The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Growth Conditions
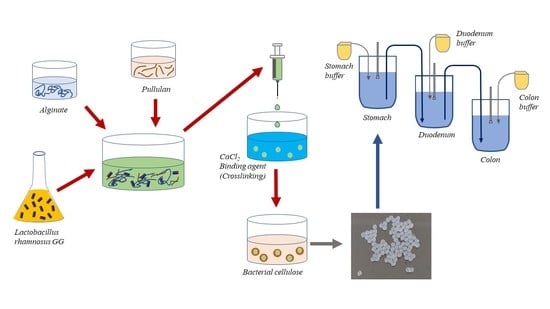
2.2. Preparation of BC
2.3. Probiotics Encapsulation
2.4. Scanning Electron Microscopy (SEM)
2.5. Mechanical Characterization
2.6. Encapsulation Efficiency
2.7. Survival of LGG in Simulated Gastric Fluid (SGF) and Simulated Duodenum Fluid (SDF)
2.8. Preparation of Enzymatic Fecal Extracts (Fecalase) and Release of LGG into Simulated Colon Fluid (SCF)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Obtaining and Characterization of Probiotic Beads
3.2. Survival of Free and Encapsulated Bacteria in SGF and SDF
3.3. Release of LGG into SCF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Chen, Y.F.; Kwok, L.Y.; Li, M.H.; Sun, T.; Sun, C.L.; Wang, X.N.; Dan, T.; Menghebilige; Zhang, H.P.; et al. Preliminary selection for potential probiotic Bifidobacterium isolated from subjects of different Chinese ethnic groups and evaluation of their fermentation and storage characteristics in bovine milk. J. Dairy Sci. 2013, 96, 6807–6817. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Urbanska, A.M. Colon-targeted delivery of live bacterial cell biotherapeutics including microencapsulated live bacterial cells. Biol. Targets Ther. 2008, 2, 355–378. [Google Scholar] [CrossRef] [Green Version]
- Mohd, G.K.; Vinod, G.; Chandel, H.S.; Asra, A.; Kasma, T. Development of Microencapsulation: A Review of Literature. Int. J. Sci. Study 2017, 5, 264–268. [Google Scholar] [CrossRef]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar] [CrossRef]
- Zhantlessova, S.; Savitskaya, I.; Kistaubayeva, A.; Ignatova, L.; Talipova, A.; Pogrebnjak, A.; Digel, I. Advanced “Green” Prebiotic Composite of Bacterial Cellulose/Pullulan Based on Synthetic Biology-Powered Microbial Coculture Strategy. Polymers 2022, 14, 3224. [Google Scholar] [CrossRef]
- Çabuk, B.; Harsa, S.T. Protection of Lactobacillus acidophilus NRRL-B 4495 under in vitro gastrointestinal conditions with whey protein/pullulan microcapsules. J. Biosci. Bioeng. 2015, 120, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cai, D.; Song, Q.; Wang, Y.; Sun, H.; Piao, C.; Yu, H.; Liu, J.; Liu, J.; Wang, Y. Effect on Viability of Microencapsulated Lactobacillus rhamnosus with the Whey Protein-pullulan Gels in Simulated Gastrointestinal Conditions and Properties of Gels. Korean J. Food Sci. Anim. Resour. 2019, 39, 459–473. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating Polymers for Probiotics Delivery Systems: Preparation, Characterization, and Applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Gallego-Lobillo, P.; Moreno, F.J.; Villamiel, M.; Hernandez-Hernandez, O. In vitro digestibility of galactooligosaccharides: Effect of the structural features on their intestinal degradation. J. Agric. Food Chem. 2019, 67, 4662–4670. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Maathuis, A.J.; Van den Heuvel, E.G.; Schoterman, M.H.; Venema, K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a 13C-labeling technique. J. Nutr. 2012, 142, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Savitskaya, I.S.; Bondarenko, V.M. Suppression of mutagenic activity of intestinal metabolites in normobiocenosis. Microbiol. J. 2008, 3, 53–58. (In Russian) [Google Scholar]
- Rodrigues, F.; Cedran, M.; Bicas, J.; Sato, H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2020, 62, 2470–2494. [Google Scholar] [CrossRef]
- Lombardo, S.; Villares, A. Engineered multilayer microcapsules based on polysaccharides nanomaterials. Molecules 2020, 25, 4420. [Google Scholar] [CrossRef]
- Tsujisaka, Y.; Mitsuhashi, M. Pullulan. In Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: West Lafayette, IN, USA, 1993; pp. 447–460. ISBN 978-0-080-92654-4. [Google Scholar]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Mokarram, R.R.; Mortazavi, S.A.; Najafi, M.B.H.; Shahidi, F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res. Int. 2009, 42, 1040–1045. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E. Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Zhang, S.; He, H.; Guan, S.; Cai, B.; Li, Q.; Rong, S. Bacterial cellulose-alginate composite beads as Yarrowia lipolytica cell carriers for lactone production. Molecules 2020, 25, 928. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, S.B.; Singhal, R.S. Pullulan-complexed α-amylase and glucosidase in alginate beads: Enhanced entrapment and stability. Carbohydr. Polym. 2014, 105, 49–56. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, C.; Zheng, S.; Li, H.; Yu, J. Development of a microencapsulated synbiotic product and its application in yoghurt. LWT 2020, 122, 109033. [Google Scholar] [CrossRef]
- Nag, A.; Han, K.S.; Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Zheng, P.; Yu, J.; Ma, X. Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohydr. Polym. 2010, 79, 301–305. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate Based Nanocomposite for Microencapsulation of Probiotic: Effect of Cellulose Nanocrystal (CNC) and Lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Pandey, S.; Shreshtha, I.; Sachan, S.G. Pullulan: Biosynthesis, Production and Applications. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 121–141. ISBN 978-3-030-75289-7. [Google Scholar]
- Fareez, I.M.; Lim, S.M.; Zulkefli, N.A.A.; Mishra, R.K.; Ramasamy, K. Cellulose derivatives enhanced stability of alginate-based beads loaded with Lactobacillus plantarum LAB12 against low pH, high temperature and prolonged storage. Probiotics Antimicrob. Proteins 2018, 10, 543–557. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Ateeq, H.; Shah, Y.A.; Hussain, M.; Javed, A.; Ikram, A.; Raza, M.A.; Nayik, G.A.; Alfarraj, S.; et al. Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir. Biomimetics 2022, 7, 109. [Google Scholar] [CrossRef]
- Maleki, O.; Khaledabad, M.A.; Amiri, S.; Asl, A.K.; Makouie, S. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate-crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability. LWT 2020, 126, 109224. [Google Scholar] [CrossRef]
- Voropaiev, M.; Nock, D. Onset of acid-neutralizing action of a calcium/magnesium carbonate-based antacid using an artificial stomach model: An in vitro evaluation. BMC Gastroenterol. 2021, 21, 112. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.; Prapasarakul, N. The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria. Sci. Rep. 2021, 11, 13753. [Google Scholar] [CrossRef]
- Potivara, K.; Phisalaphong, M. Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber. Materials 2019, 12, 2323. [Google Scholar] [CrossRef] [Green Version]
- Brumm, P.J. Bacterial genomes: What they teach us about cellulose degradation. Biofuels 2013, 4, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Cur. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [Green Version]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bostrom, T.; Bhandari, B. Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method. Intl. J. Food Microbiol. 2011, 145, 162–168. [Google Scholar] [CrossRef]
- Qi, X.; Simsek, S.; Chen, B.; Rao, J. Alginate-based double-network hydrogel improves the viability of encapsulated probiotics during simulated sequential gastrointestinal digestion: Effect of biopolymer type and concentrations. Int. J. Biol. Macromol. 2020, 165, 1675–1685. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Gandomi, H.; Misaghi, A.; Bokaei, S.; Noori, N. The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J. Sci. Food Agric. 2014, 94, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.L.; Sun, Y.; Li, A.L.; Liu, F.; Meng, X.C. Microencapsulation of Lactobacillus rhamnosus GG by Transglutaminase Cross-Linked Soy Protein Isolate to Improve Survival in Simulated Gastrointestinal Conditions and Yoghurt. J. Food Sci. 2016, 81, M1726–M1734. [Google Scholar] [CrossRef] [PubMed]
- Siang, S.; Lai, K.; Kar, L.; Phing, L. Effect of added prebiotic (Isomalto-oligosaccharide) and Coating of Beads on the Survival of Microencapsulated Lactobacillus rhamnosus GG. Food Sci. Technol. 2019, 39, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Cordoba, A.L.; Deladino, L.; Martino, M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef]
- Zhao, M.; Qu, F.; Wu, Z.; Nishinari, K.; Phillips, G.O.; Fang, Y. Protection mechanism of alginate microcapsules with different mechanical strength for Lactobacillus plantarum ST-III. Food Hydrocoll. 2017, 66, 396–402. [Google Scholar] [CrossRef]
- Iyer, C.; Kasyphathy, K. Effect of Co-encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. Educ. 2005, 70, 18–23. [Google Scholar] [CrossRef]
- Succi, M.; Tremonte, P.; Reale, A.; Sorrentino, E.; Grazia, L.; Pacifico, S.; Coppola, R. Bile salt and acid tolerance of Lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiol. Lett. 2005, 244, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kheadr, E. Impact of acid and ox gall on antibiotic susceptibility of probiotic Lactobacilli. Afr. J. Agric. Res. 2006, 1, 172–181. [Google Scholar]
- Karu, R.; Sumeri, I. Survival of Lactobacillus rhamnosus GG during simulated gastrointestinal conditions depending on food matrix. J. Food Res. 2016, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.; Morsy, O.; Abdelmonem, M.; Elsabagh, R. Anthocyanin-Colored Microencapsulation Effects on Survival Rate of Lactobacillus rhamnosus GG, Color Stability, and Sensory Parameters in Strawberry Nectar Model. Food Bioprocess Technol. 2022, 15, 352–367. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Youssef, M.; Korin, A.; Zhan, F.; Hady, E.; Ahmed, H.Y.; Geng, F.; Chen, Y.; Li, B. Encapsulation of Lactobacillus salivarius in single and dual biopolymer. J. Food Eng. 2021, 294, 110398. [Google Scholar] [CrossRef]
- Wang, J.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Entrapment, survival and release of Bifidobacterium adolescentis within chickpea protein-based microcapsules. Food Res. Int. 2014, 55, 20–27. [Google Scholar] [CrossRef]
- Klemmer, K.J.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Pea protein-based capsules for probiotic and prebiotic delivery. Int. J. Food Sci. Technol. 2011, 46, 2248–2256. [Google Scholar] [CrossRef]
- Tanimura, A.; Liu, W.; Yamada, K.; Kishida, T.; Toyohara, H. Animal cellulases with a focus on aquatic invertebrates. Fish. Sci. 2013, 79, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Piancone, E.; Fosso, B.; Marzano, M.; De Robertis, M.; Notario, E.; Oranger, A.; Manzari, C.; Bruno, S.; Visci, G.; Defazio, G.; et al. Natural and after colon washing fecal samples: The two sides of the coin for investigating the human gut microbiome. Sci. Rep. 2022, 12, 17909. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Boudechicha, A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Screening of Cellulolytic Bacteria from Various Ecosystems and Their Cellulases Production under Multi-Stress Conditions. Catalysts 2022, 12, 769. [Google Scholar] [CrossRef]
- Omura, T.; Imagawa, K.; Kono, K.; Suzuki, T.; Minami, H. Encapsulation of Either Hydrophilic or Hydrophobic Substances in Spongy Cellulose Particles. ACS Appl. Mater. Interfaces 2017, 9, 944–949. [Google Scholar] [CrossRef]






| Type | Concentration, g/100 mL | Ee, % | Size, µm | Mechanical Strength, MPa | ||
|---|---|---|---|---|---|---|
| PUL | BC | Dry | Wet | |||
| Alg, 2% | - | - | 78.8 ± 3.88 | 801 ± 55.1 | 2550 ± 127.1 | 26.6 ± 0.62 |
| Alg, 2% + BC | - | 0.5 | 81.2 ± 4.01 | 897 ± 60.1 | 2820 ± 143.0 | 28.8 ± 0.83 * |
| - | 1 | 80.2 ± 4.13 | 878 ± 59.9 | 2819 ± 140.1 | 26.9 ± 0.63 | |
| - | 2 | 77.2 ± 3.89 | 871 ± 71.4 | 2815 ± 168.9 | 24.9 ± 0.55 | |
| Alg, 2% + PUL/BC | 1 | 0.5 | 88.3 ± 4.41 * | 908 ± 61.1 * | 3341 ± 233.4 * | 36.8 ± 0.58 * |
| 1 | 1 | 87.6 ± 4.33 * | 903 ± 72.7 * | 3367 ± 167.1 * | 35.2 ± 0.68 * | |
| 1 | 2 | 87.1 ± 4.36 * | 887 ± 97.3 | 3371 ± 235.9 * | 35.8 ± 0.49 * | |
| 2 | 0.5 | 89.1 ± 4.47 * | 921 ± 61.0 * | 3401 ± 204.0 * | 37.1 ± 0.77 * | |
| 2 | 1 | 87.8 ± 4.39 * | 910 ± 85.4 * | 3351 ± 134.4 * | 34.1 ± 0.73 * | |
| 2 | 2 | 87.6 ± 4.33 * | 820 ± 61.3 | 3373 ± 168.1 * | 34.6 ± 0.69 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kistaubayeva, A.; Abdulzhanova, M.; Zhantlessova, S.; Savitskaya, I.; Karpenyuk, T.; Goncharova, A.; Sinyavskiy, Y. The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival. Polymers 2023, 15, 1752. https://doi.org/10.3390/polym15071752
Kistaubayeva A, Abdulzhanova M, Zhantlessova S, Savitskaya I, Karpenyuk T, Goncharova A, Sinyavskiy Y. The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival. Polymers. 2023; 15(7):1752. https://doi.org/10.3390/polym15071752
Chicago/Turabian StyleKistaubayeva, Aida, Malika Abdulzhanova, Sirina Zhantlessova, Irina Savitskaya, Tatyana Karpenyuk, Alla Goncharova, and Yuriy Sinyavskiy. 2023. "The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival" Polymers 15, no. 7: 1752. https://doi.org/10.3390/polym15071752
APA StyleKistaubayeva, A., Abdulzhanova, M., Zhantlessova, S., Savitskaya, I., Karpenyuk, T., Goncharova, A., & Sinyavskiy, Y. (2023). The Effect of Encapsulating a Prebiotic-Based Biopolymer Delivery System for Enhanced Probiotic Survival. Polymers, 15(7), 1752. https://doi.org/10.3390/polym15071752