Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Functionalization
2.2. Animal Experimentation
2.3. Surgical Procedure
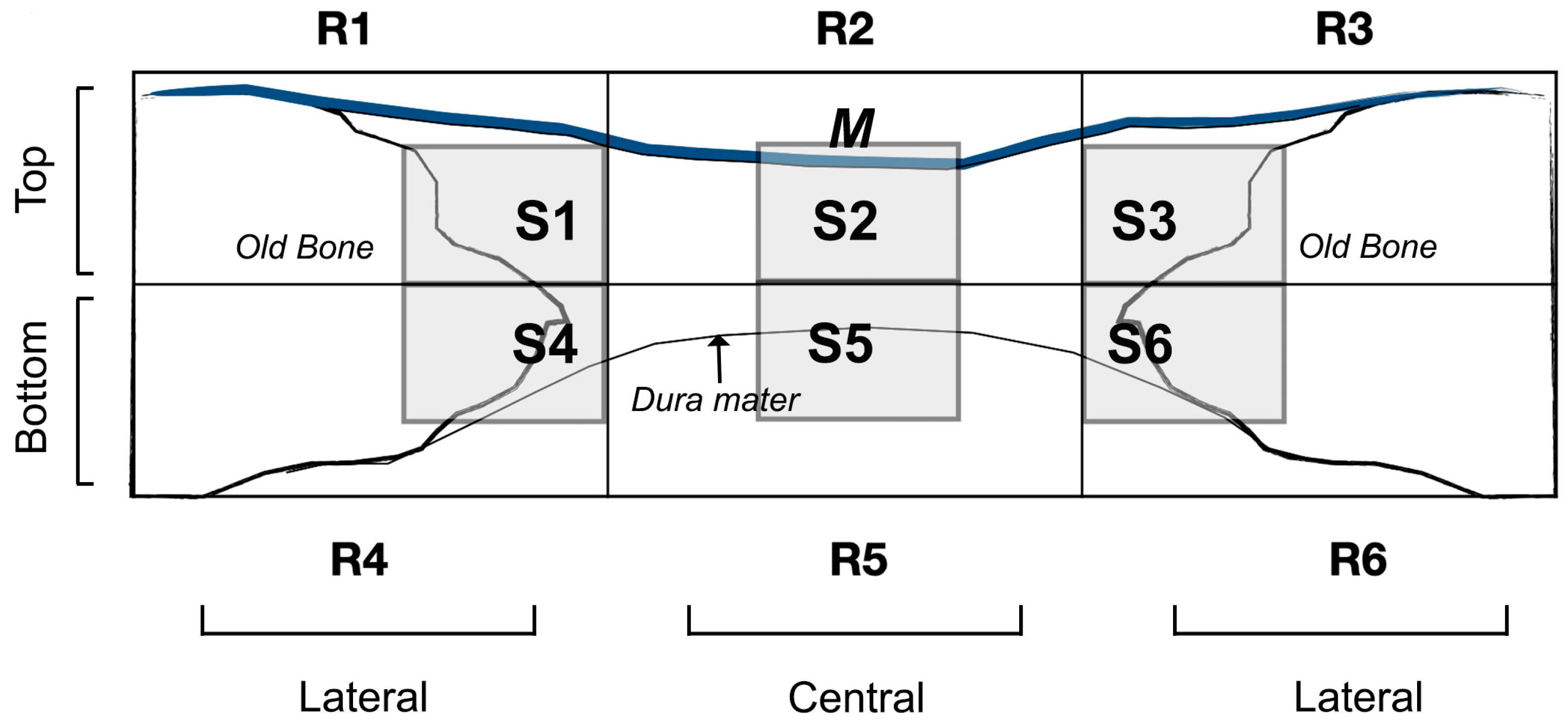
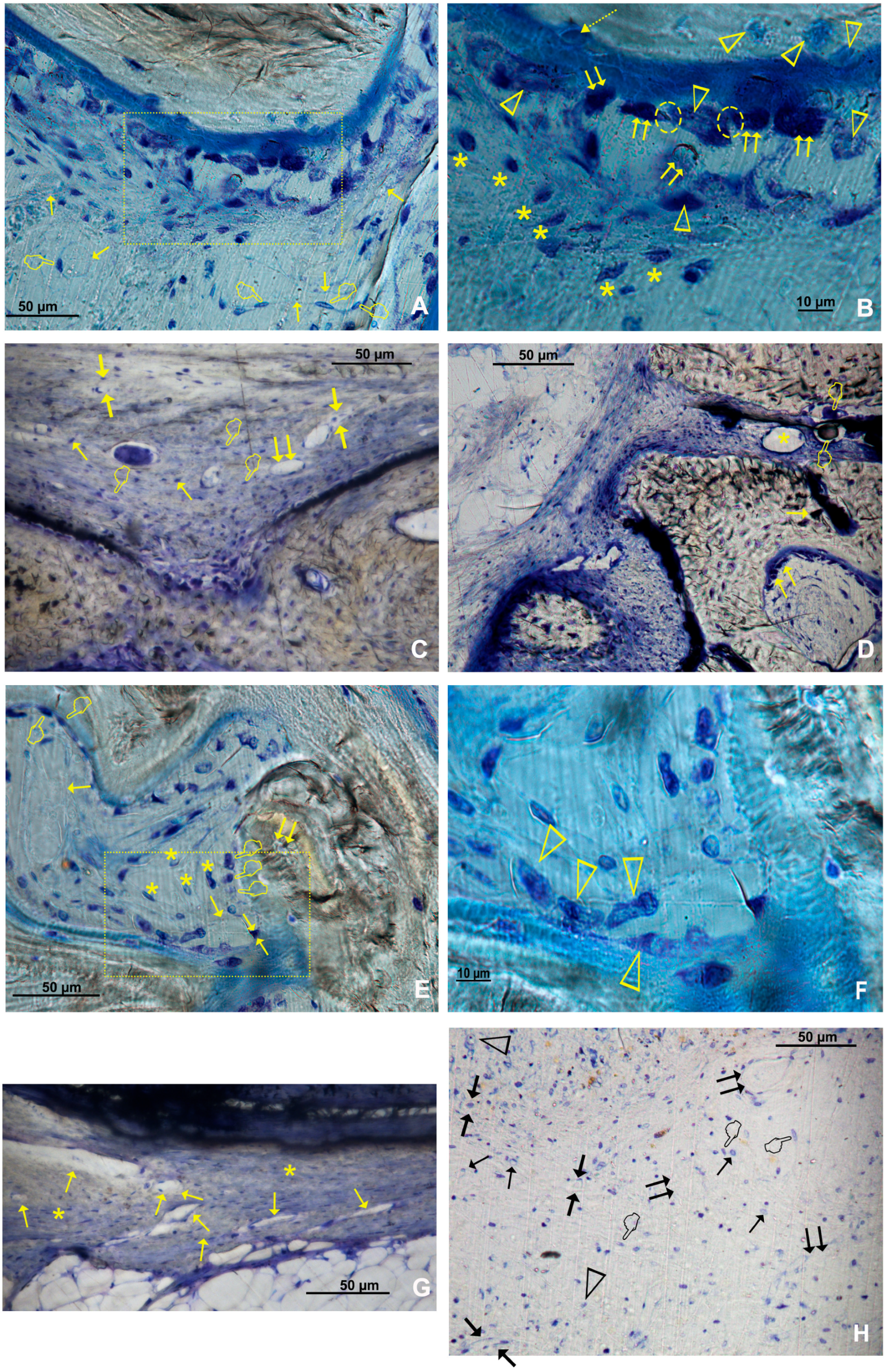
2.4. Histology
2.5. Statistical Analysis
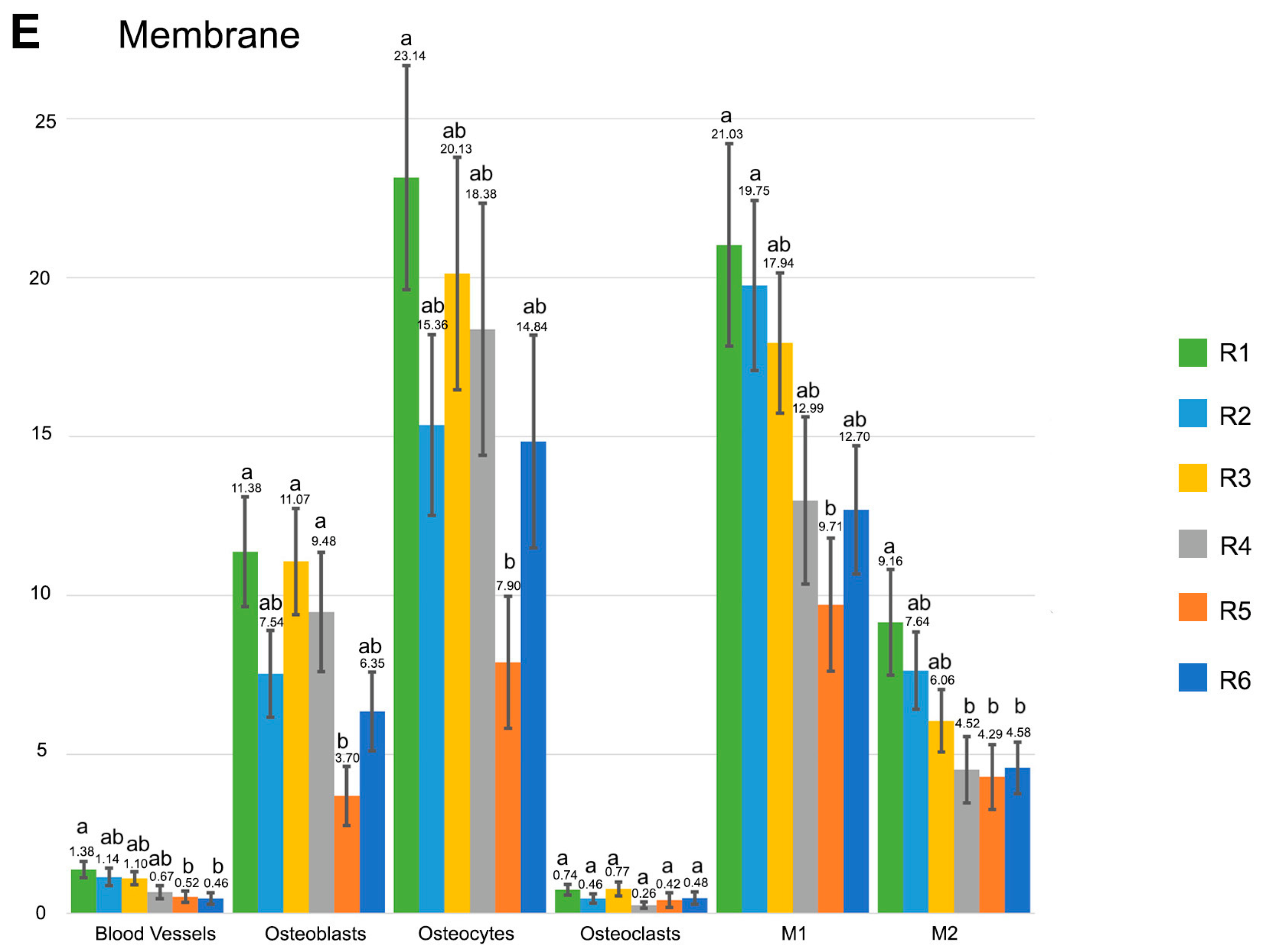
3. Results
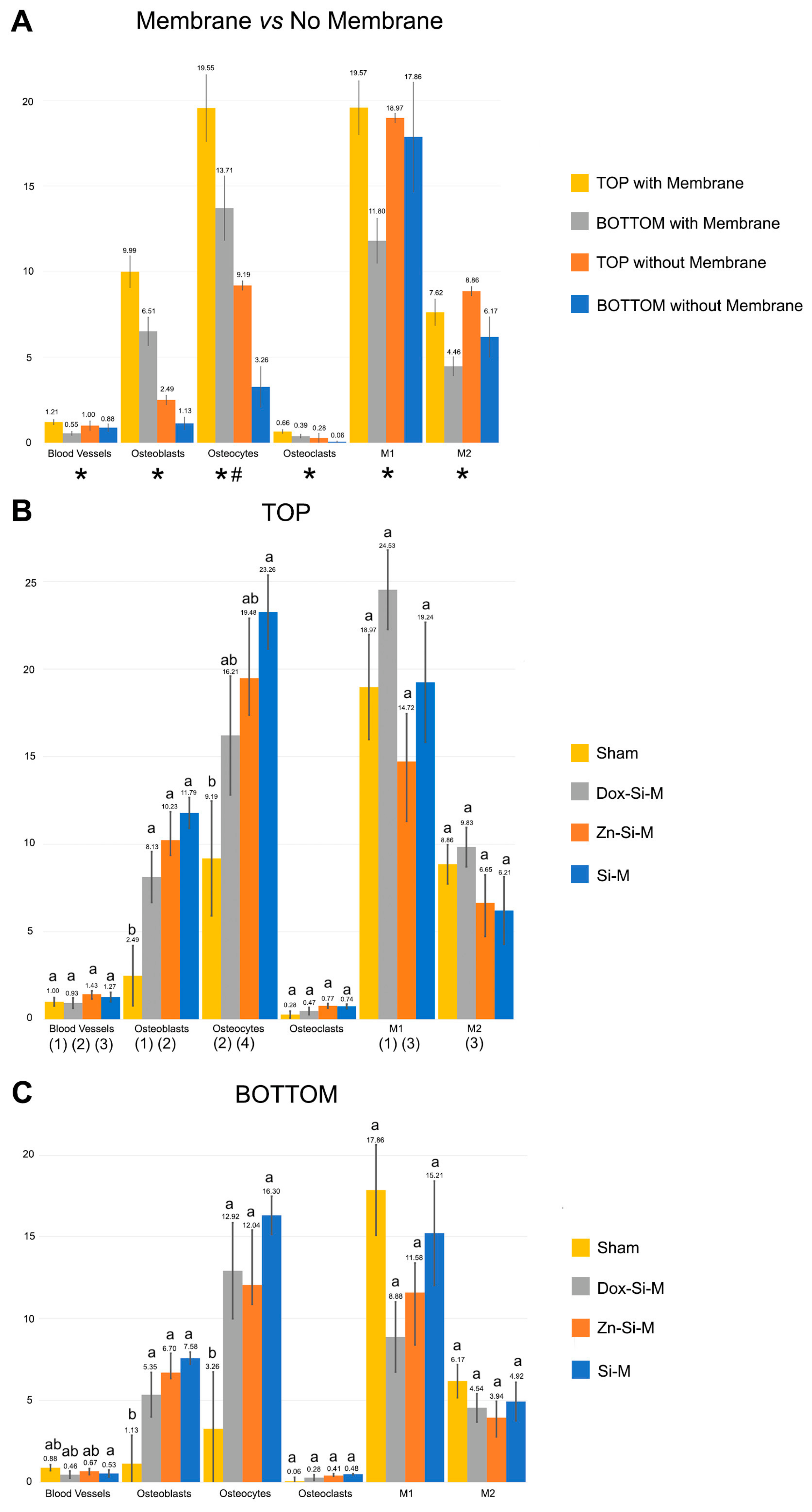
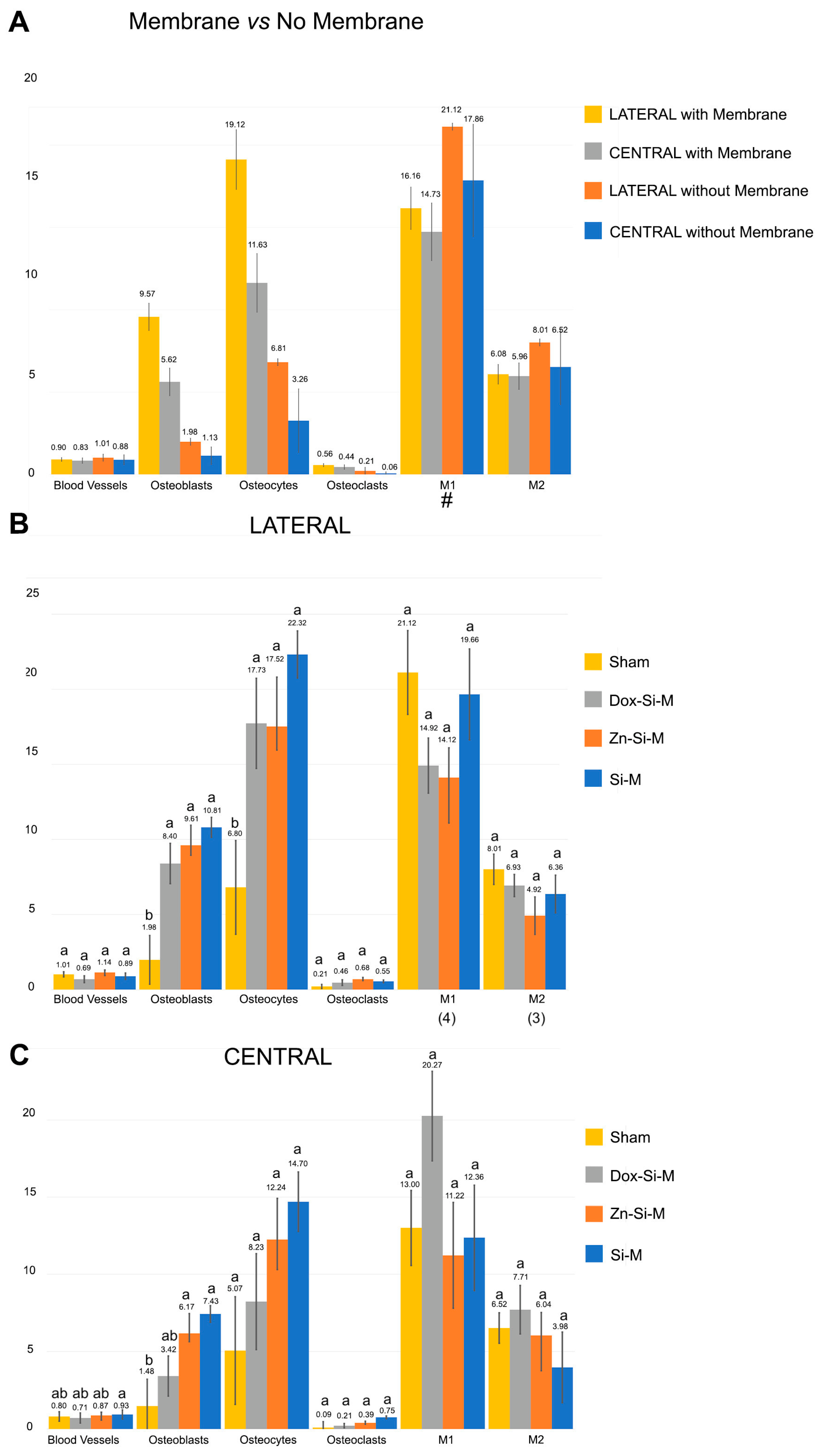
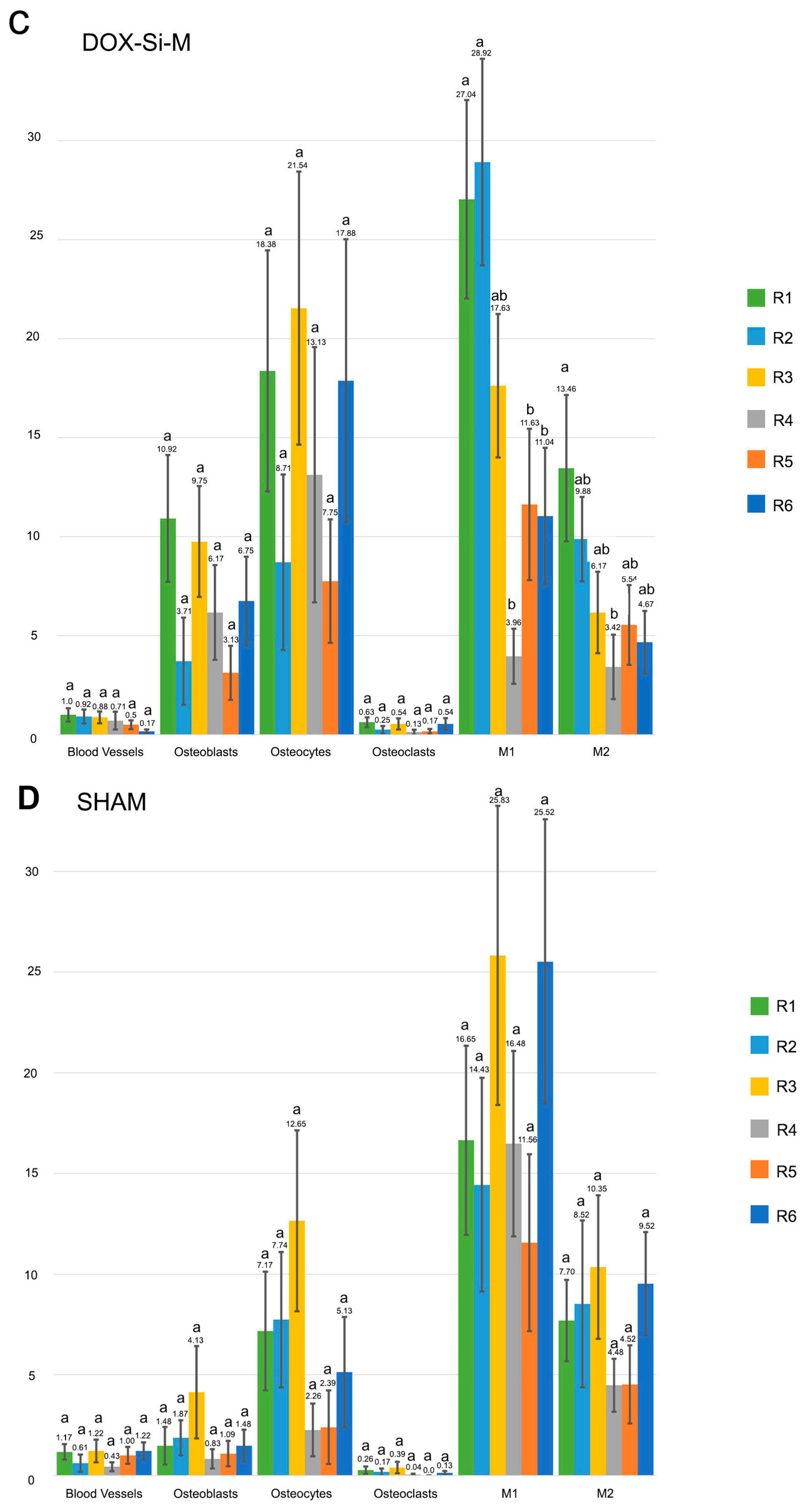
3.1. Blood Vessel Assessment
3.2. Osteoblast Assessment
3.3. Osteoclast Assessment
3.4. Osteocytes
3.5. M1, M2, M1/M2 Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| AFM | Atomic Force Microscopy (AFM) |
| BMP | Bone Morphogenetic Protein |
| CM | Collagen Membrane |
| Dox-Si-M | SiO2-NPs doped membrane functionalized with Dox |
| Dox | Doxycycline |
| FESEM | Field Emission Scanning Electron Microscopy |
| GBR | Guided Bone Regeneration |
| HCl | Hydrochloric Acid |
| HEA | Hydroxyethyl acrylate |
| HEMA | Hydroxyethyl methacrylate |
| IL-1 | Interleukin 1 |
| IL-10 | Interleukin 10 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| M1 | Macrophage phenotype 1 |
| M2 | Macrophage phenotype 2 |
| MA | Methyl acrylate |
| MMA | Methyl methacrylate |
| MMP | Matrix Metalloprotease |
| pHEMA-co-MAA | poly (2-hydroxyethyl methacrylate-comethacrylic acid) |
| PTFE | Polytetrafluoroethylene |
| SD | Standard deviation |
| Si-M | SiO2-NPs doped membrane |
| SiO2-NPs | silicon oxide nanoparticles |
| TB | Toluidine Blue |
| TNF-α | Tumor Necrosis Factor-α |
| VEGF | Vascular Endothelial Grow Factor |
| Zn-Si-M | SiO2-NPs doped membrane functionalized with Zn |
| Zn | Zinc |
References
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Ruiz, C.; Toledano, M.; Osorio, R. Testing Active Membranes for Bone Regeneration: A Review. J. Dent. 2021, 105, 103580. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and Release Kinetics of Growth Factors on Barrier Membranes for Guided Tissue/Bone Regeneration: A Systematic Review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Quirino, L.C.; de Azambuja Carvalho, P.H.; Neto, R.T.A.; Comachio, C.A.; Monteiro, N.G.; Ervolino-Silva, A.C.; Okamoto, R.; Pereira-Filho, V.A. Polydioxanone Membrane Compared with Collagen Membrane for Bone Regeneration. Polymers 2023, 15, 868. [Google Scholar] [CrossRef] [PubMed]
- de Souza Balbinot, G.; Mendes Nobre do Espírito Santo, C.; Leitune, V.C.B.; Visioli, F.; Duarte Soares, R.M.; Sauro, S.; Collares, F.M. Antibacterial Effect of Triazine in Barrier Membranes with Therapeutic Activity for Guided Bone Regeneration. Polymers 2022, 14, 4482. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, M.; Osorio, R. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.-L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Pre-Prosthetic Use of Poly (Lactic-Co-Glycolic Acid) Membranes Treated with Oxygen Plasma and TiO2 Nanocomposite Particles for Guided Bone Regeneration Processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Luo, J.; Liu, X.; Yang, Q.; Shi, X.; Wang, Y. Black Phosphorus Nanosheets-Enabled DNA Hydrogel Integrating 3D-Printed Scaffold for Promoting Vascularized Bone Regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef]
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. [Google Scholar] [CrossRef]
- Yu, H.; VandeVord, P.J.; Mao, L.; Matthew, H.W.; Wooley, P.H.; Yang, S.-Y. Improved Tissue-Engineered Bone Regeneration by Endothelial Cell Mediated Vascularization. Biomaterials 2009, 30, 508–517. [Google Scholar] [CrossRef]
- Schilling, K.; Zhai, Y.; Zhou, Z.; Zhou, B.; Brown, E.; Zhang, X. High-Resolution Imaging of the Osteogenic and Angiogenic Interface at the Site of Murine Cranial Bone Defect Repair via Multiphoton Microscopy. Elife 2022, 11, e83146. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus Immune System. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Y.; Shen, J.; Chen, Z.; Xie, Z. Extracellular Vesicles in Bone Homeostasis: Emerging Mediators of Osteoimmune Interactions and Promising Therapeutic Targets. Int. J. Biol. Sci. 2022, 18, 4088–4100. [Google Scholar] [CrossRef]
- Vig, S.; Fernandes, M.H. Bone Cell Exosomes and Emerging Strategies in Bone Engineering. Biomedicines 2022, 10, 767. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Shah, R.; Mahajan, A.; Shah, N.; Dadhania, A.P. Preemptive Analgesia in Third Molar Impaction Surgery. Natl. J. Maxillofac. Surg. 2012, 3, 144–147. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key Players around Bone Biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]
- Morón-Calvente, V.; Romero-Pinedo, S.; Toribio-Castelló, S.; Plaza-Díaz, J.; Abadía-Molina, A.C.; Rojas-Barros, D.I.; Beug, S.T.; LaCasse, E.C.; MacKenzie, A.; Korneluk, R.; et al. Inhibitor of Apoptosis Proteins, NAIP, CIAP1 and CIAP2 Expression during Macrophage Differentiation and M1/M2 Polarization. PLoS ONE 2018, 13, e0193643. [Google Scholar] [CrossRef]
- He, Y.; Luo, J.; Zhang, Y.; Li, Z.; Chen, F.; Song, W.; Zhang, Y. The Unique Regulation of Implant Surface Nanostructure on Macrophages M1 Polarization. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110221. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of Macrophage Phenotype by Cell Shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchacher, T.; Ohradanova-Repic, A.; Stockinger, H.; Fischer, M.B.; Weber, V. M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia Pneumoniae. PLoS ONE 2015, 10, e0143593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punet, X.; Mauchauffé, R.; Rodríguez-Cabello, J.C.; Alonso, M.; Engel, E.; Mateos-Timoneda, M.A. Biomolecular Functionalization for Enhanced Cell-Material Interactions of Poly (Methyl Methacrylate) Surfaces. Regen. Biomater. 2015, 2, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, R.; Carrasco-Carmona, Á.; Toledano, M.; Osorio, E.; Medina-Castillo, A.L.; Iskandar, L.; Marques, A.; Deb, S.; Toledano-Osorio, M. Ex Vivo Investigations on Bioinspired Electrospun Membranes as Potential Biomaterials for Bone Regeneration. J. Dent. 2020, 98, 103359. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Carrasco-Carmona, Á.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Osorio, R. Protein Adsorption and Bioactivity of Functionalized Electrospun Membranes for Bone Regeneration. J. Dent. 2020, 102, 103473. [Google Scholar] [CrossRef]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Osorio, E.; Medina-Castillo, A.L.; Alaminos, M.; Toledano-Osorio, M.; Toledano, M. Novel Potential Scaffold for Periodontal Tissue Engineering. Clin. Oral Investig. 2017, 21, 2695–2707. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-Doped Membranes Induced Osteogenic Gene Expression on Osteoblastic Cells. J. Dent. 2021, 109, 103676. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C.; Osorio, R. Doxycycline-Doped Polymeric Membranes Induced Growth, Differentiation and Expression of Antigenic Phenotype Markers of Osteoblasts. Polymers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Toledano, M.; Gutierrez-Pérez, J.L.; Gutierrez-Corrales, A.; Serrera-Figallo, M.A.; Toledano-Osorio, M.; Rosales-Leal, J.I.; Aguilar, M.; Osorio, R.; Torres-Lagares, D. Novel Non-Resorbable Polymeric-Nanostructured Scaffolds for Guided Bone Regeneration. Clin. Oral Investig. 2020, 24, 2037–2049. [Google Scholar] [CrossRef]
- Ma, S.-F.; Chen, Y.-J.; Zhang, J.-X.; Shen, L.; Wang, R.; Zhou, J.-S.; Hu, J.-G.; Lü, H.-Z. Adoptive Transfer of M2 Macrophages Promotes Locomotor Recovery in Adult Rats after Spinal Cord Injury. Brain Behav. Immun. 2015, 45, 157–170. [Google Scholar] [CrossRef]
- Udagawa, A.; Sato, S.; Hasuike, A.; Kishida, M.; Arai, Y.; Ito, K. Micro-CT Observation of Angiogenesis in Bone Regeneration. Clin. Oral Implant. Res. 2013, 24, 787–792. [Google Scholar] [CrossRef]
- Sam, G.; Pillai, B.R.M. Evolution of Barrier Membranes in Periodontal Regeneration—“Are the Third Generation Membranes Really Here?”. J. Clin. Diagn. Res. 2014, 8, ZE14–ZE17. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Vallecillo, C.; Vallecillo-Rivas, M.; Manzano-Moreno, F.-J.; Osorio, R. Antibiotic-Loaded Polymeric Barrier Membranes for Guided Bone/Tissue Regeneration: A Mini-Review. Polymers 2022, 14, 840. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Toledano, M.; Manzano-Moreno, F.J.; Vallecillo, C.; Vallecillo-Rivas, M.; Rodriguez-Archilla, A.; Osorio, R. Alveolar Bone Ridge Augmentation Using Polymeric Membranes: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 1172. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [Green Version]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Using Resorbable Membrane and Different Bone Substitutes: Early Histological and Molecular Events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef]
- Maggiano, I.S.; Maggiano, C.M.; Clement, J.G.; Thomas, C.D.L.; Carter, Y.; Cooper, D.M.L. Three-Dimensional Reconstruction of Haversian Systems in Human Cortical Bone Using Synchrotron Radiation-Based Micro-CT: Morphology and Quantification of Branching and Transverse Connections across Age. J. Anat. 2016, 228, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Roger, P.-M. From Crosstalk between Immune and Bone Cells to Bone Erosion in Infection. Int. J. Mol. Sci. 2019, 20, 5154. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Vallecillo, C.; Gutierrez-Corrales, A.; Torres-Lagares, D.; Toledano-Osorio, M.; Serrera-Figallo, M.-A. Histomorphometric Analysis of Differential Regional Bone Regeneration Induced by Distinct Doped Membranes. Polymers 2022, 14, 2078. [Google Scholar] [CrossRef]
- Schaller, B.; Fujioka-Kobayashi, M.; Zihlmann, C.; Schuler, V.C.; Katagiri, H.; Lang, N.P.; Saulacic, N. Effects of Additional Collagen in Biphasic Calcium Phosphates: A Study in a Rabbit Calvaria. Clin. Oral Investig. 2020, 24, 3093–3103. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual Functional Approaches for Osteogenesis Coupled Angiogenesis in Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109761. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc Reduces Collagen Degradation in Demineralized Human Dentin Explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Bhattacharya, H.S.; Srikanth, G.; Singh, A. Comparative Evaluation of Decalcified Freeze Dried Bone Allograft with and without Local Doxycycline in Non-Contained Human Periodontal Infrabony Defects. J. Indian Soc. Periodontol. 2013, 17, 490–494. [Google Scholar] [CrossRef]
- Kameo, Y.; Ozasa, M.; Adachi, T. Computational Framework for Analyzing Flow-Induced Strain on Osteocyte as Modulated by Microenvironment. J. Mech. Behav. Biomed. Mater. 2022, 126, 105027. [Google Scholar] [CrossRef]
- Hong, I.; Khalid, A.W.; Pae, H.-C.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Jung, U.-W.; Choi, S.-H. Distinctive Bone Regeneration of Calvarial Defects Using Biphasic Calcium Phosphate Supplemented Ultraviolet-Crosslinked Collagen Membrane. J. Periodontal Implant. Sci. 2020, 50, 14–27. [Google Scholar] [CrossRef]
- Toledano, M.; Vallecillo-Rivas, M.; Osorio, M.T.; Muñoz-Soto, E.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Lynch, C.D.; Serrera-Figallo, M.-A.; Osorio, R. Zn-Containing Membranes for Guided Bone Regeneration in Dentistry. Polymers 2021, 13, 1797. [Google Scholar] [CrossRef]
- Zheng, J.; Li, B.; Yan, Y.; Huang, X.; Zhang, E. β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-ΚB Signaling. Int. J. Mol. Sci. 2022, 24, 444. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-Inflammatory M2, but Not pro-Inflammatory M1 Macrophages Promote Angiogenesis in Vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key Components of Engineering Vascularized 3-Dimensional Bioprinted Bone Constructs. Transl. Res. 2020, 216, 57–76. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Zhan, P.; Cui, Y.; Cao, Y.; Bao, X.; Wu, M.; Yang, Q.; Yang, J.; Zheng, H.; Zou, J.; Xie, T.; et al. PGE2 Promotes Macrophage Recruitment and Neovascularization in Murine Wet-Type AMD Models. Cell Commun. Signal. 2022, 20, 155. [Google Scholar] [CrossRef]
- Chen, X.; Wan, Z.; Yang, L.; Song, S.; Fu, Z.; Tang, K.; Chen, L.; Song, Y. Exosomes Derived from Reparative M2-like Macrophages Prevent Bone Loss in Murine Periodontitis Models via IL-10 MRNA. J. Nanobiotechnol. 2022, 20, 110. [Google Scholar] [CrossRef]
- Shortridge, C.; Akbari Fakhrabadi, E.; Wuescher, L.M.; Worth, R.G.; Liberatore, M.W.; Yildirim-Ayan, E. Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold. Int. J. Mol. Sci. 2021, 22, 1134. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, Á.; Vallecillo, C.; Toledano, R.; Medina-Castillo, A.L.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part II: Synthetic Polymers-Based Biomaterials. Polymers 2020, 12, 1845. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic Scaffolds as Functional Templates for Cardiac Tissue Engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Wuescher, L.M.; Worth, R.; Yildirim-Ayan, E. Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization. Ann. Biomed. Eng. 2019, 47, 2213–2231. [Google Scholar] [CrossRef]
- Galhano, G.Á.P.; Pellizzer, E.P.; Mazaro, J.V.Q. Optical Impression Systems for CAD-CAM Restorations. J. Craniofacial Surg. 2012, 23, e575–e579. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Ramakrishna, S. What Is Next for Electrospinning? Matter 2020, 2, 279–283. [Google Scholar] [CrossRef] [Green Version]

| A | |||
|---|---|---|---|
| M1 | M2 | M1/M2 | |
| Si-M | 17.23 ± 2.05 | 5.57 ± 0.75 | 4.38 ± 0.69 |
| Zn-Si-M | 13.15 ± 1.56 | 5.30 ± 0.71 | 3.67 ± 0.71 |
| Dox-Si-M | 16.70 ± 1.75 | 7.19 ± 0.96 | 4.07 ± 0.63 |
| Sham | 18.41 ± 2.33 | 7.51 ± 1.13 | 2.42 ± 0.28 |
| B | |||
| M1 | M2 | M1/M2 | |
| Si-M vs. Zn-Si-M | 0.08 | 0.45 | 0.63 |
| Si-M vs. Dox-Si-M | 0.78 | 0.54 | 0.71 |
| Si-M vs. Sham | 0.68 | 0.39 | 0.27 |
| Zn-Si-M vs. Dox-Si-M | 0.34 | 0.47 | 0.85 |
| Zn-Si-M vs. Sham | 0.31 | 0.10 | 0.37 |
| Dox-Si-M vs. Sham | 0.64 | 0.92 | 0.51 |
| C | |||
| M1/M2 TOP | M1/M2 BOTTOM | p-value pairwise | |
| Si-M | 5.12 ± 0.93 | 3.63 ± 1.02 | 0.69 |
| Zn-Si-M | 4.16 ± 1.24 | 3.17 ± 0.69 | 0.57 |
| Dox-Si-M | 6.08 ± 1.18 | 2.06 ± 0.31 | 0.00 |
| Sham | 2.23 ± 0.33 | 2.60 ± 0.45 | 0.55 |
| p-value ANOVA | 0.04 | 0.35 | 0.03 |
| D | |||
| M1/M2 LATERAL | M1/M2 CENTRAL | p-value pairwise | |
| Si-M | 3.94 ± 0.63 | 5.26 ± 1.64 | 0.51 |
| Zn-Si-M | 4.61 ± 1.04 | 1.79 ± 0.31 | 0.41 |
| Dox-Si-M | 4.15 ± 0.78 | 3.91 ± 1.09 | 0.33 |
| Sham | 2.78 ± 0.40 | 1.69 ± 0.23 | 0.05 |
| p-value ANOVA | 0.360 | 0.031 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano, M.; Vallecillo, C.; Serrera-Figallo, M.-A.; Vallecillo-Rivas, M.; Gutierrez-Corrales, A.; Lynch, C.D.; Toledano-Osorio, M. Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration. Polymers 2023, 15, 1726. https://doi.org/10.3390/polym15071726
Toledano M, Vallecillo C, Serrera-Figallo M-A, Vallecillo-Rivas M, Gutierrez-Corrales A, Lynch CD, Toledano-Osorio M. Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration. Polymers. 2023; 15(7):1726. https://doi.org/10.3390/polym15071726
Chicago/Turabian StyleToledano, Manuel, Cristina Vallecillo, María-Angeles Serrera-Figallo, Marta Vallecillo-Rivas, Aida Gutierrez-Corrales, Christopher D. Lynch, and Manuel Toledano-Osorio. 2023. "Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration" Polymers 15, no. 7: 1726. https://doi.org/10.3390/polym15071726
APA StyleToledano, M., Vallecillo, C., Serrera-Figallo, M. -A., Vallecillo-Rivas, M., Gutierrez-Corrales, A., Lynch, C. D., & Toledano-Osorio, M. (2023). Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration. Polymers, 15(7), 1726. https://doi.org/10.3390/polym15071726









