Melt Memory Effect in Polyethylene Random Terpolymer with Small Amount of 1-Octene and 1-Hexene Co-Units: Non-Isothermal and Isothermal Investigations
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
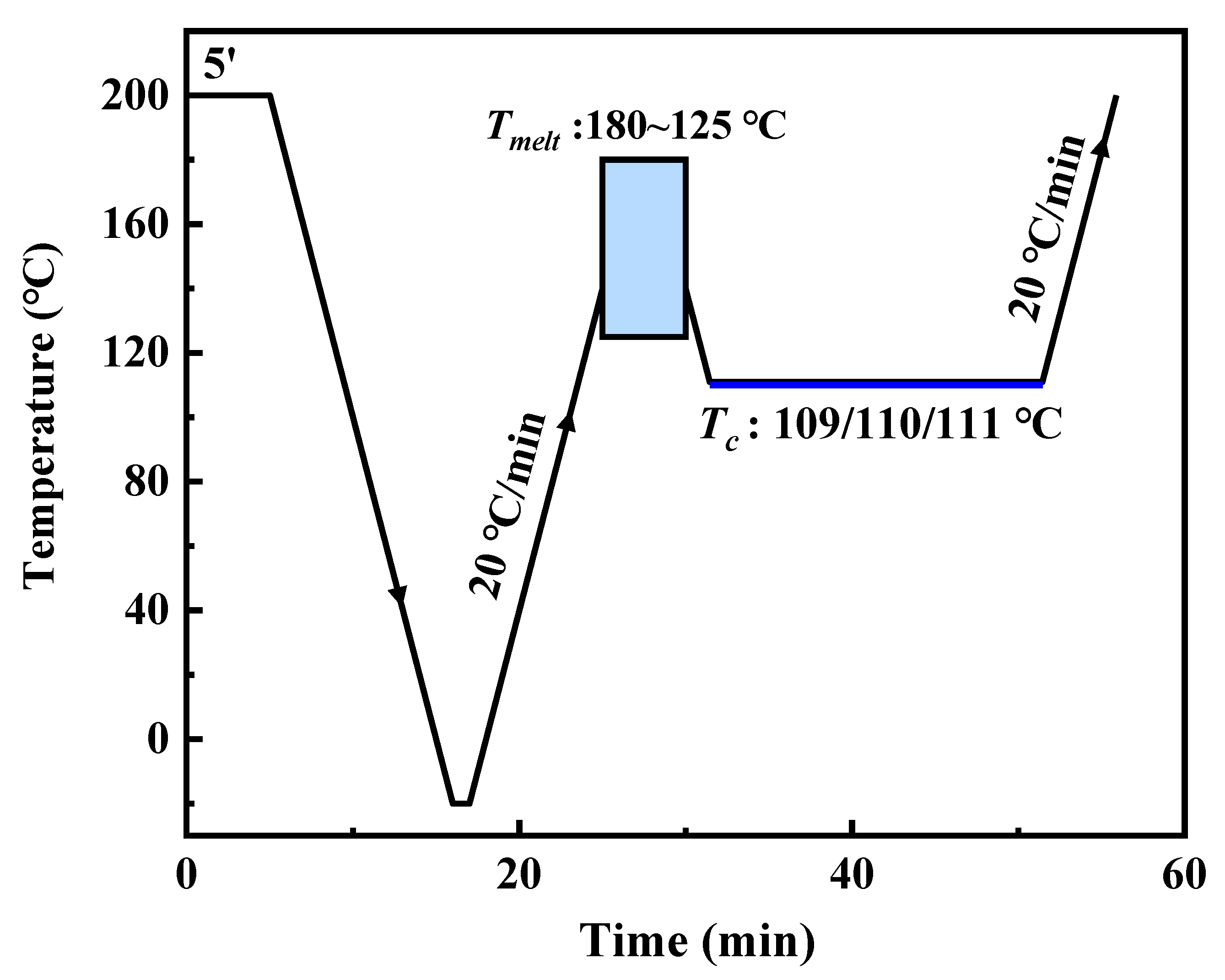
2.2. DSC Tests
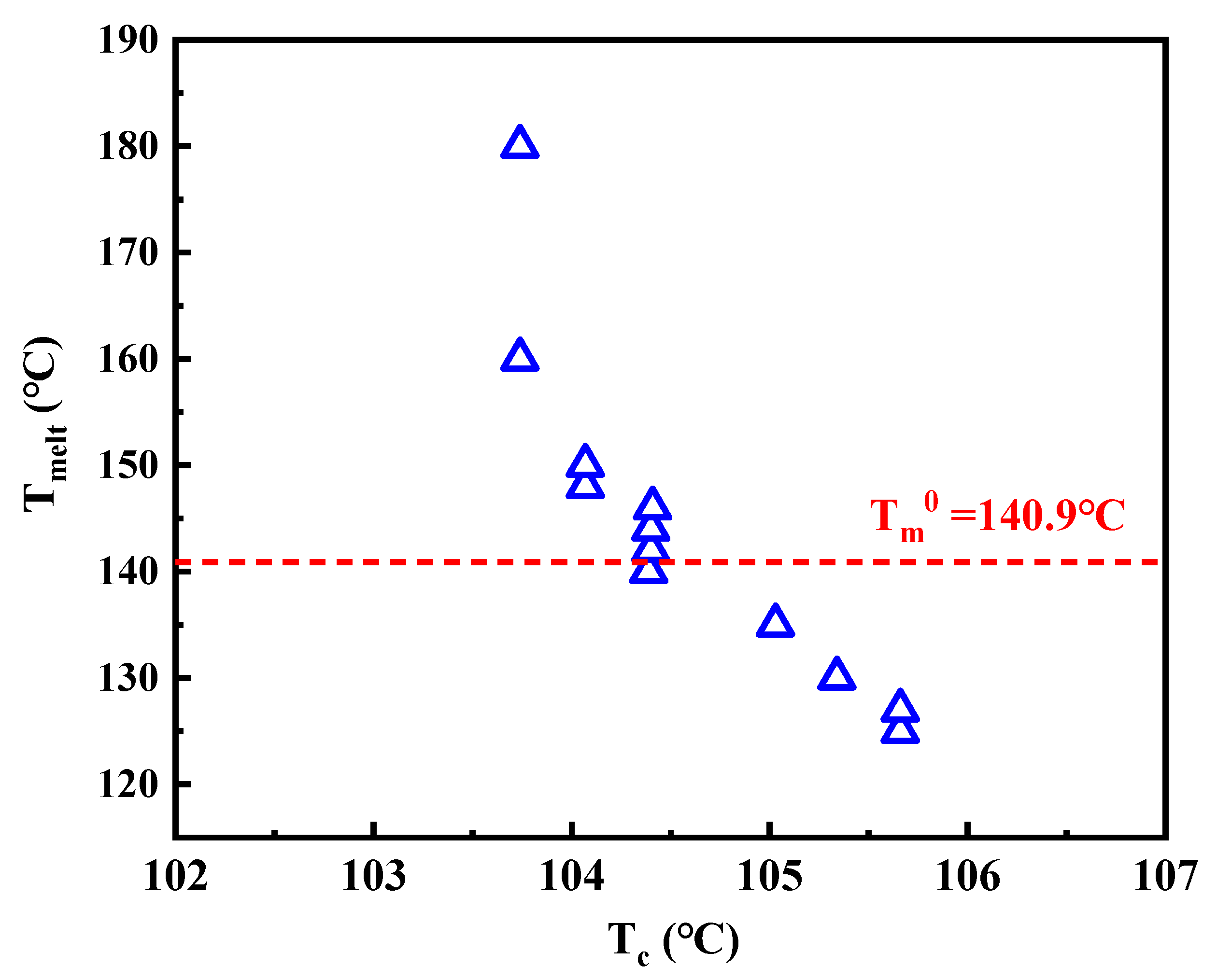
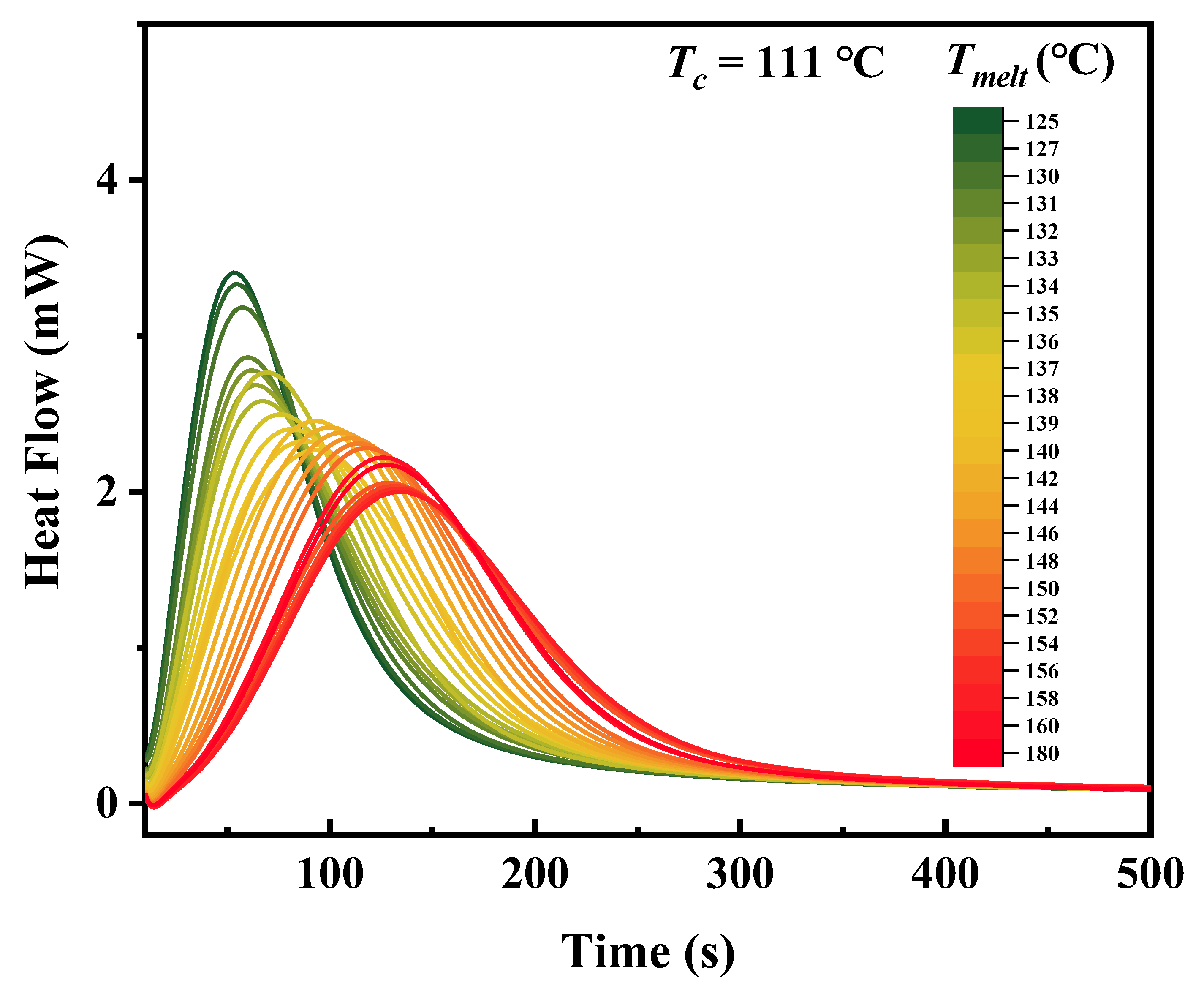
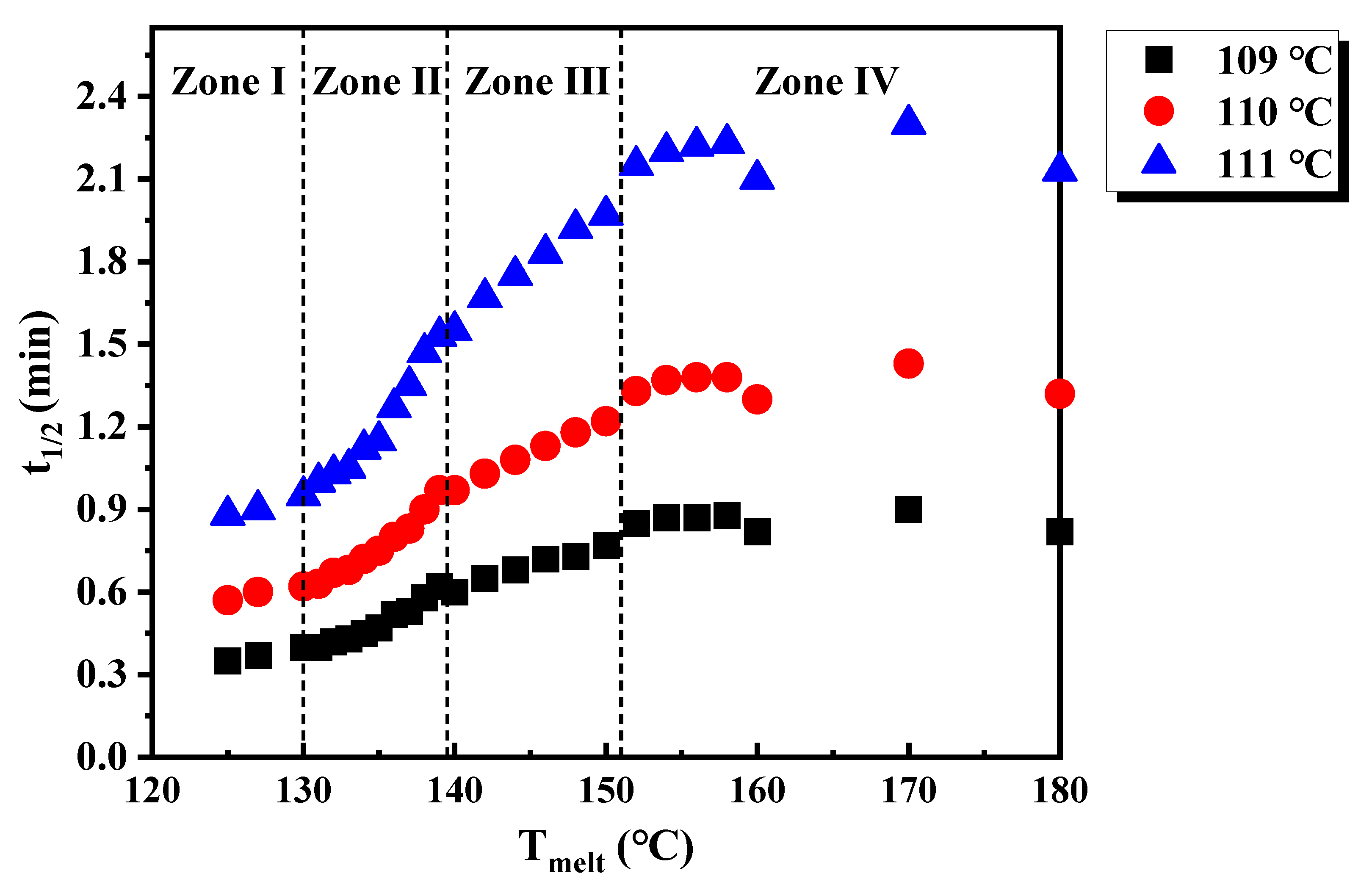
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, H.; Vadlamudi, M.; Alamo, R.G.; Hu, W. Monte Carlo Simulations of Strong Memory Effect of Crystallization in Random Copolymers. Macromolecules 2013, 46, 6498–6506. [Google Scholar] [CrossRef]
- Richardson, M.J.; Flory, P.J.; Jackson, J.B. Crystallization and melting of copolymers of polymethylene. Polymer 1963, 4, 221–236. [Google Scholar] [CrossRef]
- Alamo, R.G.; Mandelkern, L. Thermodynamic and structural properties of ethylene copolymers. Macromolecules 1989, 22, 1273–1277. [Google Scholar] [CrossRef]
- Mathot, V.B.F.; Pijpers, M.F.J. Molecular Structure, Melting Behavior, and crystallinity of VLDPE. J. Appl. Polym. Sci. 1990, 39, 979–994. [Google Scholar] [CrossRef]
- Alamo, R.G.; Mandelkern, L. Crystallization kinetics of random ethylene copolymers. Macromolecules 1991, 24, 6480–6493. [Google Scholar] [CrossRef]
- Alamo, R.G.; Chan, E.K.M.; Mandelkern, L.; Voigt-Martin, I.G. Influence of molecular weight on the melting and phase structure of random copolymers of ethylene. Macromolecules 1992, 25, 6381–6394. [Google Scholar] [CrossRef]
- Alamo, R.G.; Mandelkern, L. The crystallization behavior of random copolymers of ethylene. Thermochim. Acta 1994, 238, 155–201. [Google Scholar] [CrossRef]
- Lu, L.; Alamo, R.G.; Mandelkern, L. Lamellar Thickness Distribution in Linear Polyethylene and Ethylene Co-polymers. Macromolecules 1994, 27, 6571–6576. [Google Scholar] [CrossRef]
- Hauser, G.; Schmidtke, J.; Strobl, G. The Role of Co-Units in Polymer Crystallization and Melting: New Insights from Studies on Syndiotactic Poly(propene-co-octene). Macromolecules 1998, 31, 6250–6258. [Google Scholar] [CrossRef]
- Crist, B.; Howard, P.R. Crystallization and Melting of Model Ethylene−Butene Copolymers. Macromolecules 1999, 32, 3057–3067. [Google Scholar] [CrossRef]
- Häfele, A.; Heck, B.; Hippler, T.; Kawai, T.; Kohn, P.; Strobl, G. Crystallization of poly(ethylene-co-octene): II Melt memory effects on first order kinetics. Eur. Phys. J. E 2005, 16, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, J.I.; Hoffman, J.D. Formation of Polymer Crystals with Folded Chains from Dilute Solution. J. Chem. Phys. 1959, 31, 1680–1681. [Google Scholar] [CrossRef]
- Mandelkern, L.; Fatou, J.G.; Howard, C. The Nucleation of Long-Chain Molecules. J. Pshysical. Chem. 1965, 69, 956–959. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetic of crystallization from the melt and chain folding in polyethylene fractions revisited: Theory and experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Haigh, J.; Nguyen, C.; Alamo, R.; Mandelkern, L. Crystallization and melting of model polyethylenes with different chain structures. J. Therm. Anal. Calorim. 2000, 59, 435–450. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B. Analysis of the Degree of Reversibility of Crystallization and Melting in Poly(ethylene-co-1-octene). Macromolecules 2000, 33, 9076–9089. [Google Scholar] [CrossRef]
- Alamo, R.G. The role of defect microstructure in the crystallization behavior of metallocene and MgCl2-supported Ziegler-Natta isotactic poly(propylenes). Polímeros 2003, 13, 270–275. [Google Scholar] [CrossRef]
- Alamo, R.G.; Blanco, J.A.; Agarwal, P.K.; Randall, J.C. Crystallization Rates of Matched Fractions of MgCl2-Supported Ziegler Natta and Metallocene Isotactic Poly(Propylene)s. 1. The Role of Chain Microstructure. Macromolecules 2003, 36, 1559–1571. [Google Scholar] [CrossRef]
- Hu, W.; Mathot, V.B.F.; Frenkel, D. Phase Transitions of Bulk Statistical Copolymers Studied by Dynamic Monte Carlo Simulations. Macromolecules 2003, 36, 2165–2175. [Google Scholar] [CrossRef]
- Randall, J.C.; Alamo, R.G.; Agarwal, P.K.; Ruff, C.J. Crystallization Rates of Matched Fractions of MgCl2-Supported Ziegler-Natta and Metallocene Isotactic Poly(propylene)s. 2. Chain Microstructures from a Supercritical Fluid Fractionation of a MgCl2-Supported Ziegler−Natta Isotactic Poly(propylene). Macromolecules 2003, 36, 1572–1584. [Google Scholar] [CrossRef]
- Razavi-Nouri, M. Studies of comonomer distributions and molecular segregations in metallocene-prepared poly-ethylenes by DSC. Polym. Test. 2006, 25, 1052–1058. [Google Scholar] [CrossRef]
- Strobl, G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Reid, B.O.; Vadlamudi, M.; Mamun, A.; Janani, H.; Gao, H.; Hu, W.; Alamo, R.G. Strong memory effect of crystallization above the equilibrium melting point of random copolymers. Macromolecules 2013, 46, 6485–6497. [Google Scholar] [CrossRef]
- Mamun, A.; Chen, X.; Alamo, R.G. Interplay between a Strong Memory Effect of Crystallization and Liquid–Liquid Phase Separation in Melts of Broadly Distributed Ethylene–1-Alkene Copolymers. Macromolecules 2014, 47, 7958–7970. [Google Scholar] [CrossRef]
- Liu, P.; Xue, Y.; Men, Y. Melt Memory Effect beyond the Equilibrium Melting Point in Commercial Isotactic Polybutene-1. Ind. Eng. Chem. Res. 2019, 58, 5472–5478. [Google Scholar] [CrossRef]
- Sangroniz, L.; Cavallo, D.; Müller, A.J. Self-Nucleation Effects on Polymer Crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Zhao, X.; Han, S.; Song, W.; Liu, L.; Men, Y. Self-nucleation and heterogeneous nucleation in ethylene/1-octene random copolymer and its linear polyethylene blends. Thermochim. Acta 2022, 718, 179392. [Google Scholar] [CrossRef]
- Zhao, X.-T.; Men, Y.-F. Fractionation of Polyolefin Elastomer by a Modified SSA Technique. Chin. J. Polym. Sci. 2022, 40, 1252–1258. [Google Scholar] [CrossRef]
- Zhao, X.T.; Men, Y.F. Thermal Fractionation of Polyolefins: Brief History, New Developments and Future Perspective. Polym. Sci. Ser. A 2022, in press. [CrossRef]
- Chen, X.; Mamun, A.; Alamo, R.G. Effect of Level of Crystallinity on Melt Memory Above the Equilibrium Melting Temperature in a Random Ethylene 1-Butene Copolymer. Macromol. Chem. Phys. 2015, 216, 1220–1226. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhao, J.; Jiang, Z.; Men, Y. Direct Formation of Different Crystalline Forms in Butene-1/Ethylene Copolymer via Manipulating Melt Temperature. Macromolecules 2014, 47, 8653–8662. [Google Scholar] [CrossRef]



| Sample | Ethylene (mol%) | Octene (mol%) | Hexene (mol%) | Mw (g/mol) | Mn (g/mol) | Mw/Mn | Tm a (°C) | Φw b (%) |
|---|---|---|---|---|---|---|---|---|
| P(E-co-O-co-H) | 98.9 | 0.8 | 0.3 | 106,600 | 41,700 | 2.55 | 116.2 | 42.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, S.; Lu, Y.; Wang, J.; Men, Y. Melt Memory Effect in Polyethylene Random Terpolymer with Small Amount of 1-Octene and 1-Hexene Co-Units: Non-Isothermal and Isothermal Investigations. Polymers 2023, 15, 1721. https://doi.org/10.3390/polym15071721
Wang D, Li S, Lu Y, Wang J, Men Y. Melt Memory Effect in Polyethylene Random Terpolymer with Small Amount of 1-Octene and 1-Hexene Co-Units: Non-Isothermal and Isothermal Investigations. Polymers. 2023; 15(7):1721. https://doi.org/10.3390/polym15071721
Chicago/Turabian StyleWang, Dengfei, Shiyan Li, Ying Lu, Jian Wang, and Yongfeng Men. 2023. "Melt Memory Effect in Polyethylene Random Terpolymer with Small Amount of 1-Octene and 1-Hexene Co-Units: Non-Isothermal and Isothermal Investigations" Polymers 15, no. 7: 1721. https://doi.org/10.3390/polym15071721
APA StyleWang, D., Li, S., Lu, Y., Wang, J., & Men, Y. (2023). Melt Memory Effect in Polyethylene Random Terpolymer with Small Amount of 1-Octene and 1-Hexene Co-Units: Non-Isothermal and Isothermal Investigations. Polymers, 15(7), 1721. https://doi.org/10.3390/polym15071721





