Fabricating Well-Dispersed Poly(Vinylidene Fluoride)/Expanded Graphite Composites with High Thermal Conductivity by Melt Mixing with Maleic Anhydride Directly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Composites
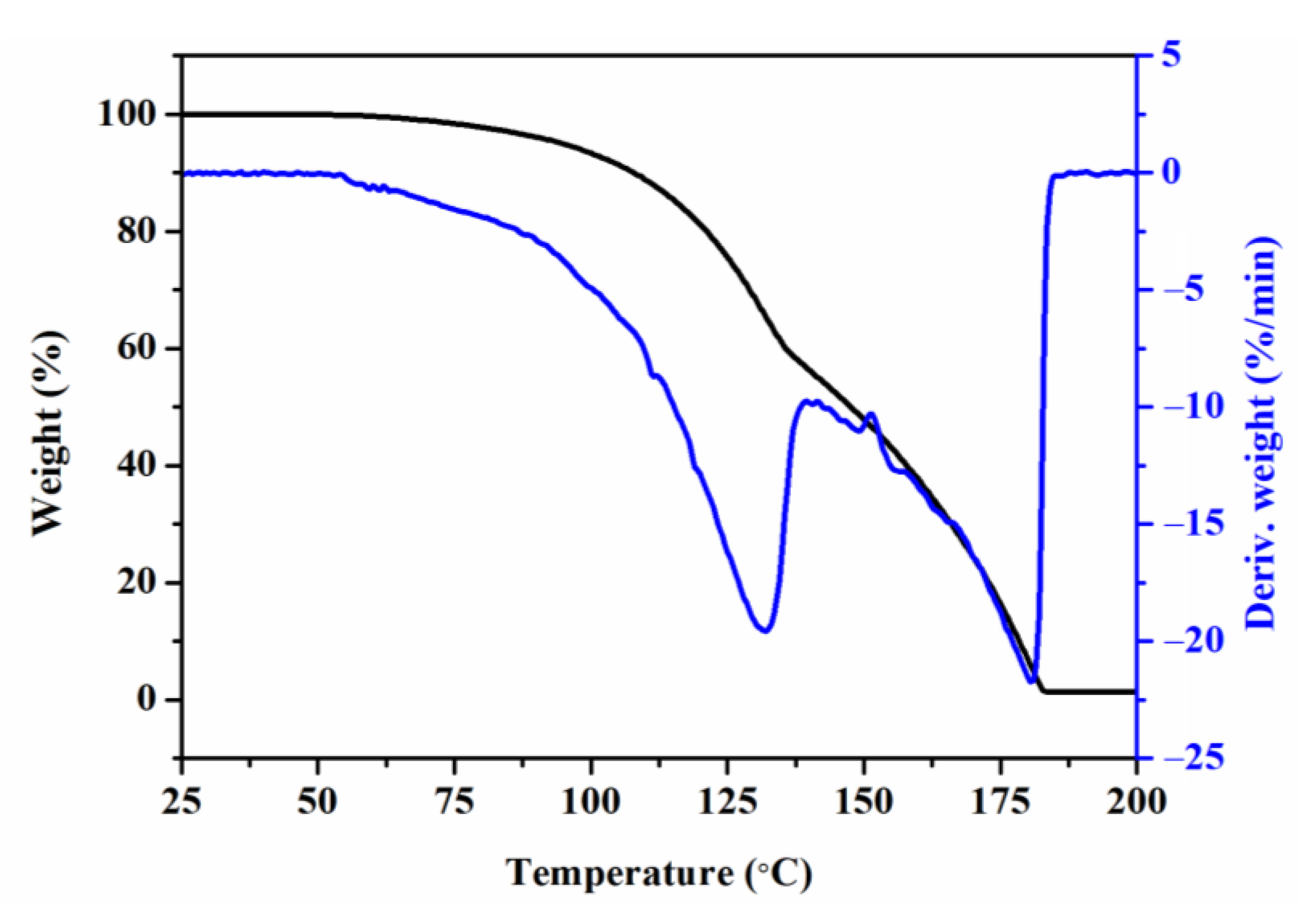
2.3. Characterization
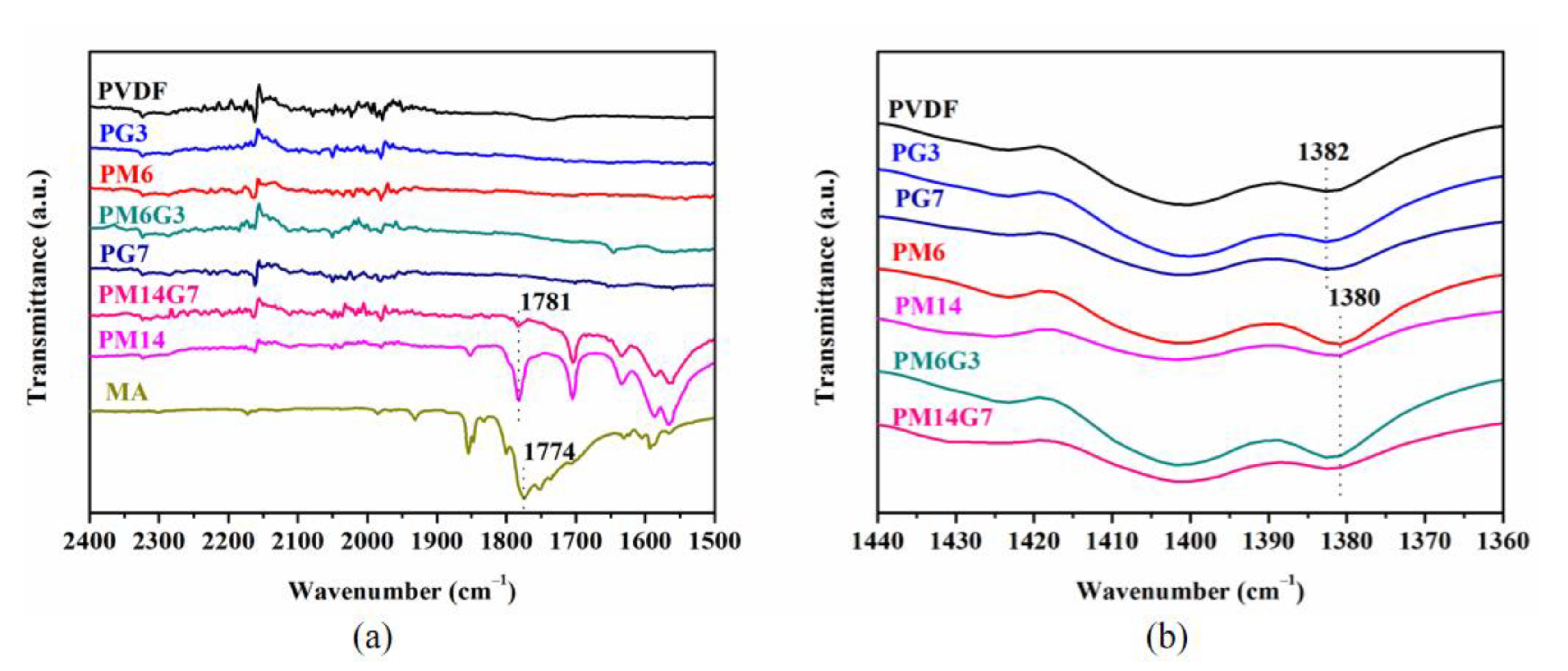
3. Results and Discussion
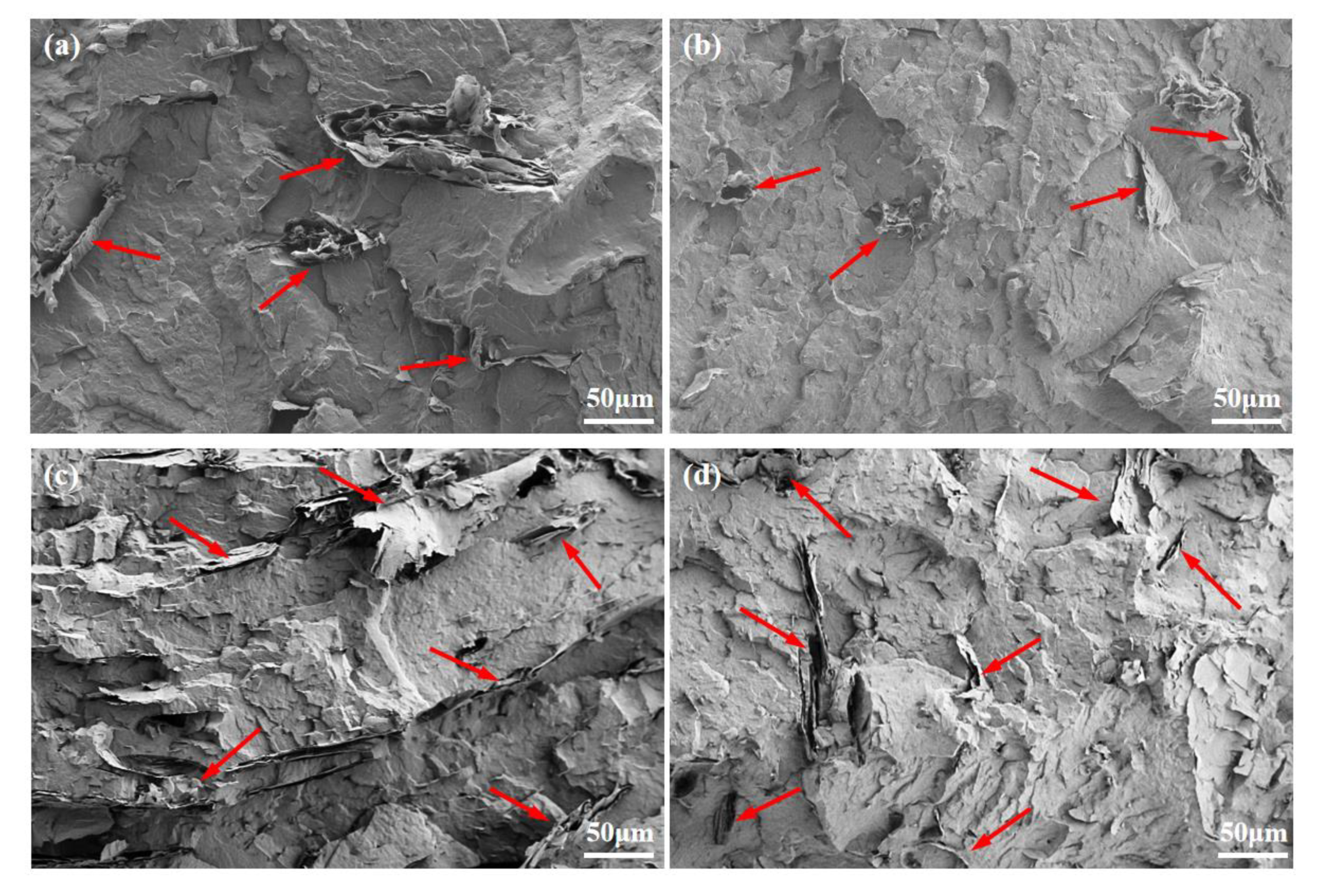
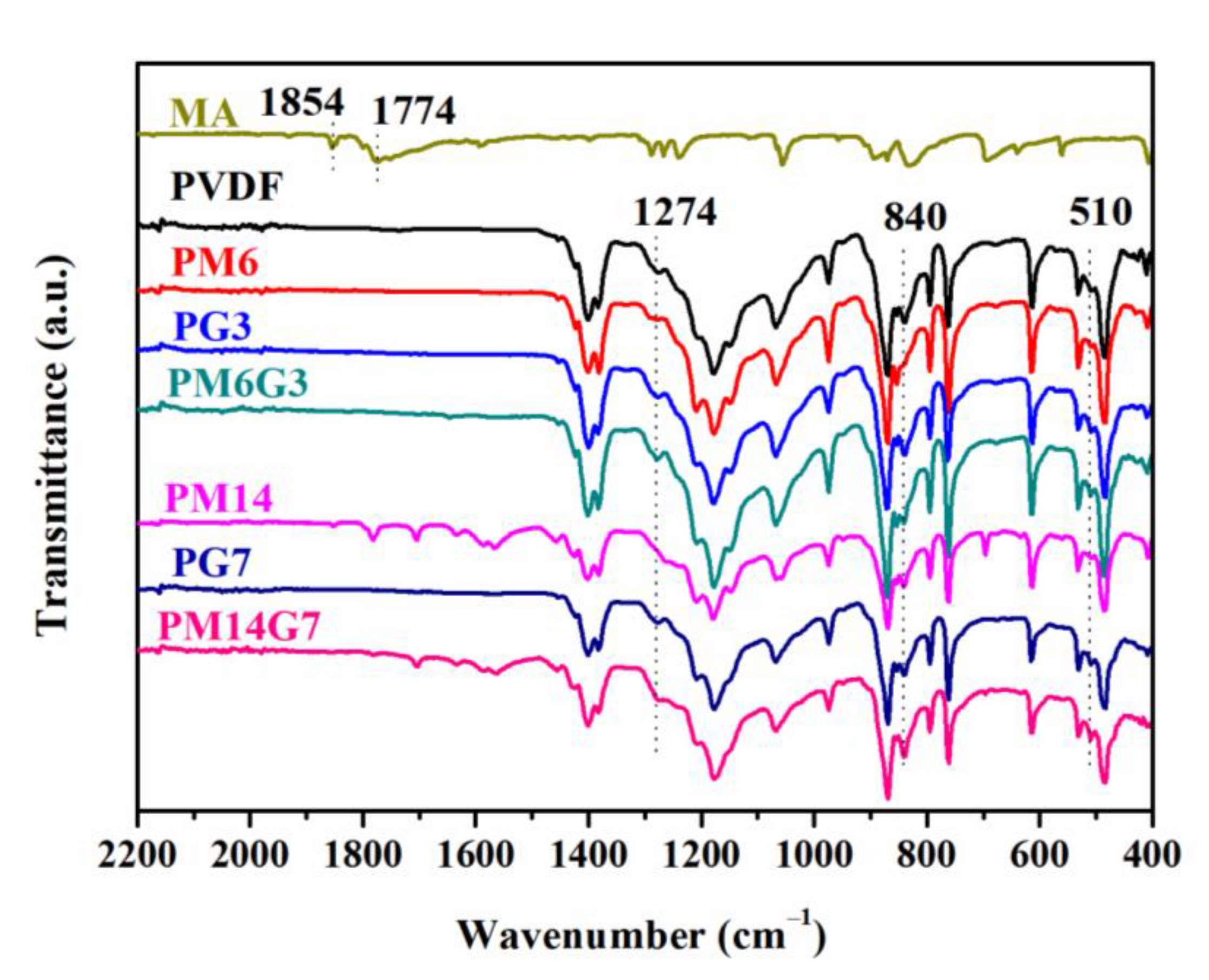
3.1. Dispersion State of EG
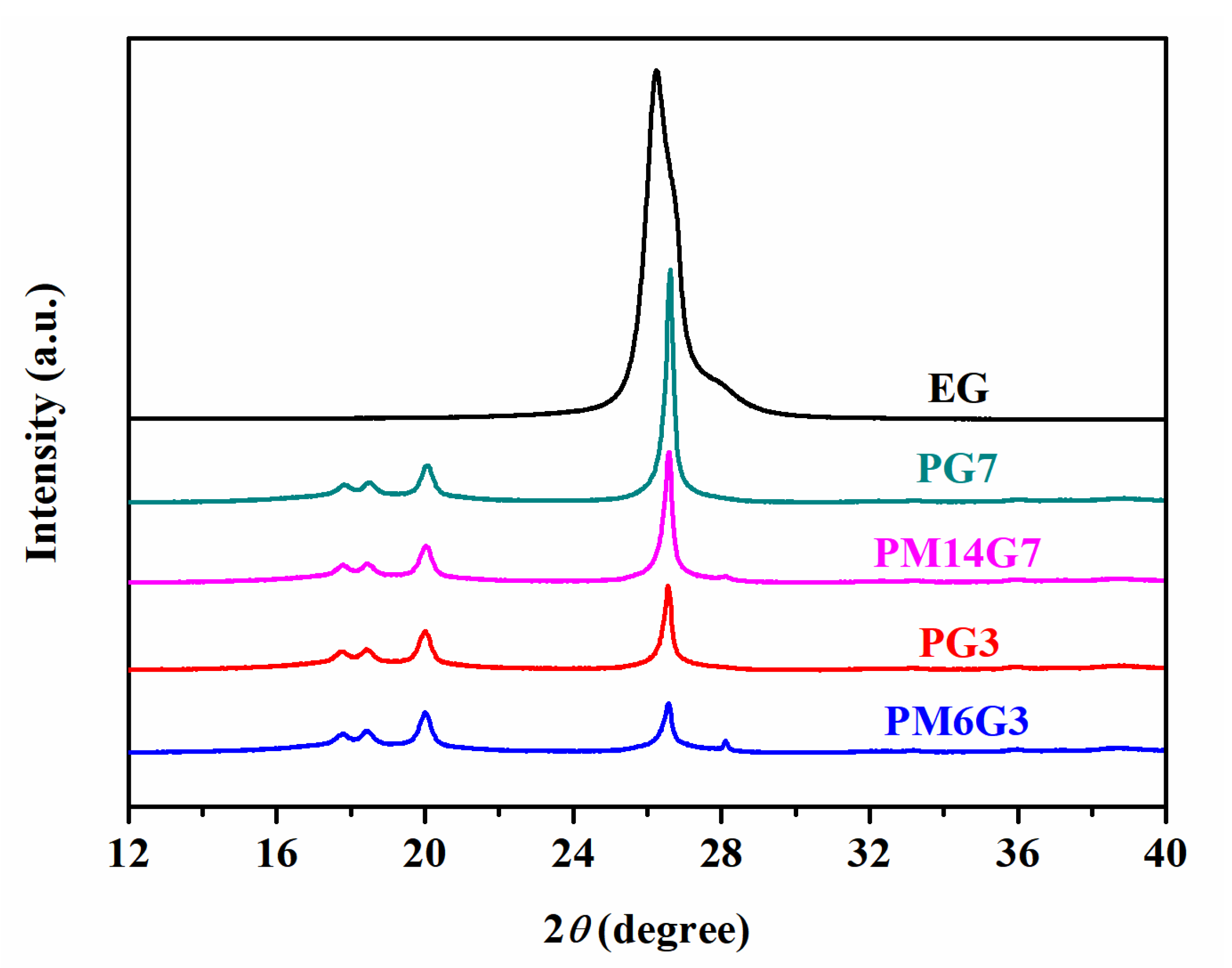
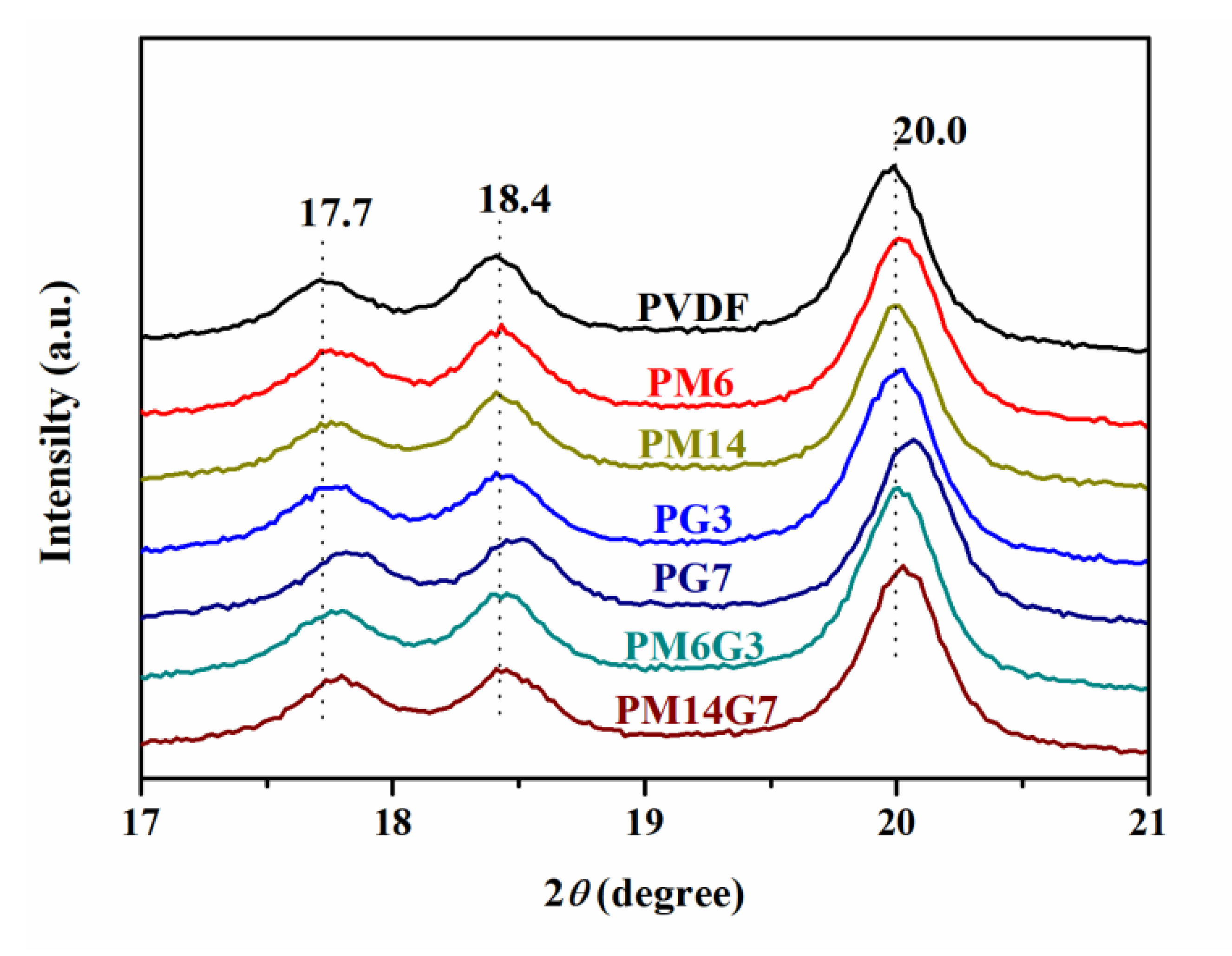
3.2. Crystallization Behavior and Crystal Structure
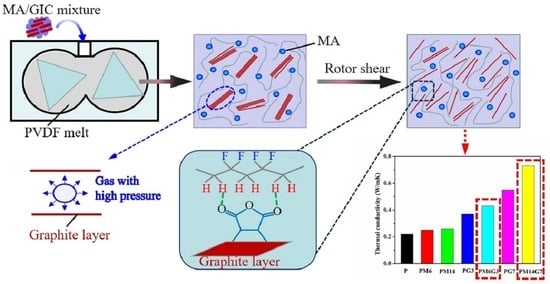
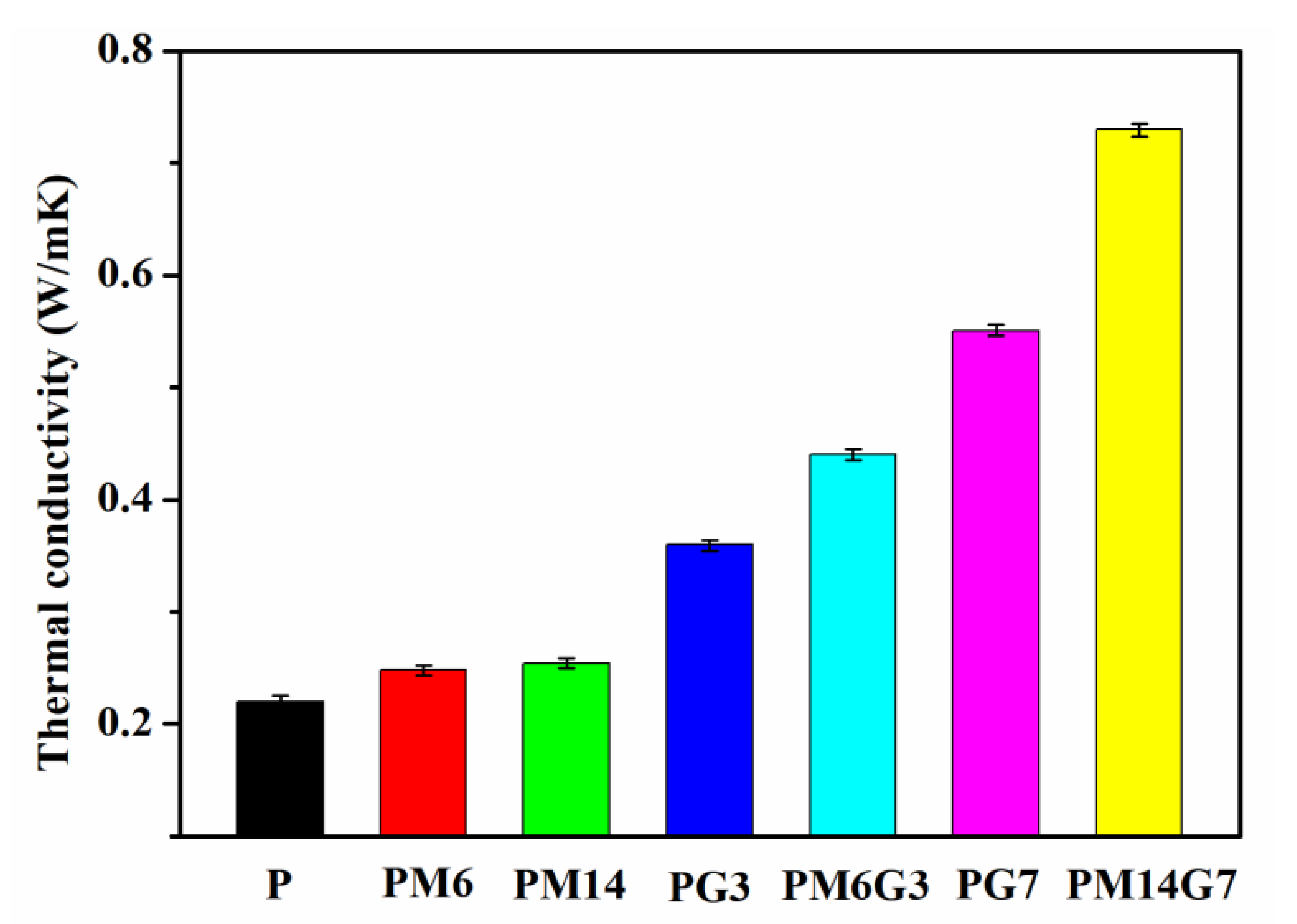
3.3. Thermal Conductivity
3.4. Mechanism for EG Dispersion during Melt Mixing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Wang, W.; Chen, X.; Lin, T.; Zhang, Y.; Yang, J.; Wang, Y.; Zhou, Z. Hybrid network structure and thermal conductive properties in poly(vinylidene fluoride) composites based on carbon nanotubes and graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2016, 90, 614–625. [Google Scholar] [CrossRef]
- Cho, E.C.; Huang, J.H.; Li, C.P.; Chang-Jian, C.W.; Lee, K.C.; Hsiao, Y.S.; Huang, J.H. Graphene based thermoplastic composites and their application for LED thermal management. Carbon 2016, 102, 66–73. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, C.; Li, Q.; Li, J.; Zhu, Y. A novel approach to align carbon nanotubes via water-assisted shear stretching. Compos. Sci. Technol. 2018, 164, 1–7. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. Prediction of electrical conductivity of carbon fiber-carbon nanotubereinforced polymer hybrid composites. Compos. Part B Eng. 2019, 167, 728–735. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.J.; Chen, X.Q.; Weng, G.J. A Monte Carlo model with equipotential approximation and tunneling resistance for the electrical conductivity of carbon nanotube polymer composites. Carbon 2019, 146, 125–138. [Google Scholar] [CrossRef]
- Zheng, W.G.; Wong, S.C.; Sue, H.J. Transport behavior of PMMA/expanded graphite nanocomposites. Polymer 2002, 43, 6767–6773. [Google Scholar] [CrossRef]
- Chen, G.H.; Wu, C.L.; Weng, W.G.; Wu, D.J.; Yan, W.L. Preparation of polystyrene/graphite nanosheet composite. Polymer 2003, 44, 1781–1784. [Google Scholar] [CrossRef]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. A green method to prepare nanosilica modified graphene oxide to inhibit nanoparticles re-aggregation during melt processing. Chem. Eng. J. 2017, 308, 1034–1047. [Google Scholar] [CrossRef] [Green Version]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kobayashi, S.; AbdurRahim, M.A.; Zhang, M.J.; Khusainova, A.; Hillmyer, M.A.; Abdala, A.A.; Macosko, C.W. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer 2011, 52, 1837–1846. [Google Scholar] [CrossRef]
- Soares, B.G. Ionic liquid: A smart approach for developing conducting polymer composites A review. J. Mol. Liq. 2018, 262, 8–18. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced thermal conductivity of epoxy/graphene composites by using non-oxidized graphene flakes with non-covalent functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Gui, H.; Wang, X.; Hu, Y.; Ding, Y. Improved dielectric properties of nanocomposites based on polyvinylidene fluoride and ionic liquid-functionalized graphene. Compos. Sci. Technol. 2015, 117, 282–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.J. Imidazolium-optimized conductive interfaces in multilayer graphene nanoplatelet/epoxy composites for thermal management applications and electroactive devices. Polymer 2019, 168, 53–60. [Google Scholar] [CrossRef]
- Kaper, H.; Grandjean, A.; Weidenthaler, C.; Schüth, F.; Goettmann, F. Surface Diels–Alder reactions as an effective method to synthesize functional carbon materials. Chem. Eur. J. 2011, 18, 4099–4106. [Google Scholar] [CrossRef]
- Sarkar, S.; Bekyarova, E.; Niyogi, S.; Haddon, R.C. Diels–Alder chemistry of graphite and graphene: Graphene as diene and dienophile. J. Am. Chem. Soc. 2011, 133, 3324–3327. [Google Scholar] [CrossRef]
- Sarkar, S.; Bekyarova, E.; Haddon, R.C. Chemistry at the Dirac point: Diels–Alder reactivity of graphene. Acc. Chem. Res. 2012, 45, 673–682. [Google Scholar] [CrossRef]
- Seo, J.M.; Jeon, I.-Y.; Baek, J.B. Mechanochemically driven solid-state Diels-Alder reaction of graphite into graphene nanoplatelets. Chem. Sci. 2013, 4, 4273–4277. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Chen, J.; Huang, L.; Shi, G. High-yield production of highly conductive graphene via reversible covalent chemistry. Chem. Commun. 2015, 51, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.V.; Magedov, I.V.; Harper, A.; Jha, S.K.; Ovezmyradov, M.; Chandler, G.; Garcia, J.; Bethke, D.; Shaner, E.A.; Vasiliev, I.; et al. Tetracyanoethylene oxide-functionalized graphene and graphite characterized by Raman and Auger spectroscopy. Carbon 2015, 81, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabihi, O.; Ahmadi, M.; Abdollahi, T.; Nikafshar, S.; Naebe, M. Collision-induced activation: Towards industrially scalable approach to graphite nanoplatelets functionalization for superior polymer nanocomposites. Sci. Rep. 2017, 7, 3560–3572. [Google Scholar] [CrossRef] [Green Version]
- Dang, Z.M.; Yan, W.T.; Xu, H.P. Novel High-dielectric permittivity poly (vinylidene fluoride)/polypropylene blend composites: The influence of the poly (vinylidene fluoride) concentration and compatibilizer. J. Appl. Polym. Sci. 2007, 105, 3649–3655. [Google Scholar] [CrossRef]
- Iqbal, M.; Chuai, C.Z.; Huang, Y.; Chi, C.H. Modification of low-density polyethylene by graft copolymerization with maleic anhydride and blends with polyamide 6. J. Appl. Polym. Sci. 2010, 116, 1558–1565. [Google Scholar] [CrossRef]
- Ismail, H.; Mathialagan, M. Compatibilization of bentonite filled ethylene-propylene-diene monomer composites: Effect of maleic anhydride grafted EPDM. J. Appl. Polym. Sci. 2013, 127, 1164–1171. [Google Scholar] [CrossRef]
- Park, H.Y.; Hwang, S.W.; Ryu, H.C.; Kim, S.W.; Seo, K.H. Effect of maleated EVA on compatibility and toughness of PETG/EVA blend. J. Appl. Polym. Sci. 2013, 127, 2600–2606. [Google Scholar] [CrossRef]
- Ye, C.; Yu, Q.; He, T.; Shen, J.; Li, Y.; Li, J. Physical and rheological properties of maleic anhydrideIncorporated PVDF: Does MAH act as a physical crosslinking point for PVDF molecular chains? ACS Omega 2019, 4, 21540–21547. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Feng, C. An attempt towards fabricating reduced graphene oxide composites with traditional polymer processing techniques by adding chemical reduction agents. Compos. Sci. Technol. 2017, 140, 16–22. [Google Scholar] [CrossRef]
- Yu, J.; Qian, R.; Jiang, P. Enhanced thermal conductivity for PVDF composites with a hybrid functionalized graphene sheet-nanodiamond filler. Fiber. Polym. 2013, 14, 1317–1323. [Google Scholar] [CrossRef]
- Teyssedre, G.; Bernes, A.; Lacabanne, C. Influence of the crystalline phase on the molecular mobility of PVDF. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 2027–2034. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, J.; Li, W.; Zhang, H.; Hu, M.; Chen, H.; He, H. Highly enhancing electrical, thermal, and mechanical properties of polypropylene/graphite intercalation compound composites by in situ expansion during melt mixing. Polymers 2021, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, H.X.; Tong, J.; Ke, D.Y. Enhancing thermal conductivity and mechanical properties of poly (methyl methacrylate) via adding expanded graphite and injecting water. Compos. Part A 2017, 102, 228–235. [Google Scholar] [CrossRef]
- Dini, M.; Mousavand, T.; Carreau, P.; Kamal, M.; Ton-That, M. Effect of water assisted extrusion and solid-state polymerization on the microstructure of PET/clay nanocomposites. Polym. Eng. Sci. 2014, 54, 1723–1736. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Ionic liquid integrated multiwalled carbon nanotube in a poly(vinylidene fluoride) matrix: Formation of a piezoelectric β-polymorph with significant reinforcement and conductivity improvement. ACS Appl. Mater. Inter. 2013, 5, 747–760. [Google Scholar] [CrossRef]
- Rafeie, O.; Aghjeh, M.K.R.; Tavakoli, A.; Salami-Kalajahi, M.; Jameie-Oskooie, A.; Ghayoumi, M. Study on crystalline structure of poly(vinylidene fluoride)/polyethylene/graphene blend-nanocomposites. Polym. Compos. 2019, 40, 4402–4415. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Guthy, C.; Lukes, J.R.; Fischer, J.E.; Winey, K.I. Single wall carbon nanotube/polyethylene nanocomposites: Thermal and electrical conductivity. Macromolecules 2007, 40, 2417–2421. [Google Scholar] [CrossRef]
- Cai, D.Y.; Song, M. Latex technology as a simple route to improve the thermal conductivity of a carbon nanotube/polymer composite. Carbon 2008, 46, 2107–2112. [Google Scholar] [CrossRef]
- Deng, S.; Zhu, Y.; Qi, X.; Yu, W.; Chen, F.; Fu, Q. Preparation of polyvinylidene fluoride/expanded graphite composites with enhanced thermal conductivity via ball milling treatment. RSC Adv. 2016, 6, 45578–45584. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.; Wen, B. Highly thermally conductive and superior electrical insulation polymer composites via in situ thermal expansion of expanded graphite and in situ oxidation of aluminum nanoflakes. ACS Appl. Mater. Inter. 2021, 13, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Barra, G.M.O.; Crespo, J.S.; Bertolino, J.R.; Soldi, V.; Pires, A.T.N. Maleic anhydride grafting on EPDM: Qualitative and quantitative determination. J. Braz. Chem. Soc. 1999, 10, 31–34. [Google Scholar] [CrossRef]
- Yang, L.Q.; Zhang, F.R.; Endo, T.; Hirotsu, T. Microstructure of maleic anhydride grafted polyethylene by high-resolution solution-state NMR and FTIR spectroscopy. Macromolecules 2003, 36, 4709–4718. [Google Scholar] [CrossRef]
- Samadaei, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Radical coupling of maleic anhydride onto graphite to fabricate oxidized graphene nanolayers. Bull. Mater. Sci. 2016, 39, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.B.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
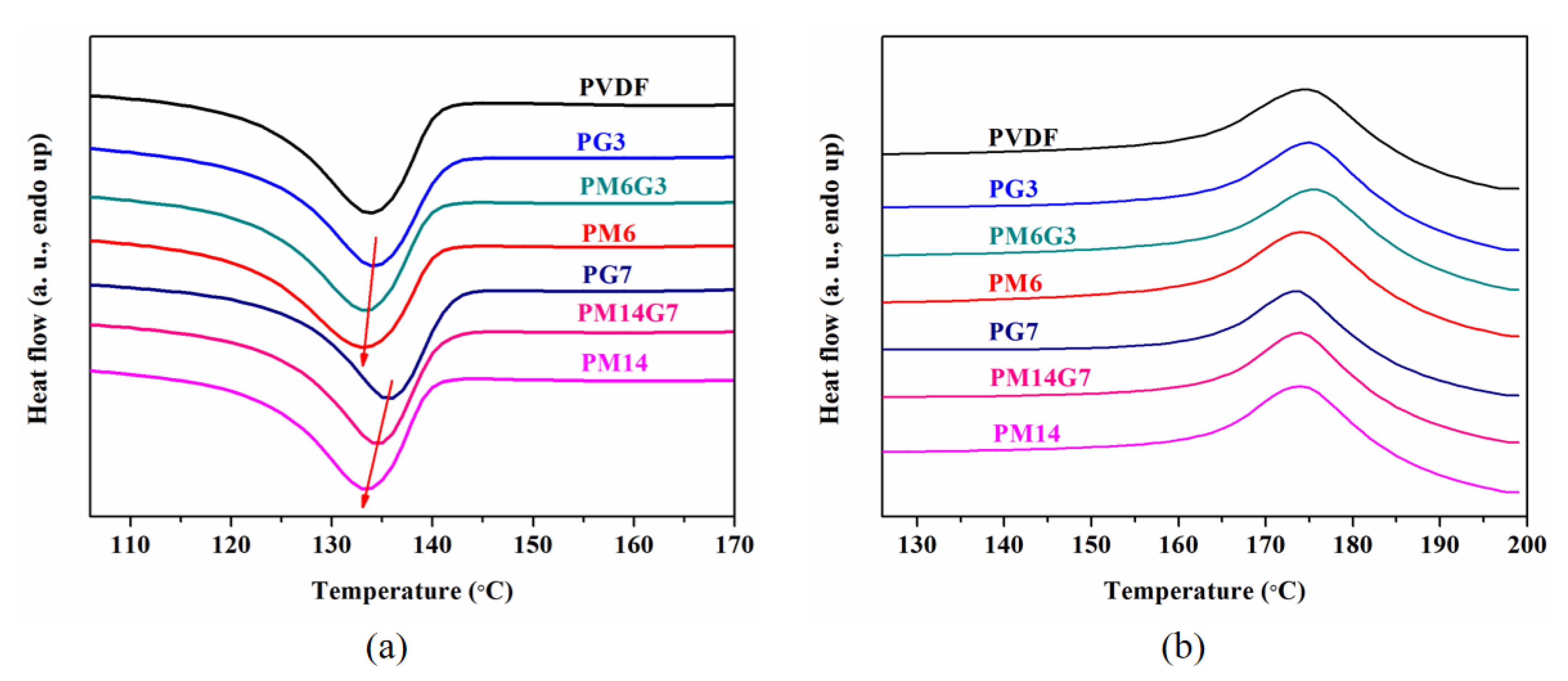


| Sample | Tc (°C) | Tm (°C) | Hm (J/g) | Xc (%) |
|---|---|---|---|---|
| PVDF | 133.8 | 175.8 | 21.97 | 21.0 |
| PG3 | 134.2 | 175.1 | 20.71 | 20.4 |
| PM6G3 | 133.2 | 176.0 | 19.89 | 20.7 |
| PM6 | 132.9 | 174.6 | 22.71 | 23.0 |
| PG7 | 135.5 | 173.7 | 19.50 | 19.9 |
| PM14G7 | 134.5 | 174.1 | 20.36 | 23.6 |
| PM14 | 133.4 | 174.2 | 21.36 | 23.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, J.; Zheng, H.; Fan, J.; Li, W.; Wang, Z.; Zhang, H.; Dai, Y.; Chen, H.; Zhu, Z. Fabricating Well-Dispersed Poly(Vinylidene Fluoride)/Expanded Graphite Composites with High Thermal Conductivity by Melt Mixing with Maleic Anhydride Directly. Polymers 2023, 15, 1747. https://doi.org/10.3390/polym15071747
Tong J, Zheng H, Fan J, Li W, Wang Z, Zhang H, Dai Y, Chen H, Zhu Z. Fabricating Well-Dispersed Poly(Vinylidene Fluoride)/Expanded Graphite Composites with High Thermal Conductivity by Melt Mixing with Maleic Anhydride Directly. Polymers. 2023; 15(7):1747. https://doi.org/10.3390/polym15071747
Chicago/Turabian StyleTong, Jun, Huannan Zheng, Jinwei Fan, Wei Li, Zhifeng Wang, Haichen Zhang, Yi Dai, Haichu Chen, and Ziming Zhu. 2023. "Fabricating Well-Dispersed Poly(Vinylidene Fluoride)/Expanded Graphite Composites with High Thermal Conductivity by Melt Mixing with Maleic Anhydride Directly" Polymers 15, no. 7: 1747. https://doi.org/10.3390/polym15071747
APA StyleTong, J., Zheng, H., Fan, J., Li, W., Wang, Z., Zhang, H., Dai, Y., Chen, H., & Zhu, Z. (2023). Fabricating Well-Dispersed Poly(Vinylidene Fluoride)/Expanded Graphite Composites with High Thermal Conductivity by Melt Mixing with Maleic Anhydride Directly. Polymers, 15(7), 1747. https://doi.org/10.3390/polym15071747