Coagulative Nucleation in the Copolymerization of Methyl Methacrylate–Butyl Acrylate under Monomer-Starved Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Latex Preparation
2.3. Analysis
2.4. Characterization
2.4.1. Transmission Electron Microscopy
2.4.2. Glass Transition Temperature
2.4.3. Nuclear Magnetic Resonance
3. Results and Discussion
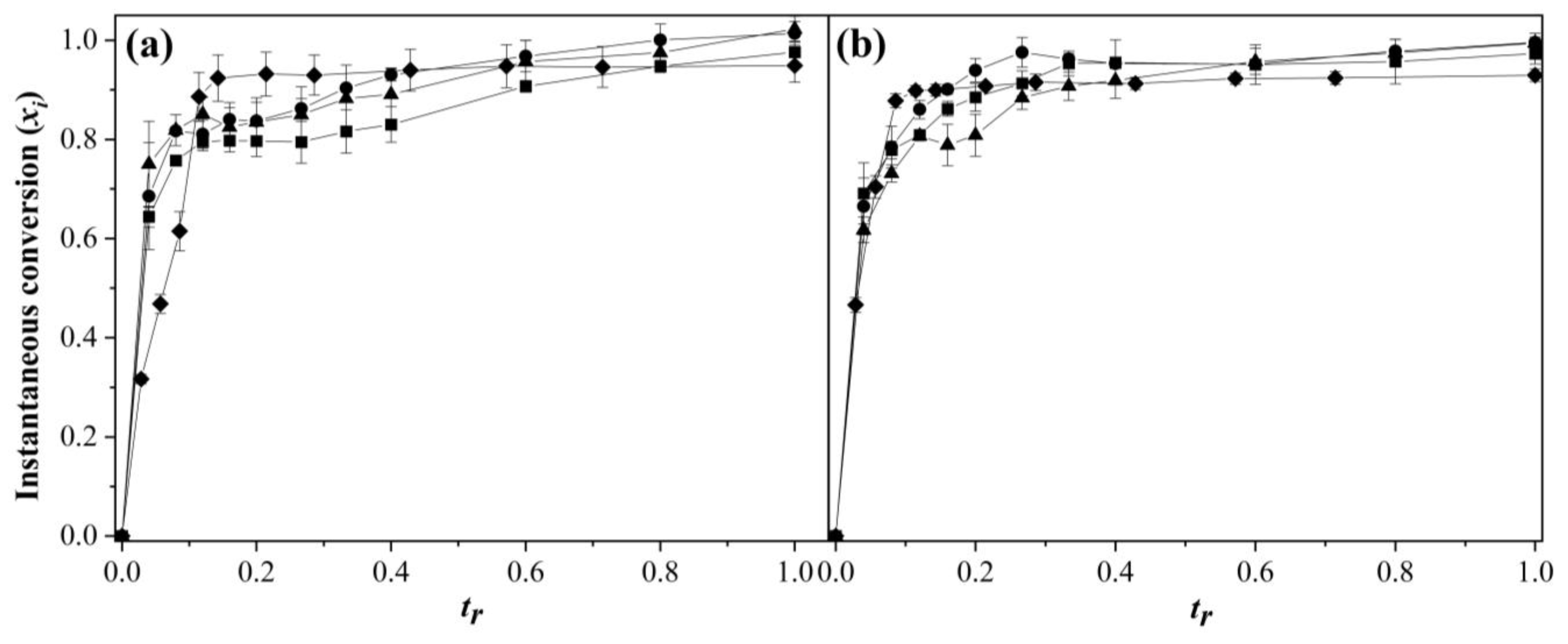
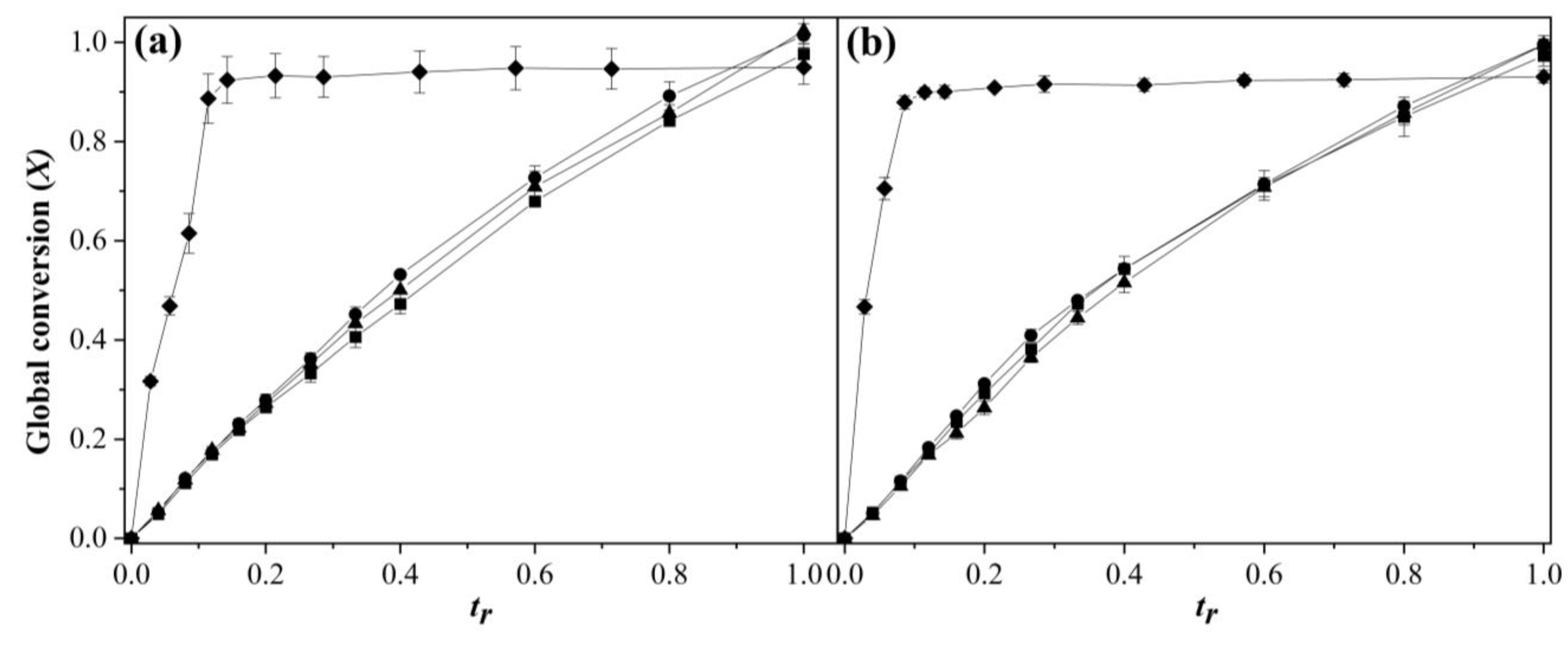
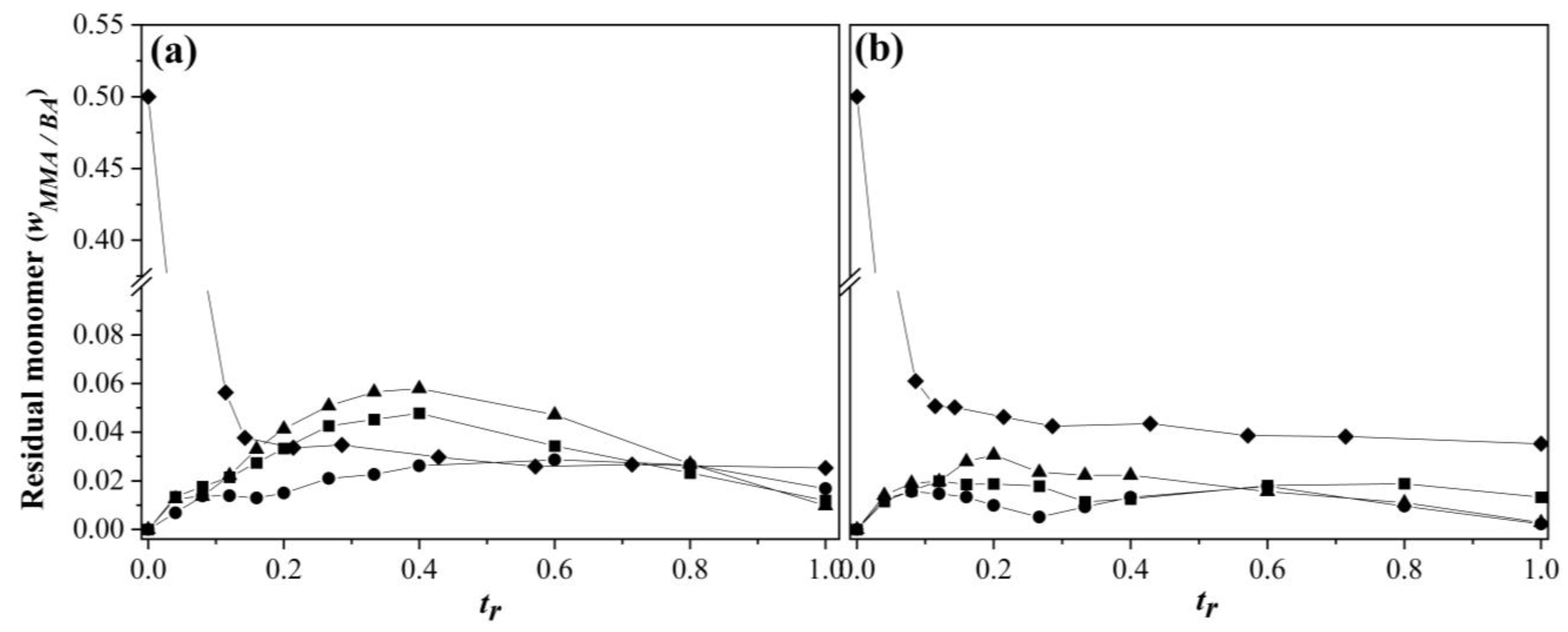
3.1. Comonomers Conversion
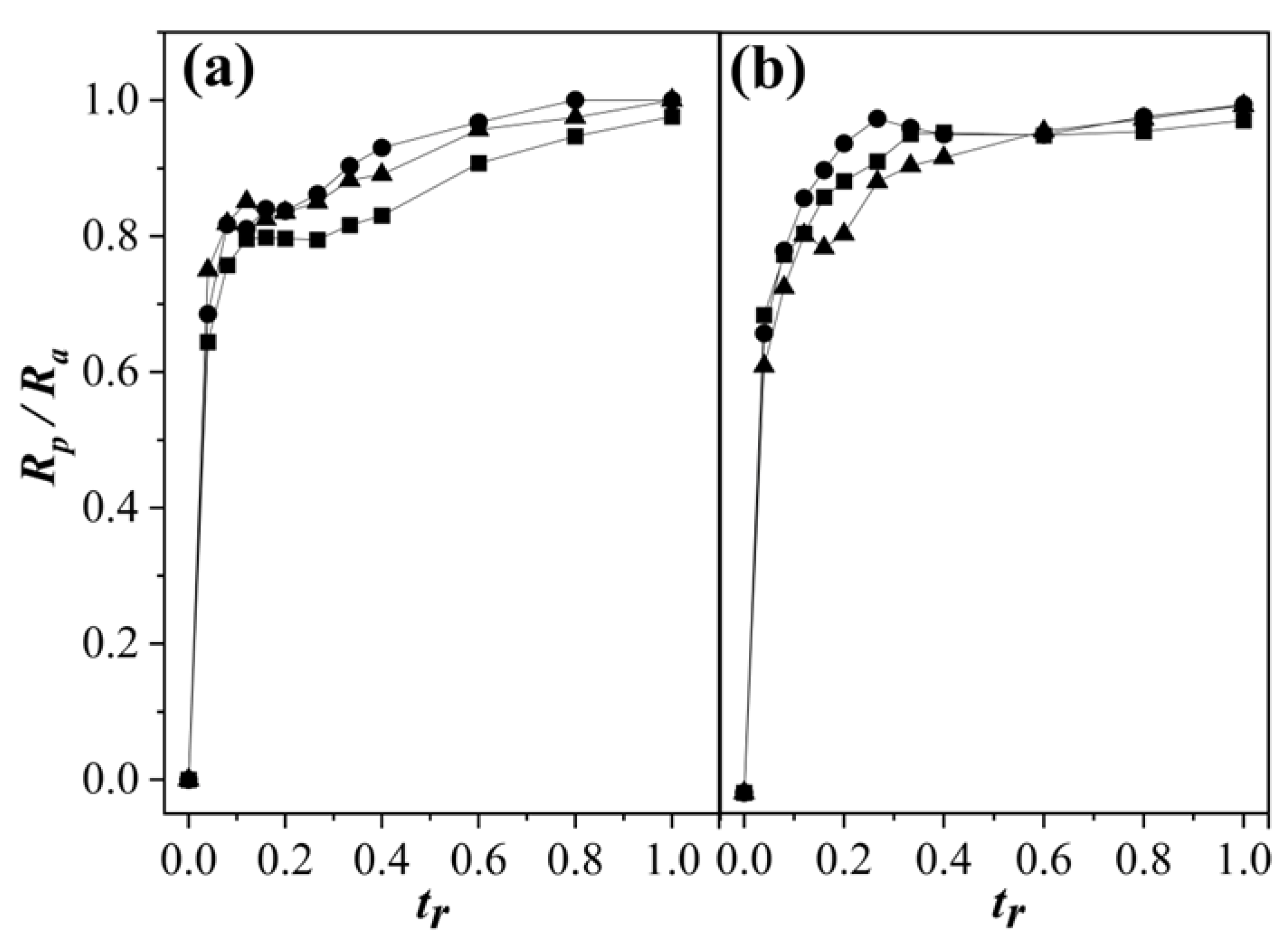
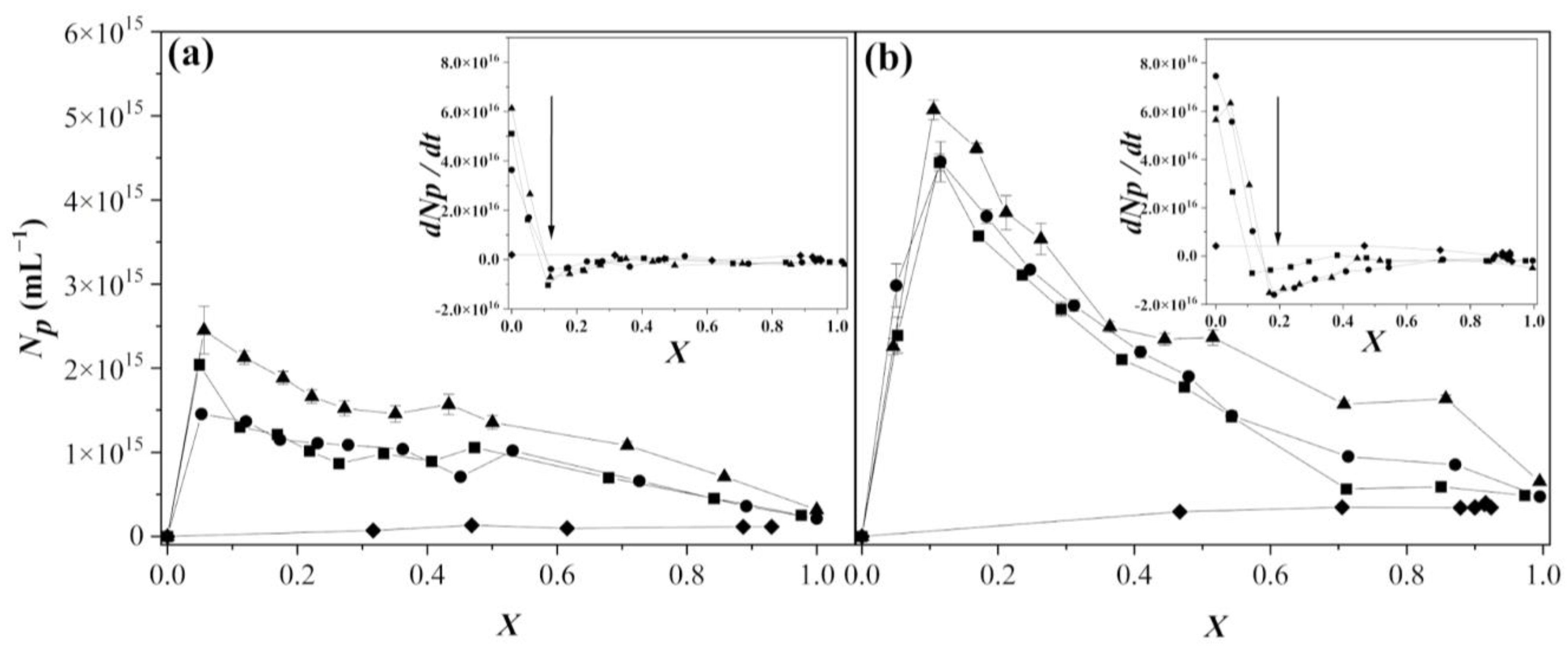
3.2. Polymerization Rate Analysis
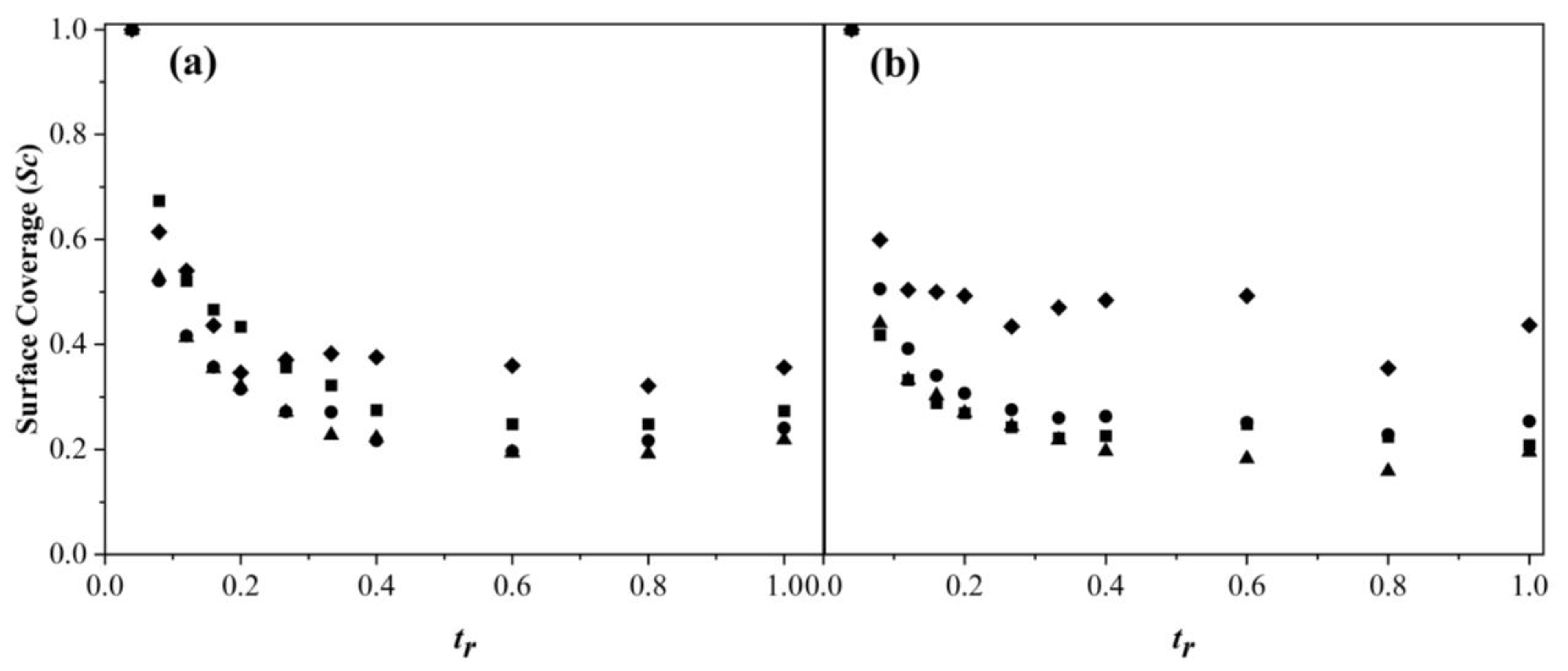
3.3. Particle Size and Surfactant Coverage Ratio
3.4. Coagulation Mechanism Analysis
3.5. Thermic Copolymer Behavior
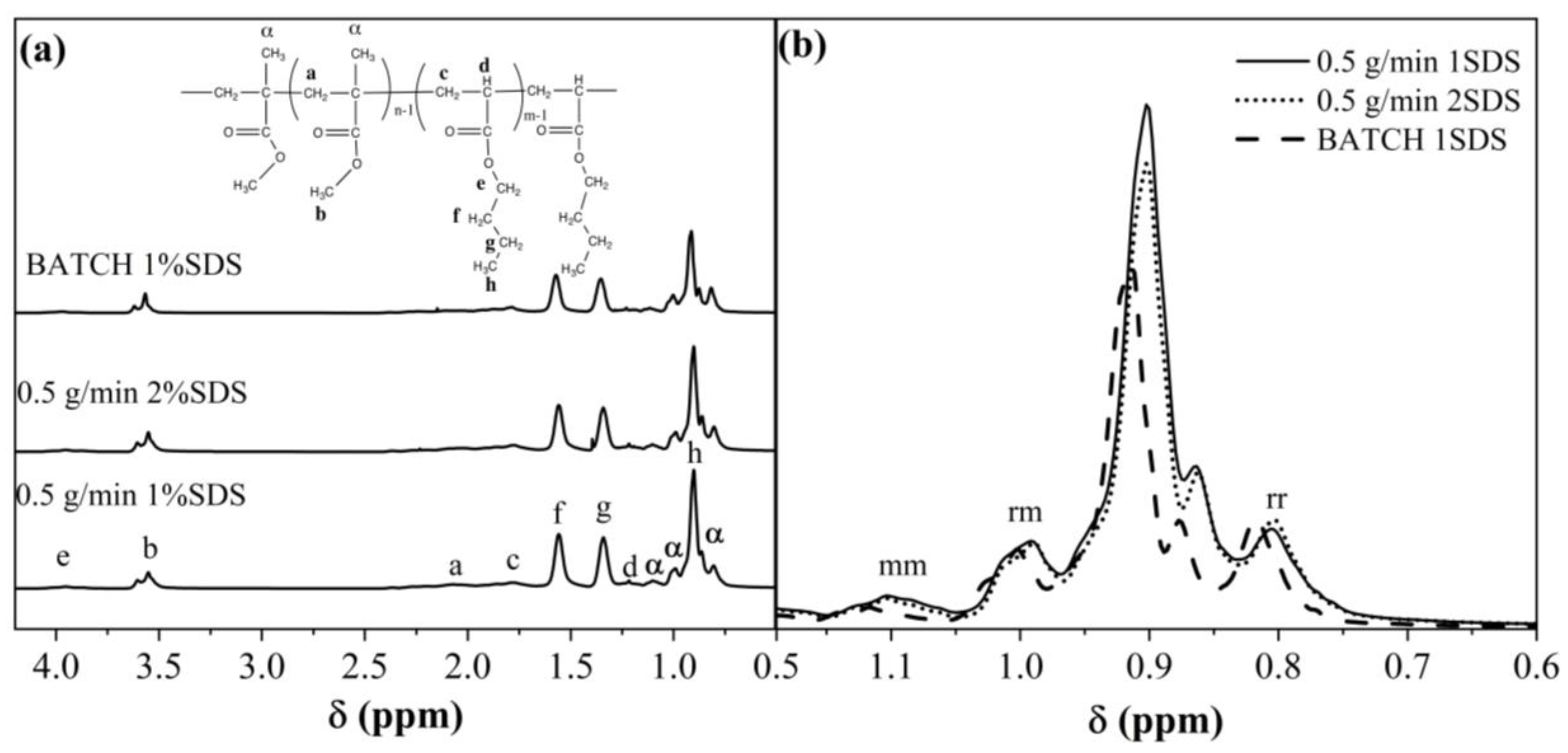
3.6. Stereoregularity Analysis
3.7. Copolymer Particles Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capek, I.; Potisk, P. Microemulsion and Emulsion Polymerization of Butyl Acrylate-I. Effect of the Initiator Type and Tem-perature. Eur. Polym. J. 1995, 31, 1269–1277. [Google Scholar] [CrossRef]
- Aguilar, J.; Rabelero, M.; Nuño-Donlucas, S.M.; Mendizábal, E.; Martínez-Richa, A.; López, R.G.; Arellano, M.; Puig, J.E. Narrow size-distribution poly(methyl methacrylate) nanoparticles made by semicontinuous heterophase polymerization. J. Appl. Polym. Sci. 2011, 119, 1827–1834. [Google Scholar] [CrossRef]
- Lichti, G.; Gilbert, R.G.; Napper, D.H. The mechanisms of latex particle formation and growth in the emulsion polymerization of styrene using the surfactant sodium dodecyl sulfate. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 269–291. [Google Scholar] [CrossRef]
- Sosa, N.; Peralta, R.D.; López, R.G.; Ramos, L.F.; Katime, I.; Cesteros, C.; Mendizábal, E.; Puig, J.E. A Comparison of the Characteristics of Poly(Vinyl Acetate) Latex with High Solid Content Made by Emulsion and Semi-Continuous Microemul-sion Polymerization. Polymer 2001, 42, 6923–6928. [Google Scholar] [CrossRef]
- Pérez-García, M.G.; Alvarado, A.G.; Rabelero, M.; Arellano, M.; Pérez-Carrillo, L.A.; López-Serrano, F.; Lopez, R.G.; Mendizabal, E.; Puig, J.E. Semicontinuous Heterophase Polymerization of Methyl and Hexyl Methacrylates to Produce Latexes with High Nanoparticles Content. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 144–155. [Google Scholar] [CrossRef]
- Sajjadi, S. Nanoparticle Formation by Monomer-Starved Semibatch Emulsion Polymerization. Langmuir 2007, 23, 1018–1024. [Google Scholar] [CrossRef]
- Sajjadi, S.; Chen, Y.; Jahanzad, F. Ultrafine nanolatexes made via monomer-starved semicontinuous emulsion polymerisation in the presence of a water-soluble chain transfer agent. Eur. Polym. J. 2016, 80, 89–98. [Google Scholar] [CrossRef]
- Aguilar, J.; Rabelero, M.; Mendizábal, E.; Nuño-Donlucas, S.M.; Arellano, M.; Puig, J.E. Tensile Properties of Self-Crosslinkable Poly(n-butyl methacrylate-co-N-methylolacrylamide) Films Prepared by Emulsion and Microemulsion Latexes. Macromol. Symp. 2009, 283–284, 223–229. [Google Scholar] [CrossRef]
- Sajjadi, S. Control of particle size by feed composition in the nanolatexes produced via monomer-starved semicontinuous emulsion copolymerization. J. Colloid Interface Sci. 2015, 445, 174–182. [Google Scholar] [CrossRef]
- Chen, Y.; Jahanzad, F.; Sajjadi, S. Semicontinuous Monomer-Starved Emulsion Polymerization as a Means to Produce Nanolatexes: Analysis of Nucleation Stage. Langmuir 2013, 29, 5650–5658. [Google Scholar] [CrossRef]
- Ge, L.; Texter, J. Combustion resistant nanocomposites from water/AOT/MMA reverse microemulsions. Polym. Bull. 2004, 52, 297–305. [Google Scholar] [CrossRef]
- Alvarado, A.G.; Rabelero, M.; Aguilar, J.; Mejia, J.F.; Sánchez, F.J.M. Synthesis and characterization of butyl acrylate-co-poly (ethylene glycol) dimethacrylate obtained by microemulsion polymerization. Des. Monomers Polym. 2020, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, R.; Treviño, M.E.; Elizalde, L.E.; Pérez-Carrillo, L.A.; Mendizábal, E.; Puig, J.E.; López, R.G. Semicontinuous heterophase polymerization under monomer starved conditions to prepare nanoparticles with narrow size distribution. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1463–1473. [Google Scholar] [CrossRef]
- Feeney, P.J.; Napper, D.H.; Gilbert, R.G. Coagulative nucleation and particle size distributions in emulsion polymerization. Macromolecules 1984, 17, 2520–2529. [Google Scholar] [CrossRef]
- Nomura, M.; Horie, I.; Kubo, M.; Fujita, K. Kinetics and Mechanism of Emulsion Copolymerization. IV. Kinetic Modeling of Emulsion Copolymerization of Styrene and Methyl Methacrylate. J. Appl. Polym. Sci. 1989, 37, 1029–1050. [Google Scholar] [CrossRef]
- Forcada, J.; Asúa, J.M. Modeling of unseeded emulsion copolymerization of styrene and methyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 987–1009. [Google Scholar] [CrossRef]
- Lee, K.-C.; El-Aasser, M.S.; Vanderhoff, J.W. Emulsion Copolymerization of Vinylidene Chloride and Butyl Methacrylate, 83:17 in Mol %. J. Appl. Polym. Sci. 1991, 42, 3133–3145. [Google Scholar] [CrossRef]
- Carro, S.; Herrera-Ordonez, J.; Castillo-Tejas, J. On the evolution of the rate of polymerization, number and size distribution of particles in styrene emulsion polymerization above CMC. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3152–3160. [Google Scholar] [CrossRef]
- Lee, C.-F.; Lin, K.-R.; Chiu, W.-Y. Kinetics Studies of Two-stage Soapless Emulsion Polymerization of Butyl Acrylate and Methyl Methacrylate. J. Appl. Polym. Sci. 1994, 51, 1621–1628. [Google Scholar] [CrossRef]
- Sommer, F.; Duc, T.M.; Pirri, R.; Meunier, G.; Quet, C. Surface Morphology of Poly(Butyl Acrylate)/Poly(Methyl Methacrylate) Core Shell Latex by Atomic Force Microscopy. Langmuir 1995, 11, 440–448. [Google Scholar] [CrossRef]
- Ballard, M.J.; Napper, D.H.; Gilbert, R.G. Kinetics of emulsion polymerization of methyl methacrylate. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3225–3253. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of Emulsion Polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef] [PubMed]
- Ouzineb, K.; Heredia, M.F.; Graillat, C.; Mckenna, T.F. Stabilization and kinetics in the emulsion copolymerization of butyl acrylate and methyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2832–2846. [Google Scholar] [CrossRef]
- Chern, C.S.; Hsu, H. Semibatch emulsion copolymerization of methyl methacrylate and butyl acrylate. J. Appl. Polym. Sci. 1995, 55, 571–581. [Google Scholar] [CrossRef]
- Capek, I. Emulsion Polymerization of Butyl Acrylate, 2 a) Effect of the Initiator Type and Concentration. Macromol. Chem. Phys. 1994, 195, 1137–1146. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, X.; Han, Y.; Liu, B.; Zhang, J.; Zhang, M. Facile synthesis of narrow particle size distribution, high solid content, cationic polymer latexes by macroemulsion polymerization-based particle coagulation mechanism. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 116–122. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Zhang, M.; Zhang, H. Initiator Systems Effect on Particle Coagulation and Particle Size Distribution in One-Step Emulsion Polymerization of Styrene. Polymers 2016, 8, 55. [Google Scholar] [CrossRef]
- Adelnia, H.; Pourmahdian, S. Soap-free emulsion polymerization of poly (methyl methacrylate-co-butyl acrylate): Effects of anionic comonomers and methanol on the different characteristics of the latexes. Colloid Polym. Sci. 2014, 292, 197–205. [Google Scholar] [CrossRef]
- Sood, A.; Lodhi, P.K. Modeling evidence in support of coagulative nucleation theory. J. Appl. Polym. Sci. 2011, 122, 517–531. [Google Scholar] [CrossRef]
- Feeney, P.J.; Napper, D.H.; Gilbert, R.G. Surfactant-free emulsion polymerizations: Predictions of the coagulative nucleation theory. Macromolecules 1987, 20, 2922–2930. [Google Scholar] [CrossRef]
- Dobrowolska, M.E.; van Esch, J.H.; Koper, G.J.M. Direct Visualization of “Coagulative Nucleation” in Surfactant-Free Emulsion Polymerization. Langmuir 2013, 29, 11724–11729. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Meng, W.; Wang, P.; Zhang, M.; Zhang, H. In situ charge neutralization-controlled particle coagulation and its effects on the particle size distribution in the one-step emulsion polymerization. Eur. Polym. J. 2016, 83, 278–287. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Liu, L.; Yang, W. Direct Observations of Three Nucleation/Growth Processes of Charge-Stabilized Dispersion Polymerizations with Varying Water/Methanol Ratios. J. Phys. Chem. B 2010, 114, 10970–10978. [Google Scholar] [CrossRef]
- Chou, I.-C.; Chiu, W.-Y. Novel Synthesis of Multi-Scaled, Surfactant-Free Monodisperse Latexes via Alcoholic Dispersion Polymerization in a Mixed Ionic/Nonionic Initiation System. Macromolecules 2013, 46, 3561–3569. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Yuan, L.; Chai, C. Synthesis of Acrylic Emulsion Containing High Hydroxyl Content. J. Macromol. Sci. Part A Pure Appl. Chem. 2004, 41, 15–27. [Google Scholar] [CrossRef]
- Lee, K.C.; El-Aasser, M.S.; Vanderhoff, J.W. Batch and Semicontinuous Emulsion Copolymerization of Vinylidene Chloride and Butyl Methacrylate. I. Kinetics in VDC–BMA Emulsion Polymerization and Surface and Colloidal Properties of VDC–BMA Latexes. J. Appl. Polym. Sci. 1992, 45, 2207–2219. [Google Scholar] [CrossRef]
- Sajjadi, S.; Brooks, B.W. Semibatch emulsion polymerization of butyl acrylate. I. Effect of monomer distribution. J. Appl. Polym. Sci. 1999, 74, 3094–3110. [Google Scholar] [CrossRef]
- Kukulj, D.; Davis, T.P.; Suddaby, K.G.; Haddleton, D.M.; Gilbert, R.G. Catalytic chain transfer for molecular weight control in the emulsion homo- and copolymerizations of methyl methacrylate and butyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 859–878. [Google Scholar] [CrossRef]
- Sajjadi, S. Population Balance Modeling of Particle Size Distribution in Monomer-Starved Semibatch Emulsion Polymerization. AIChE J. 2009, 55, 3191–3205. [Google Scholar] [CrossRef]
- Matijevic, E.; Pathica, B.A. The Properties of Ionized Monolayers Part 1.-Sodium Dodecyl Sulphate at the Air/Water Interface. Trans. Faraday Soc. 1958, 54, 1382–1389. [Google Scholar] [CrossRef]
- Aguilar, J.; Moscoso, F.; Rios, O.; Ceja, I.; Sánchez, J.C.; Bautista, F.; Puig, J.E.; Fernández, V.V.A. Swelling Behavior of Poly(N-isopropylacrylamide) Nanogels with Narrow Size Distribution Made by Semi-continuous Inverse Heterophase Polymerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 412–419. [Google Scholar] [CrossRef]
- Parker, A.P.; Reynolds, P.A.; Lewis, A.L.; Hughes, L. Semi-continuous emulsion co-polymerisation of methylmethacrylate and butylacrylate using zwitterionic surfactants as emulsifiers: Evidence of coagulative nucleation above the critical micelle concentration. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 162–174. [Google Scholar] [CrossRef]
- Wessling, R.A. Kinetics of continuous addition emulsion polymerization. J. Appl. Polym. Sci. 1968, 12, 309–319. [Google Scholar] [CrossRef]
- Sajjadi, S.; Brooks, B.W. Semibatch emulsion polymerisation reactors: Polybutyl acrylate case study. Chem. Eng. Sci. 2000, 55, 4757–4781. [Google Scholar] [CrossRef]
- Sajjadi, S.; Brooks, B.W. Semibatch Emulsion Polymerization of Butyl Acrylate. II. Effects of Emulsifier Distribution. J. Appl. Polym. Sci. 2001, 79, 582–597. [Google Scholar] [CrossRef]
- Sajjadi, S.; Yianneskis, M. Semibatch Emulsion Polymerization of Methyl Methacrylate with a Neat Monomer Feed. Polym. React. Eng. 2003, 11, 715–736. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Liu, Y.; Tan, Z.; Zhou, C.; Zhang, H. Particle Nucleation and Growth in the Emulsion Polymerization of Styrene: Effect of Monomer/Water Ratio and Electrolyte Concentration. J. Macromol. Sci. Part A Pure Appl. Chem. 2015, 52, 147–154. [Google Scholar] [CrossRef]
- Dobrowolska, M.E.; Koper, G.J.M. Optimal ionic strength for nonionically initiated polymerization. Soft Matter 2014, 10, 1151–1154. [Google Scholar] [CrossRef]
- Liu, B.J.; Deng, Y.J.; Sun, S.L.; Zhang, M.Y.; Lin, R.Q.; Zhang, H.X. A Novel Approach to Prepare Large-Scale and Nar-row-Dispersed Latex Particles by Emulsion Polymerization Based on Particle Coagulation Mechanism. Des. Monomers Polym. 2016, 19, 119–127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos, S.G.; Fernández-Escamilla, V.V.A.; Corona-Rivera, M.Á.; González-Iñiguez, K.J.; Barrera, A.; Moscoso-Sánchez, F.J.; Figueroa-Ochoa, E.B.; Ceja, I.; Rabelero, M.; Aguilar, J. Coagulative Nucleation in the Copolymerization of Methyl Methacrylate–Butyl Acrylate under Monomer-Starved Conditions. Polymers 2023, 15, 1628. https://doi.org/10.3390/polym15071628
Castellanos SG, Fernández-Escamilla VVA, Corona-Rivera MÁ, González-Iñiguez KJ, Barrera A, Moscoso-Sánchez FJ, Figueroa-Ochoa EB, Ceja I, Rabelero M, Aguilar J. Coagulative Nucleation in the Copolymerization of Methyl Methacrylate–Butyl Acrylate under Monomer-Starved Conditions. Polymers. 2023; 15(7):1628. https://doi.org/10.3390/polym15071628
Chicago/Turabian StyleCastellanos, Sujey G., V. Vladimir A. Fernández-Escamilla, Miguel Á. Corona-Rivera, Karla J. González-Iñiguez, Arturo Barrera, Francisco J. Moscoso-Sánchez, Edgar B. Figueroa-Ochoa, Israel Ceja, Martín Rabelero, and Jacobo Aguilar. 2023. "Coagulative Nucleation in the Copolymerization of Methyl Methacrylate–Butyl Acrylate under Monomer-Starved Conditions" Polymers 15, no. 7: 1628. https://doi.org/10.3390/polym15071628
APA StyleCastellanos, S. G., Fernández-Escamilla, V. V. A., Corona-Rivera, M. Á., González-Iñiguez, K. J., Barrera, A., Moscoso-Sánchez, F. J., Figueroa-Ochoa, E. B., Ceja, I., Rabelero, M., & Aguilar, J. (2023). Coagulative Nucleation in the Copolymerization of Methyl Methacrylate–Butyl Acrylate under Monomer-Starved Conditions. Polymers, 15(7), 1628. https://doi.org/10.3390/polym15071628






