sadhukhan, A.; Gupta, P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Niezgoda, A.; Deng, Y.; Sabatier, F.; Ansart, R. From end-of-life tires to storable energy carriers. J. Environ. Manag. 2020, 276, 111318. [Google Scholar] [CrossRef] [PubMed]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T. Application of coal conversion technology to tire processing. Fuel Process. Technol. 1999, 60, 231–242. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Lv, D.; Ren, D.; Zhai, T.; Sun, C.; Liu, H. Rubber reclamation with high bond-breaking selectivity using a low-temperature mechano-chemical devulcanization method. J. Clean. Prod. 2021, 279, 123266. [Google Scholar] [CrossRef]
- Martínez, J.D.; Campuzano, F.; Cardona-Uribe, N.; Arenas, C.N.; Muñoz-Lopera, D. Waste tire valorization by intermediate pyrolysis using a continuous twin-auger reactor: Operational features. Waste Manag. 2020, 113, 404–412. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, T.J. Scrap Tyre Management Pathways and Their Use as a Fuel—A Review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Rawat, M.; Agrawal, A. Tyre pyrolysis oil as an alternative fuel: A review. Mater. Today Proc. 2020, 28, 2481–2484. [Google Scholar] [CrossRef]
- Conesa, J.A.; Gálvez, A.; Mateos, F.; Martín-Gullón, I.; Font, R. Organic and inorganic pollutants from cement kiln stack feeding alternative fuels. J. Hazard. Mater. 2008, 158, 585–592. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Gao, Y.; Tao, Y. Comparison of end-of-life tire treatment technologies: A Chinese case study. Waste Manag. 2010, 30, 2235–2246. [Google Scholar] [CrossRef]
- Xu, S.; Lai, D.; Zeng, X.; Zhang, L.; Han, Z.; Cheng, J.; Wu, R.; Mašek, O.; Xu, G. Pyrolysis characteristics of waste tire particles in fixed-bed reactor with internals. Carbon Resour. Convers. 2018, 1, 228–237. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265, 121639. [Google Scholar] [CrossRef]
- Santella, C.; Cafiero, L.; De Angelis, D.; La Marca, F.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and catalytic pyrolysis of a mixture of plastics from small waste electrical and electronic equipment (WEEE). Waste Manag. 2016, 54, 143–152. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Cardoso, A.; Han, Y.; Bikane, K.; Sun, L. Catalytic pyrolysis of rubbers and vulcanized rubbers using modified zeolites and mesoporous catalysts with Zn and Cu. Energy 2019, 188, 116117. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Ullah, N.; Awan, I.A.; Iqbal, M.; Shah, A.; Sirajuddin; Sayed, M.; Mahmood, T.; Khan, M.S. Pyrolysis of waste tire rubber: Influence of temperature on pyrolysates yield. J. Environ. Chem. Eng. 2018, 6, 3469–3473. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Lin, F.; Zhong, L.; Ma, W.; Chen, G. Study of scrap tires pyrolysis—Products distribution and mechanism. Energy 2020, 213, 119038. [Google Scholar] [CrossRef]
- Sarkar, M.D.; Mukunda, P.G.; De, P.P.; Bhowmick, A.K. Degradation of Hydrogenated Styrene—Butadiene Rubber at High Temperature. Rubber Chem. Technol. 1997, 70, 855–870. [Google Scholar] [CrossRef]
- Wu, X.; Formela, K.; Rasool, R.t.; Wang, S. Evaluation of structural change during fast transformation process of cross-linked NR into liquid NR by light pyrolysis. Polym. Degrad. Stab. 2017, 136, 48–57. [Google Scholar] [CrossRef]
- Wei, X.; Zhong, H.; Yang, Q.; Yao, E.; Zhang, Y.; Zou, H. Studying the mechanisms of natural rubber pyrolysis gas generation using RMD simulations and TG-FTIR experiments. Energy Convers. Manag. 2019, 189, 143–152. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhang, B.; Song, Z.; Qian, X. Pyrolysis Characteristics of Waste Tire in an Analytical Pyrolyzer Coupled with Gas Chromatography/Mass Spectrometry. Energy Fuels 2015, 29, 3181–3187. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Aguado, R.; Bilbao, J. Continuous pyrolysis of waste tyres in a conical spouted bed reactor. Fuel 2010, 89, 1946–1952. [Google Scholar] [CrossRef]
- Tamri, Z.; Yazdi, A.V.; Haghighi, M.N.; Abbas-Abadi, M.S.; Heidarinasab, A. The effect of temperature, heating rate, initial cross-linking and zeolitic catalysts as key process and structural parameters on the degradation of natural rubber (NR) to produce the valuable hydrocarbons. J. Anal. Appl. Pyrolysis 2018, 134, 35–42. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Evaluation of pyrolysis product of virgin high density polyethylene degradation using different process parameters in a stirred reactor. Fuel Process. Technol. 2013, 109, 90–95. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H.; Ullah, S. Recycling of the glass/carbon fibre reinforced polymer composites: A step towards the circular economy. Polym.-Plast. Technol. Mater. 2022, 61, 761–788. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T.; Snape, C.E. Influence of Process Variables on Oils from Tire Pyrolysis and Hydropyrolysis in a Swept Fixed Bed Reactor. Energy Fuels 2000, 14, 739–744. [Google Scholar] [CrossRef]
- de Marco Rodriguez, I.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomón, M.J.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; Canito, J.L.; Rodríguez, J.J. Pyrolysis of automobile tyre waste. Influence of operating variables and kinetics study. J. Anal. Appl. Pyrolysis 2001, 58–59, 667–683. [Google Scholar] [CrossRef]
- Murillo, R.; Aylón, E.; Navarro, M.V.; Callén, M.S.; Aranda, A.; Mastral, A.M. The application of thermal processes to valorise waste tyre. Fuel Process. Technol. 2006, 87, 143–147. [Google Scholar] [CrossRef]
- Aylón, E.; Callén, M.S.; López, J.M.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Stelmach, S. Assessment of tire devolatilization kinetics. J. Anal. Appl. Pyrolysis 2005, 74, 259–264. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, S.; Zhong, H.; Liu, T.; Yao, E.; Zhang, Y.; Zou, H.; Du, W. Gas products generation mechanism during co-pyrolysis of styrene-butadiene rubber and natural rubber. J. Hazard. Mater. 2021, 401, 123302. [Google Scholar] [CrossRef]
- Han, J.; Li, W.; Liu, D.; Qin, L.; Chen, W.; Xing, F. Pyrolysis characteristic and mechanism of waste tyre: A thermogravimetry-mass spectrometry analysis. J. Anal. Appl. Pyrolysis 2018, 129, 1–5. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Fuel production from pyrolysis of natural and synthetic rubbers. Fuel 2017, 191, 403–410. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.N.; Mustafi, N.N.; Rahim, M.A.; Haniu, H. Thermal Recycling of Solid Tire Wastes for Alternative Liquid Fuel: The First Commercial Step in Bangladesh. Procedia Eng. 2013, 56, 573–582. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1,3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis 2001, 58–59, 803–811. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C.; Zhang, Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J. Anal. Appl. Pyrolysis 2009, 85, 334–337. [Google Scholar] [CrossRef]
- Witpathomwong, C.; Longloilert, R.; Wongkasemjit, S.; Jitkarnka, S. Improving Light Olefins and Light Oil Production Using Ru/MCM-48 in Catalytic Pyrolysis of Waste Tire. Energy Procedia 2011, 9, 245–251. [Google Scholar] [CrossRef]
- Mastral, A.M.; Callen, M.S.; García, T.; Navarro, M.V. Improvement of liquids from coal–tire co-thermolysis. Characterization of the obtained oils. Fuel Process. Technol. 2000, 64, 135–140. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Catalytic pyrolysis of tyres: Influence of catalyst temperature. Fuel 2002, 81, 2425–2434. [Google Scholar] [CrossRef]
- Díez, C.; Martínez, O.; Calvo, L.F.; Cara, J.; Morán, A. Pyrolysis of tyres. Influence of the final temperature of the process on emissions and the calorific value of the products recovered. Waste Manag. 2004, 24, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of rubber tyre wastes: A kinetic study. Fuel 2013, 103, 542–552. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; de Marco, I.; Torres, A.; Caballero, B.; Cabrero, M.A.; Chomón, M.J. Chromatographic analysis of the gases obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2000, 55, 43–54. [Google Scholar] [CrossRef]
- Yazdani, E.; Hashemabadi, S.H.; Taghizadeh, A. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature. Waste Manag. 2019, 85, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Umeki, E.R.; de Oliveira, C.F.; Torres, R.B.; dos Santos, R.G. Physico-chemistry properties of fuel blends composed of diesel and tire pyrolysis oil. Fuel 2016, 185, 236–242. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.S.Z.; Abbas-Abadi, M.S.; Haghighi, M.N.; Abedini, H. The effect of different zeolite based catalysts on the pyrolysis of poly butadiene rubber. Fuel 2015, 160, 544–548. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Gardy, J.; Nizami, A.S. Effect of advanced catalysts on tire waste pyrolysis oil. Process Saf. Environ. Prot. 2018, 116, 542–552. [Google Scholar] [CrossRef]
- Boxiong, S.; Chunfei, W.; Binbin, G.; Rui, W.; Liang, C. Pyrolysis of waste tyres with zeolite USY and ZSM-5 catalysts. Appl. Catal. B Environ. 2007, 73, 150–157. [Google Scholar] [CrossRef]
- İlkılıç, C.; Aydın, H. Fuel production from waste vehicle tires by catalytic pyrolysis and its application in a diesel engine. Fuel Process. Technol. 2011, 92, 1129–1135. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, D.; Huo, P.; Wang, M.; Han, L.; Chen, G.; Li, H.; Li, X.; Wang, Y.; et al. Derived oil production by catalytic pyrolysis of scrap tires. Chin. J. Catal. 2016, 37, 526–532. [Google Scholar] [CrossRef]
- Olazar, M.; Aguado, R.; Arabiourrutia, M.; Lopez, G.; Barona, A.; Bilbao, J. Catalyst Effect on the Composition of Tire Pyrolysis Products. Energy Fuels 2008, 22, 2909–2916. [Google Scholar] [CrossRef]
- Boxiong, S.; Chunfei, W.; Cai, L.; Binbin, G.; Rui, W. Pyrolysis of waste tyres: The influence of USY catalyst/tyre ratio on products. J. Anal. Appl. Pyrolysis 2007, 78, 243–249. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, X.; Liu, P.; Li, J.; Liu, G.; Wang, K.; Li, M.; Zhong, Z.; Xu, J.; et al. Catalytic conversion of rubber wastes to produce aromatic hydrocarbons over USY zeolites: Effect of SiO2/Al2O3 mole ratio. Energy Convers. Manag. 2019, 197, 111857. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-ong, D.; Sricharoenchaikul, V.; Atong, D. Effect of CV-ZSM-5, Ni-ZSM-5 and FA-ZSM-5 catalysts for selective aromatic formation from pyrolytic vapors of rubber wastes. J. Anal. Appl. Pyrolysis 2017, 124, 733–741. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.D.; Marques, M.d.F.V.; Henriques, C.A. Degradation of Polypropylene and Polyethylene Wastes Over HZSM-5 and USY Zeolites. Catal. Lett. 2019, 149, 798–812. [Google Scholar] [CrossRef]
- Manchantrarat, N.; Jitkarnka, S. Impact of HY as an additive in Pd/HBETA catalyst on waste tire pyrolysis products. Chem. Eng. Trans. 2012, 29, 733–738. [Google Scholar]
- Arabiourrutia, M.; Olazar, M.; Aguado, R.; López, G.; Barona, A.; Bilbao, J. HZSM-5 and HY Zeolite Catalyst Performance in the Pyrolysis of Tires in a Conical Spouted Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 7600–7609. [Google Scholar] [CrossRef]
- Hijazi, A.; Al-Muhtaseb, A.a.H.; Aouad, S.; Ahmad, M.N.; Zeaiter, J. Pyrolysis of waste rubber tires with palladium doped zeolite. J. Environ. Chem. Eng. 2019, 7, 103451. [Google Scholar] [CrossRef]
- Hijazi, A.; Boyadjian, C.; Ahmad, M.N.; Zeaiter, J. Solar pyrolysis of waste rubber tires using photoactive catalysts. Waste Manag. 2018, 77, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Basagiannis, A.C.; Verykios, X.E. Influence of the carrier on steam reforming of acetic acid over Ru-based catalysts. Appl. Catal. B Environ. 2008, 82, 77–88. [Google Scholar] [CrossRef]
- Ali, M.A.; Kimura, T.; Suzuki, Y.; Al-Saleh, M.A.; Hamid, H.; Inui, T. Hydrogen spillover phenomenon in noble metal modified clay-based hydrocracking catalysts. Appl. Catal. A Gen. 2002, 227, 63–72. [Google Scholar] [CrossRef]
- Dũng, N.A.; Klaewkla, R.; Wongkasemjit, S.; Jitkarnka, S. Light olefins and light oil production from catalytic pyrolysis of waste tire. J. Anal. Appl. Pyrolysis 2009, 86, 281–286. [Google Scholar] [CrossRef]
- Khalil, U.; Vongsvivut, J.; Shahabuddin, M.; Samudrala, S.P.; Srivatsa, S.C.; Bhattacharya, S. A study on the performance of coke resistive cerium modified zeolite Y catalyst for the pyrolysis of scrap tyres in a two-stage fixed bed reactor. Waste Manag. 2020, 102, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Elbaba, I.F.; Williams, P.T. High yield hydrogen from the pyrolysis–catalytic gasification of waste tyres with a nickel/dolomite catalyst. Fuel 2013, 106, 528–536. [Google Scholar] [CrossRef]
- Seng-eiad, S.; Jitkarnka, S. Untreated and HNO3-treated pyrolysis char as catalysts for pyrolysis of waste tire: In-depth analysis of tire-derived products and char characterization. J. Anal. Appl. Pyrolysis 2016, 122, 151–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Williams, P.T. Carbon nanotubes and hydrogen production from the pyrolysis catalysis or catalytic-steam reforming of waste tyres. J. Anal. Appl. Pyrolysis 2016, 122, 490–501. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Fernández, A.M.; Barriocanal, C.; Alvarez, R. Pyrolysis of a waste from the grinding of scrap tyres. J. Hazard. Mater. 2012, 203–204, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.D.; Lapuerta, M.; García-Contreras, R.; Murillo, R.; García, T. Fuel Properties of Tire Pyrolysis Liquid and Its Blends with Diesel Fuel. Energy Fuels 2013, 27, 3296–3305. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Oasmaa, A.; van de Beld, B.; Saari, P.; Elliott, D.C.; Solantausta, Y. Norms, Standards, and Legislation for Fast Pyrolysis Bio-oils from Lignocellulosic Biomass. Energy Fuels 2015, 29, 2471–2484. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of waste tires in a circulating fluidized-bed reactor. Energy 2001, 26, 385–399. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; Caballero, B.M.; de Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Alguacil, F.J.; Lobato, B. Distillation of granulated scrap tires in a pilot plant. J. Hazard. Mater. 2011, 190, 285–292. [Google Scholar] [CrossRef] [PubMed]
- López, F.A.; Centeno, T.A.; Alguacil, F.J.; Lobato, B.; López-Delgado, A.; Fermoso, J. Gasification of the char derived from distillation of granulated scrap tyres. Waste Manag. 2012, 32, 743–752. [Google Scholar] [CrossRef]
- Ucar, S.; Karagoz, S.; Ozkan, A.R.; Yanik, J. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 2005, 84, 1884–1892. [Google Scholar] [CrossRef]
- Hita, I.; Gutiérrez, A.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Upgrading model compounds and Scrap Tires Pyrolysis Oil (STPO) on hydrotreating NiMo catalysts with tailored supports. Fuel 2015, 145, 158–169. [Google Scholar] [CrossRef]
- Martínez, J.D.; Veses, A.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Puy, N.; Artigues, A.; Bartrolí, J.; García, T. Co-pyrolysis of biomass with waste tyres: Upgrading of liquid bio-fuel. Fuel Process. Technol. 2014, 119, 263–271. [Google Scholar] [CrossRef]
- Costa, G.A.; Santos, R.G.d. Fractionation of tire pyrolysis oil into a light fuel fraction by steam distillation. Fuel 2019, 241, 558–563. [Google Scholar] [CrossRef]
- Jantaraksa, N.; Prasassarakich, P.; Reubroycharoen, P.; Hinchiranan, N. Cleaner alternative liquid fuels derived from the hydrodesulfurization of waste tire pyrolysis oil. Energy Convers. Manag. 2015, 95, 424–434. [Google Scholar] [CrossRef]
- Rofiqul Islam, M.; Haniu, H.; Rafiqul Alam Beg, M. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Navarro, M.V.; Murillo, R.; García, T.; Mastral, A.M. Waste Tire Pyrolysis: Comparison between Fixed Bed Reactor and Moving Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 4029–4033. [Google Scholar] [CrossRef]
- Teng, H.; Serio, M.A.; Wojtowicz, M.A.; Bassilakis, R.; Solomon, P.R. Reprocessing of used tires into activated carbon and other products. Ind. Eng. Chem. Res. 1995, 34, 3102–3111. [Google Scholar] [CrossRef]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Y.; Zhao, X.; Sun, J.; Wang, W.; Mao, Y.; Ma, C. Microwave pyrolysis of tire powders: Evolution of yields and composition of products. J. Anal. Appl. Pyrolysis 2017, 123, 152–159. [Google Scholar] [CrossRef]
- Siva, M.; Onenc, S.; Uçar, S.; Yanik, J. Influence of oily wastes on the pyrolysis of scrap tire. Energy Convers. Manag. 2013, 75, 474–481. [Google Scholar] [CrossRef]
- Choi, G.-G.; Oh, S.-J.; Kim, J.-S. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content. Appl. Energy 2016, 170, 140–147. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F. Recovery of value-added products from the catalytic pyrolysis of waste tyre. Energy Convers. Manag. 2009, 50, 991–994. [Google Scholar] [CrossRef]
- Ayanoğlu, A.; Yumrutaş, R. Production of gasoline and diesel like fuels from waste tire oil by using catalytic pyrolysis. Energy 2016, 103, 456–468. [Google Scholar] [CrossRef]
- Choi, G.-G.; Jung, S.-H.; Oh, S.-J.; Kim, J.-S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Aylón, E.; Murillo, R.; Fernández-Colino, A.; Aranda, A.; García, T.; Callén, M.S.; Mastral, A.M. Emissions from the combustion of gas-phase products at tyre pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 210–214. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Qiao, Y.; Tian, Y.; Zhang, J.; Che, Y. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS. Energy Convers. Manag. 2017, 151, 227–239. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Fluidised bed pyrolysis and catalytic pyrolysis of scrap tyres. Environ. Technol. 2003, 24, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, A.; Iovane, P.; Molino, A. High energy syngas production by waste tyres steam gasification in a rotary kiln pilot plant. Experimental and numerical investigations. Fuel 2010, 89, 2721–2728. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Nahil, M.A.; Williams, P. Pyrolysis–Catalytic Reforming/Gasification of Waste Tires for Production of Carbon Nanotubes and Hydrogen. Energy Fuels 2015, 29, 3328–3334. [Google Scholar] [CrossRef]
- Li, W.; Wei, M.; Liu, Y.; Ye, Y.; Li, S.; Yuan, W.; Wang, M.; Wang, D. Catalysts evaluation for production of hydrogen gas and carbon nanotubes from the pyrolysis-catalysis of waste tyres. Int. J. Hydrog. Energy 2019, 44, 19563–19572. [Google Scholar] [CrossRef]
- Luo, S.; Feng, Y. The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers. Manag. 2017, 136, 27–35. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Wu, C.; Williams, P.T. Catalytic Pyrolysis-Gasification of Waste Tire and Tire Elastomers for Hydrogen Production. Energy Fuels 2010, 24, 3928–3935. [Google Scholar] [CrossRef]
- Pattabhi Raman, K.; Walawender, W.P.; Fan, L.T. Gasification of waste tires in a fluid bed reactor. Conserv. Recycl. 1981, 4, 79–88. [Google Scholar] [CrossRef]
- Portofino, S.; Casu, S.; Iovane, P.; Russo, A.; Martino, M.; Donatelli, A.; Galvagno, S. Optimizing H2 Production from Waste Tires via Combined Steam Gasification and Catalytic Reforming. Energy Fuels 2011, 25, 2232–2241. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Kumkova, I.I.; Lerner, A.S.; Popov, V.E. Equilibrium analysis of hydrogen production using the steam-plasma gasification process of the used car tires. J. Phys. Conf. Ser. 2012, 406, 012023. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Yin, X.L.; Zhao, Z.L.; Xu, B.Y.; Chen, Y. Pyrolysis of tire powder: Influence of operation variables on the composition and yields of gaseous product. Fuel Process. Technol. 2002, 79, 141–155. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; García-Díaz, I.; Alguacil, F.J. Textural and fuel characteristics of the chars produced by the pyrolysis of waste wood, and the properties of activated carbons prepared from them. J. Anal. Appl. Pyrolysis 2013, 104, 551–558. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Gonçalves, M.; Mendes, B.; Pinto, F. Study of the Organic Extraction and Acidic Leaching of Chars Obtained in the Pyrolysis of Plastics, Tire Rubber and Forestry Biomass Wastes. Procedia Eng. 2012, 42, 1739–1746. [Google Scholar] [CrossRef]
- Sagar, M.; Nibedita, K.; Manohar, N.; Kumar, K.R.; Suchismita, S.; Pradnyesh, A.; Reddy, A.B.; Sadiku, E.R.; Gupta, U.N.; Lachit, P.; et al. A potential utilization of end-of-life tyres as recycled carbon black in EPDM rubber. Waste Manag. 2018, 74, 110–122. [Google Scholar] [CrossRef]
- Darmstadt, H.; Roy, C.; Kaliaguine, S. Characterization of pyrolytic carbon blacks from commercial tire pyrolysis plants. Carbon 1995, 33, 1449–1455. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Thermal plasma pyrolysis of used tires for carbon black recovery. J. Mater. Sci. 2005, 40, 3817–3819. [Google Scholar] [CrossRef]
- Sahouli, B.; Blacher, S.; Brouers, F.; Darmstadt, H.; Roy, C.; Kaliaguine, S. Surface morphology and chemistry of commercial carbon black and carbon black from vacuum pyrolysis of used tyres. Fuel 1996, 75, 1244–1250. [Google Scholar] [CrossRef]
- Chaala, A.; Darmstadt, H.; Roy, C. Acid-base method for the demineralization of pyrolytic carbon black. Fuel Process. Technol. 1996, 46, 1–15. [Google Scholar] [CrossRef]
- Martínez, J.D.; Cardona-Uribe, N.; Murillo, R.; García, T.; López, J.M. Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manag. 2019, 85, 574–584. [Google Scholar] [CrossRef]
- Darmstadt, H.; Roy, C.; Kaliaguine, S. ESCA characterization of commercial carbon blacks and of carbon blacks from vacuum pyrolysis of used tires. Carbon 1994, 32, 1399–1406. [Google Scholar] [CrossRef]
- Osayi, J.I.; Iyuke, S.; Ogbeide, S.E. Biocrude Production through Pyrolysis of Used Tyres. J. Catal. 2014, 2014, 386371. [Google Scholar] [CrossRef]
- Tian, X.; Zhuang, Q.; Han, S.; Li, S.; Liu, H.; Li, L.; Zhang, J.; Wang, C.; Bian, H. A novel approach of reapplication of carbon black recovered from waste tyre pyrolysis to rubber composites. J. Clean. Prod. 2021, 280, 124460. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sasaki, I.; Nishizaki, I.; Meiarashi, S.; Moriyoshi, A. Effects of Film Thickness, Wavelength, and Carbon Black on Photodegradation of Asphalt. J. Jpn. Pet. Inst. 2005, 48, 150–155. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sasaki, I.; Meiarashi, S. Mechanism of Asphalt Binder Aging by Ultraviolet Irradiation and Aging Resistance by Adding Carbon Black. J. Jpn. Pet. Inst. 2004, 47, 266–273. [Google Scholar] [CrossRef]
- Chaala, A.; Roy, C.; Ait-Kadi, A. Rheological properties of bitumen modified with pyrolytic carbon black. Fuel 1996, 75, 1575–1583. [Google Scholar] [CrossRef]
- Darmstadt, H.; Chaala, A.; Roy, C.; Kaliaguine, S. SIMS and ESCA characterization of bitumen reinforced with pyrolytic carbon black. Fuel 1996, 75, 125–132. [Google Scholar] [CrossRef]
- Feng, Z.-g.; Rao, W.-y.; Chen, C.; Tian, B.; Li, X.-j.; Li, P.-l.; Guo, Q.-l. Performance evaluation of bitumen modified with pyrolysis carbon black made from waste tyres. Constr. Build. Mater. 2016, 111, 495–501. [Google Scholar] [CrossRef]
- Rambau, K.M.; Musyoka, N.M.; Manyala, N.; Ren, J.; Langmi, H.W. Mechanochemical approach in the synthesis of activated carbons from waste tyres and its hydrogen storage applications. Mater. Today: Proc. 2018, 5, 10505–10513. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Adv. Colloid Interface Sci. 2014, 211, 93–101. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F.; Shahid, M. Conversion of Waste Tyres into Carbon Black and their Utilization as Adsorbent. J. Chin. Chem. Soc. 2006, 53, 1085–1089. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Ngila, J.C.; Nomngongo, P.N. Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater. Cogent Eng. 2017, 4, 1330912. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; Martinez de Yuso, A.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Nayak, A.; Agarwal, S.; Tyagi, I. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: Effect of pre-treatment conditions. J. Colloid Interface Sci. 2014, 417, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Asgari, G.; Askari, F.B.; Torbaghan, A.E. Adsorption of lead metal from aqueous solutions using activated carbon derived from scrap tires. Fresenius Environ. Bull. 2015, 24, 2341–2347. [Google Scholar]
- Trubetskaya, A.; Kling, J.; Ershag, O.; Attard, T.M.; Schröder, E. Removal of phenol and chlorine from wastewater using steam activated biomass soot and tire carbon black. J. Hazard. Mater. 2019, 365, 846–856. [Google Scholar] [CrossRef]
- Mashile, G.P.; Mpupa, A.; Nqombolo, A.; Dimpe, K.M.; Nomngongo, P.N. Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J. Water Process Eng. 2020, 33, 101011. [Google Scholar] [CrossRef]
- Molino, A.; Donatelli, A.; Marino, T.; Aloise, A.; Rimauro, J.; Iovane, P. Waste tire recycling process for production of steam activated carbon in a pilot plant. Resour. Conserv. Recycl. 2018, 129, 102–111. [Google Scholar] [CrossRef]


| Ref. | Temperature (°C) | Oil (wt%) | Pressure (Pa) |
|---|---|---|---|
| [55] | 450 | 58.1 | 101,325 |
| 525 | 56.9 | ||
| 600 | 53.1 | ||
| [56] | 450 | 49 | 101,325 |
| 500 | 45 | ||
| 600 | 40 | ||
| [41] | 400 | 36 | 101,325 |
| 500 | 44 | ||
| 600 | 45 | ||
| [57] | 350 | 30 | 101,325 |
| 450 | 33 | ||
| 550 | 38 | ||
| [58] | 450 | 43 | 8000–9000 |
| 550 | 44.6 | ||
| 650 | 42.9 | ||
| [43] | 500 | 55.4 | |
| 600 | 52.2 | ||
| 700 | 36.6 |
| Temperature (°C) | Catalysts | Yield (wt%) | Ref. | ||
|---|---|---|---|---|---|
| Oil | Char | Gas | |||
| 450 | - | 50.47 | 36.47 | 13.06 | [67] |
| 500 | - | 51.98 | 36.09 | 11.92 | |
| 550 | - | 52.61 | 35.69 | 11.70 | |
| 600 | - | 54.10 | 36.30 | 9.61 | |
| 500 | Ca(OH)2 | 40 | 48 | 12 | [68] |
| 500 | Na2CO3 | 47.8 | 37.6 | 14.6 | [64] |
| 500 | ZSM-5 | 55.6 | 37.6 | 6.5 | [69] |
| 500 | USY | 53.5 | 36.5 | 10 | |
| 450 | HZSM-5 | 50.2 | 33.1 | 16.7 | [70] |
| 450 | HY | 54.9 | 33 | 12.1 | |
| 450 | Hβ | 47.8 | 33.1 | 19.1 | |
| Pyrolysis Conditions | 550 °C, NP | 550 °C, NP | 520 °C, VP | 550 °C, VP | 650 °C, NP |
|---|---|---|---|---|---|
| S/(wt%) | 0.6 | 0.58 | 0.8 | 1.26 | 1.35 |
| H/C | 1.6 | 1.60 | 1.5 | 1.36 | 1.42 |
| Density/(kg.m−3) | 900 | 900 | 950 | 987 | 943 |
| Flash point/°C | 20 | 20 | 28 | 30 | <30 |
| Heat value/(MJ/kg) | 43.27 | 43.27 | 43.7 | 41.0 | 41.6 |
| Ref. | [94] | [95] | [20] | [58] | [96] |
| Reactors | Temperature (°C) | Fraction Content (vol%) | Ref. | ||
|---|---|---|---|---|---|
| Light (<200 °C) | Medium (200–350 °C) | Heavy (>350 °C) | |||
| FBR | 475 | 50 | 45 | 5 | [101] a |
| FBR | 500 | 30 | 55 | 15 | [68] |
| FBR | 550 | 60 | 35 | 5 | [96] b |
| MBR | 600 | 45 | 35 | 20 | [102] |
| Temperature (°C) | Carbon (wt%) | Ash (wt%) | S (wt%) | Specific Surface Area (m2/g) | Ref. |
|---|---|---|---|---|---|
| 500 | 82.18 | 14.6 | 3.6 | 43.1 | [129] |
| 550 | 77.22 | 14.58 | 2.41 | 89.1 | [58] |
| 550 | 88.0 | 13.2 | 2.5 | 65.7 | [130] |
| 550 | 86.3 | 12.5 | 2.8 | 64 | [94] |
| 600 | 86.6 | 7.10 | 2.10 | 116.3 | [36] |
| 650 | 82.60 | 14.80 | 2.30 | 63.5 | [96] |
| 700 | 83.0 | 14.8 | 2.7 | 83 | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. https://doi.org/10.3390/polym15071604
Han W, Han D, Chen H. Pyrolysis of Waste Tires: A Review. Polymers. 2023; 15(7):1604. https://doi.org/10.3390/polym15071604
Chicago/Turabian StyleHan, Wenwen, Deshang Han, and Hongbo Chen. 2023. "Pyrolysis of Waste Tires: A Review" Polymers 15, no. 7: 1604. https://doi.org/10.3390/polym15071604
APA StyleHan, W., Han, D., & Chen, H. (2023). Pyrolysis of Waste Tires: A Review. Polymers, 15(7), 1604. https://doi.org/10.3390/polym15071604







