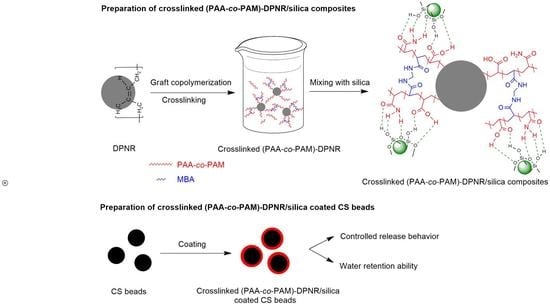
Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber
2.3. Characterization of Crosslinked (PAA-co-PAM)-DPNR
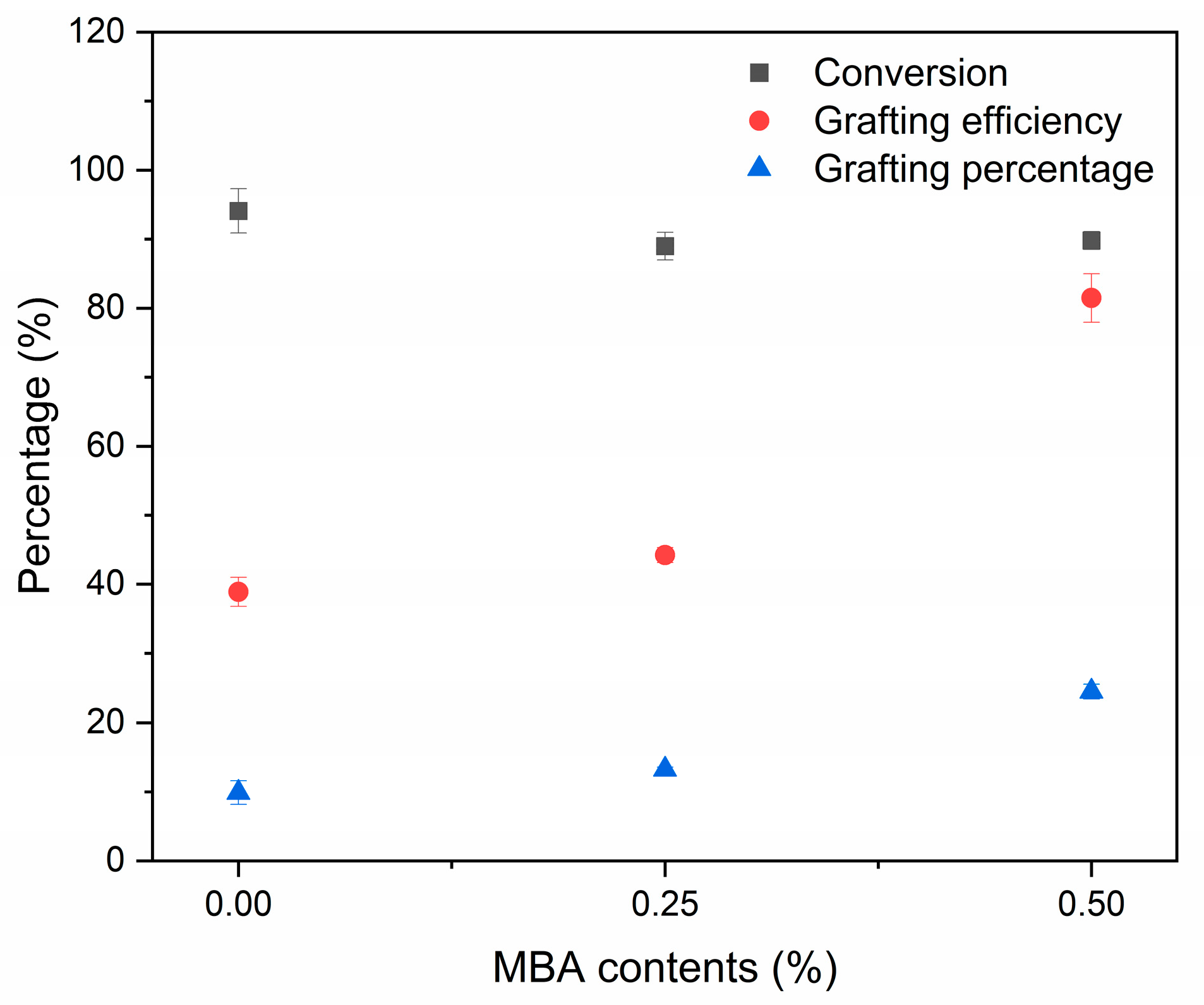
2.3.1. Determination of Monomer Conversion, Grafting Efficiency and Grafting Percentage
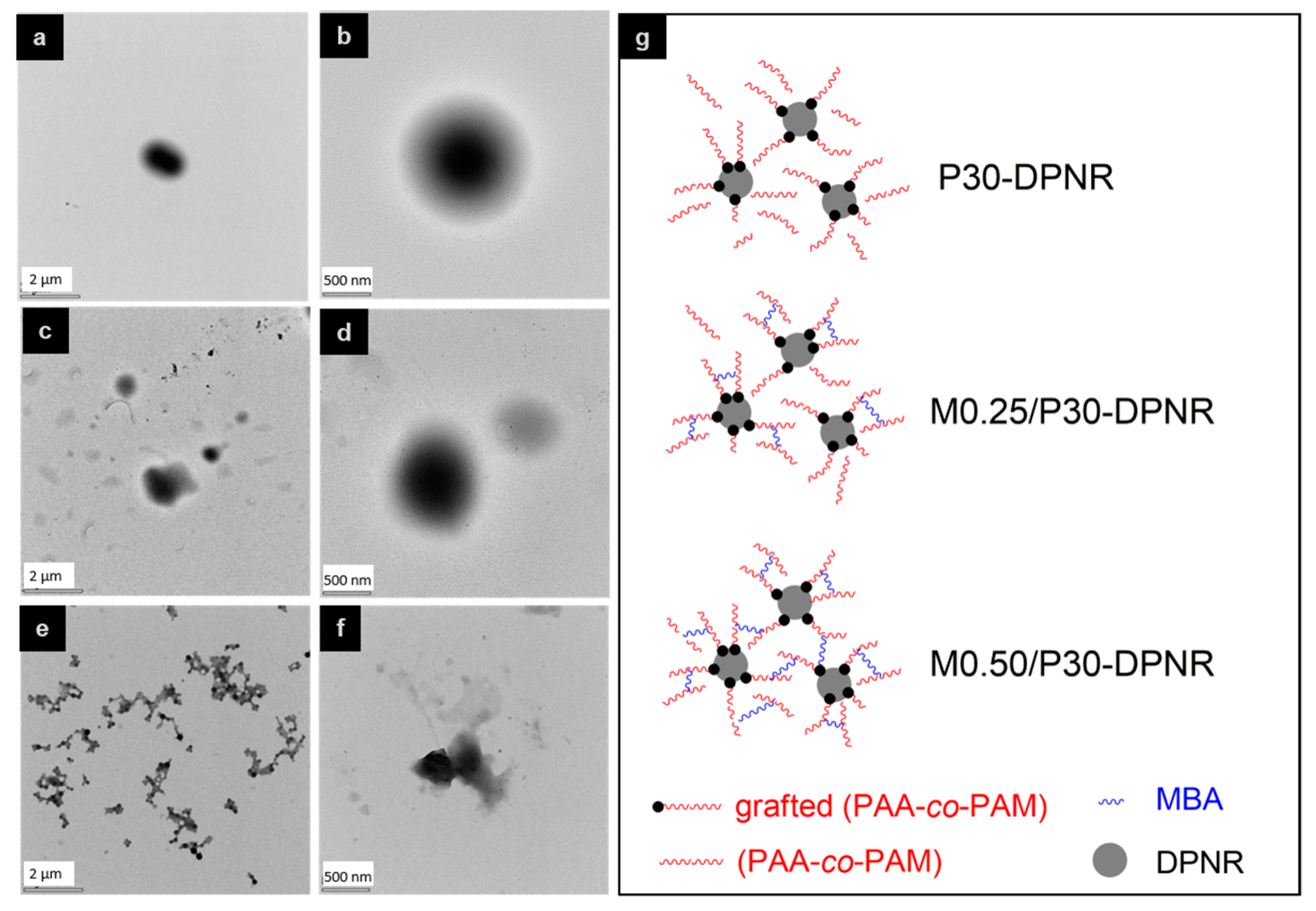
2.3.2. Morphology
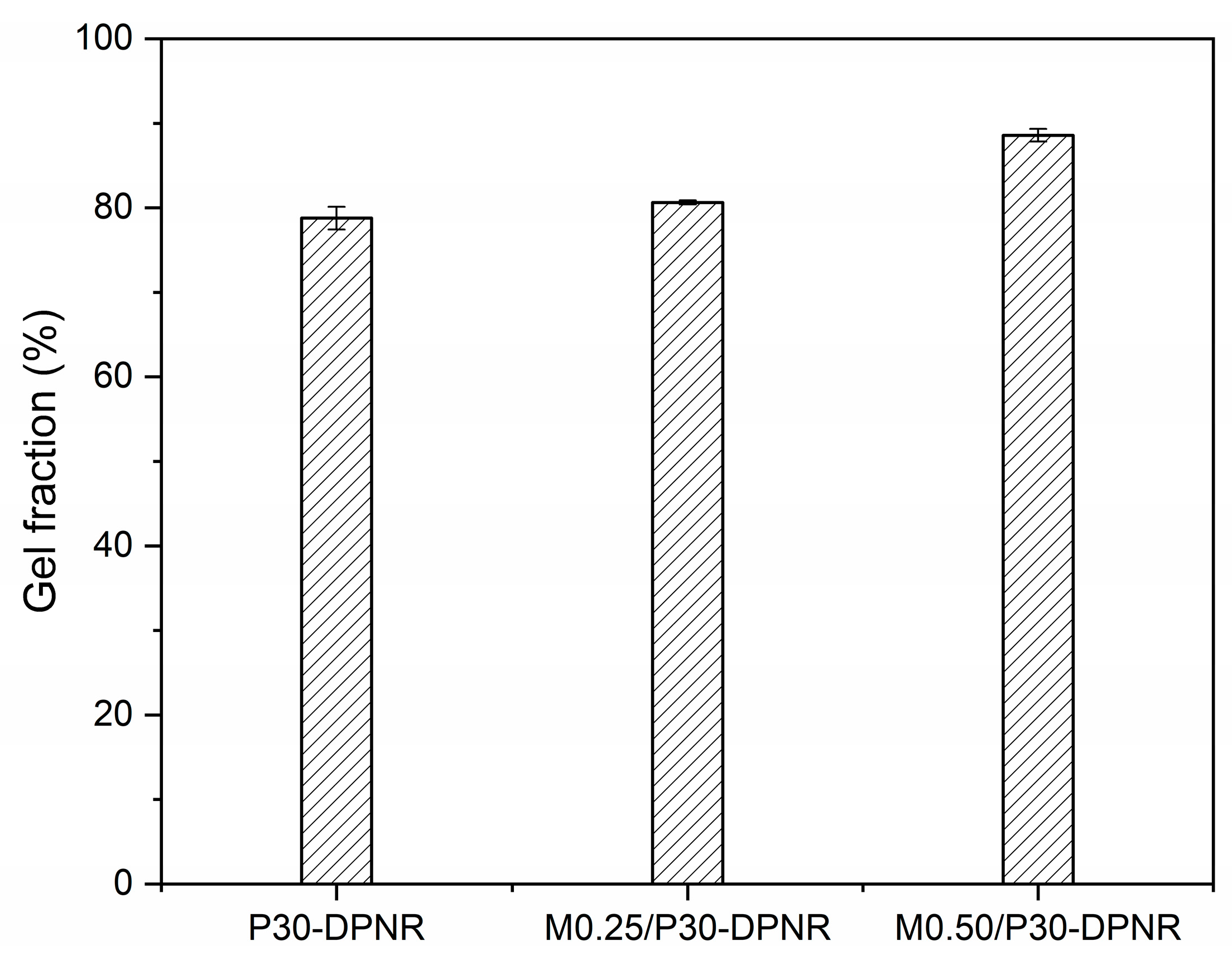
2.3.3. Gel Fraction
2.4. Preparation of Crosslinked (PAA-co-PAM)-DPNR/Silica Composites
2.5. Characterization of Crosslinked (PAA-co-PAM)-DPNR/Silica Composites
2.5.1. Fourier-Transform Infrared Spectroscopy
2.5.2. Morphology
2.5.3. Swelling Degree
2.5.4. Contact Angle
2.5.5. Thermogravimetric Analysis
2.5.6. Compressive Properties
2.6. Preparation and Characterization of Crosslinked (PAA-co-PAM)-DPNR/Silica-coated CS Beads
2.6.1. Preparation
2.6.2. Morphology
2.6.3. Water Retention
2.6.4. Loading Percentage
2.6.5. Release Behavior
3. Results and Discussion
3.1. Preparation and Characterization of Crosslinked (PAA-co-PAM)-Grafted Deproteinized Natural Rubber
3.1.1. Conversion, Grafting Efficiency and Grafting Percentage
3.1.2. Morphology
3.1.3. Gel Fraction
3.2. Preparation and Characterization of Crosslinked (PAA-co-PAM)-DPNR/Silica Composites
3.2.1. Preparation of Crosslinked (PAA-co-PAM)-DPNR/Silica Composites
3.2.2. FTIR Analysis
3.2.3. Morphology
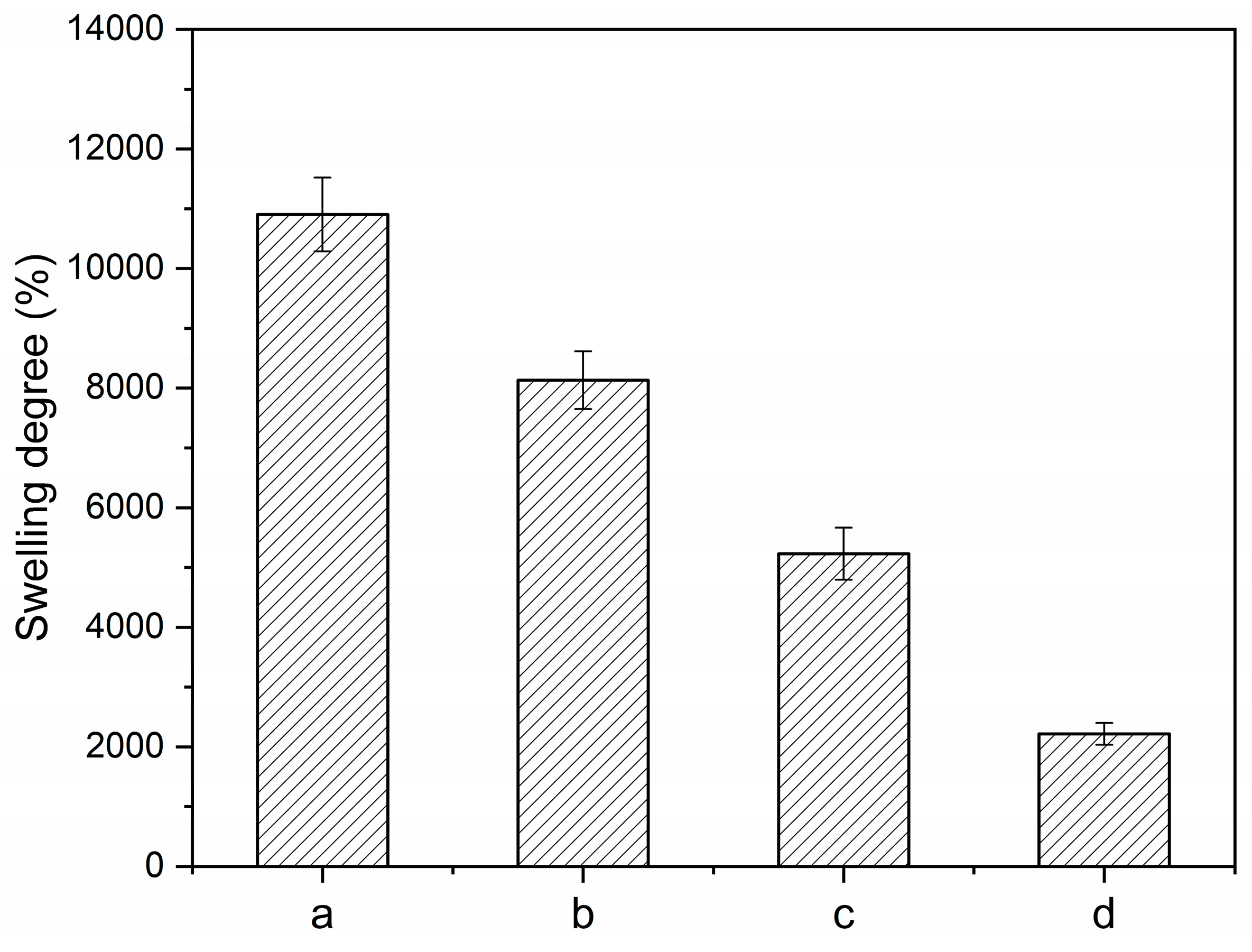
3.2.4. Swelling Degree
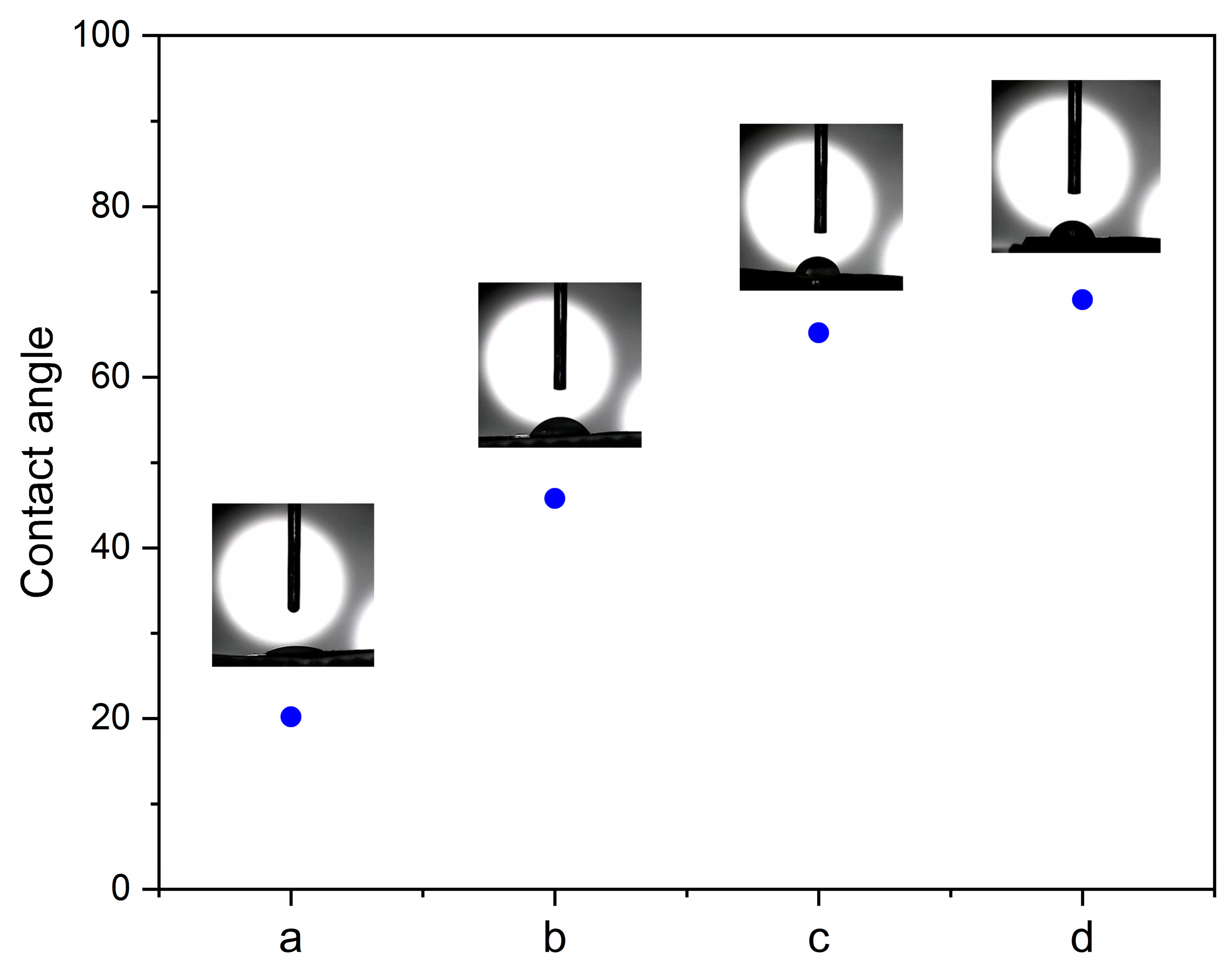
3.2.5. Contact Angle
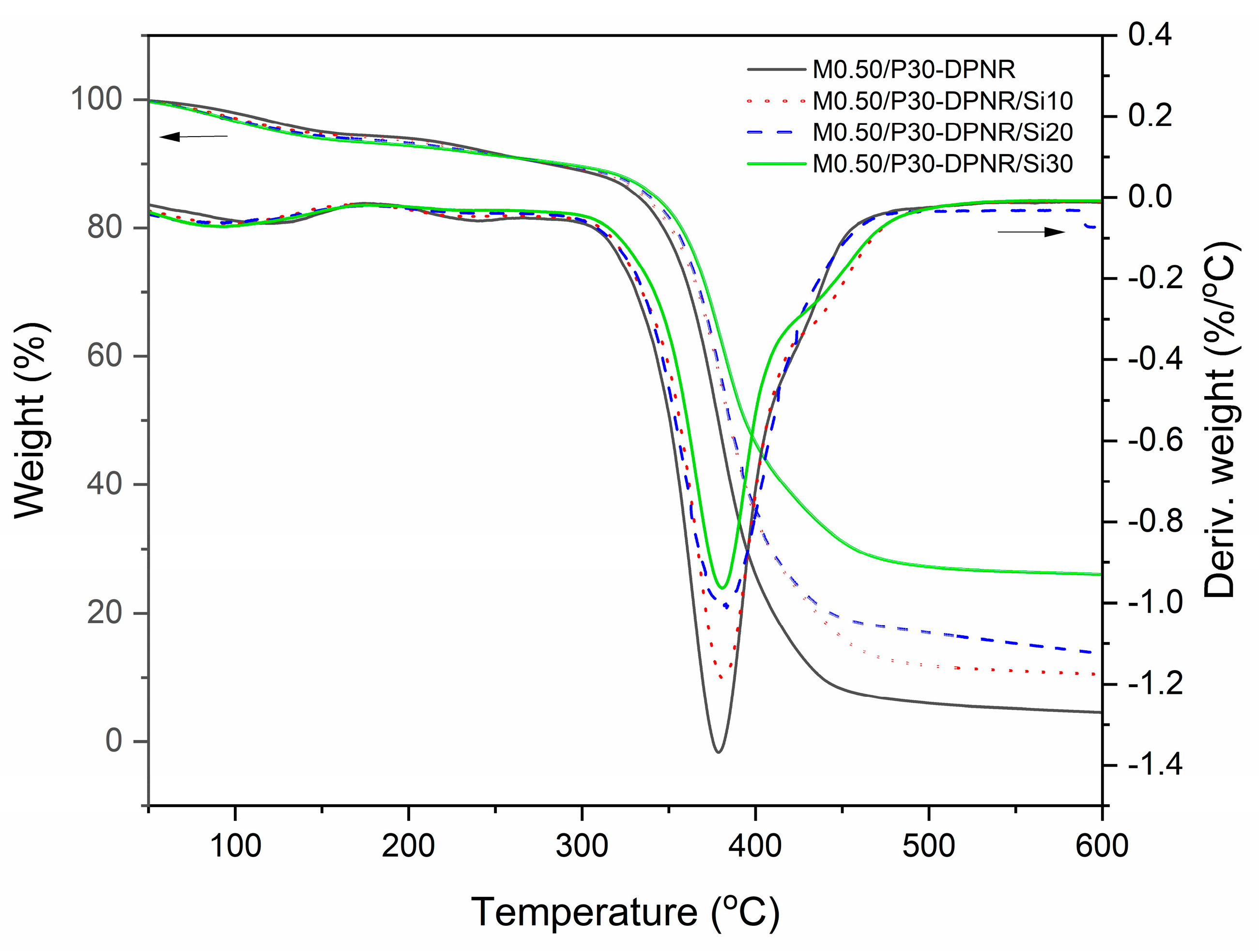
3.2.6. Thermal Properties
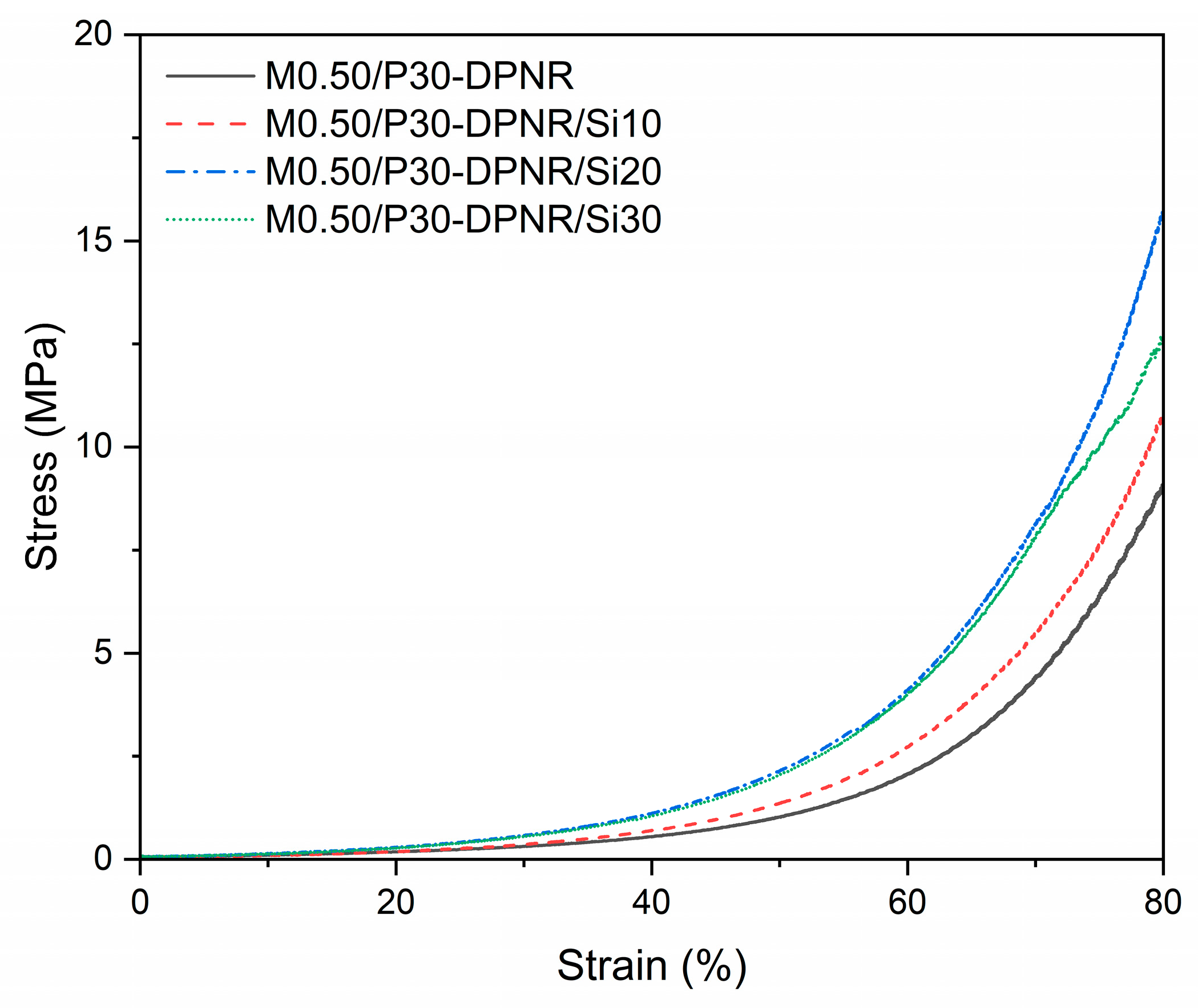
3.2.7. Compressive Properties
3.3. Fabrication of Crosslinked (PAA-co-PAM)-DPNR/Silica-Coated CS Beads
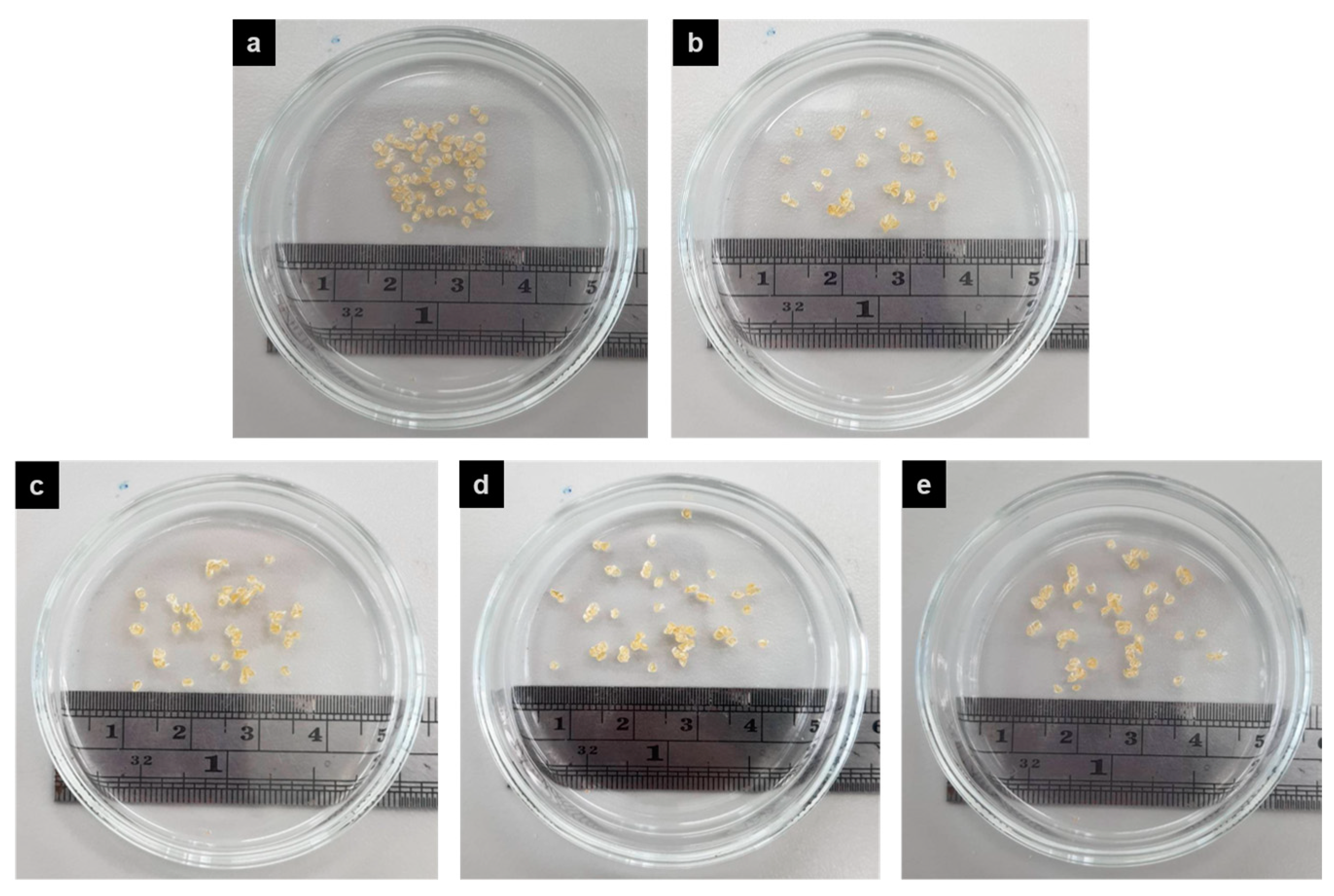
3.3.1. Morphology
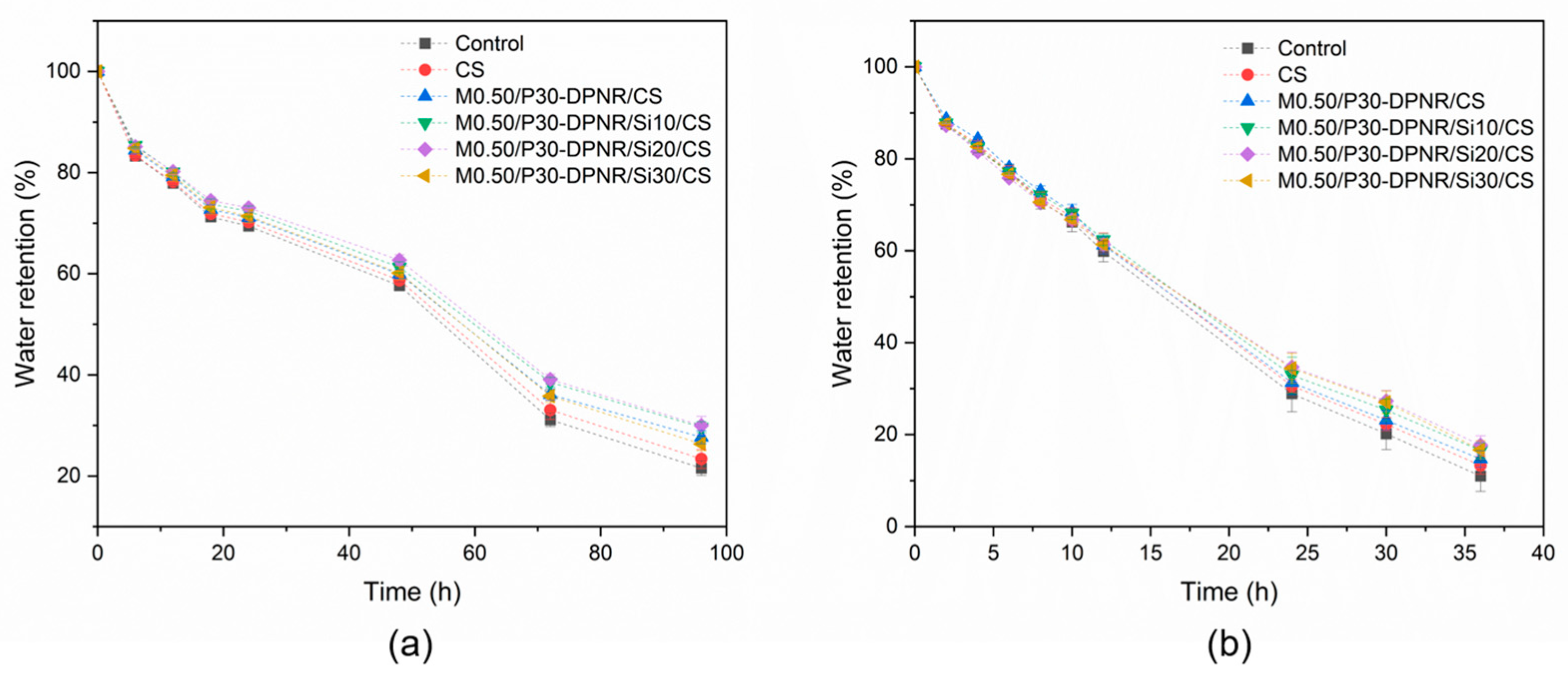
3.3.2. Water Retention
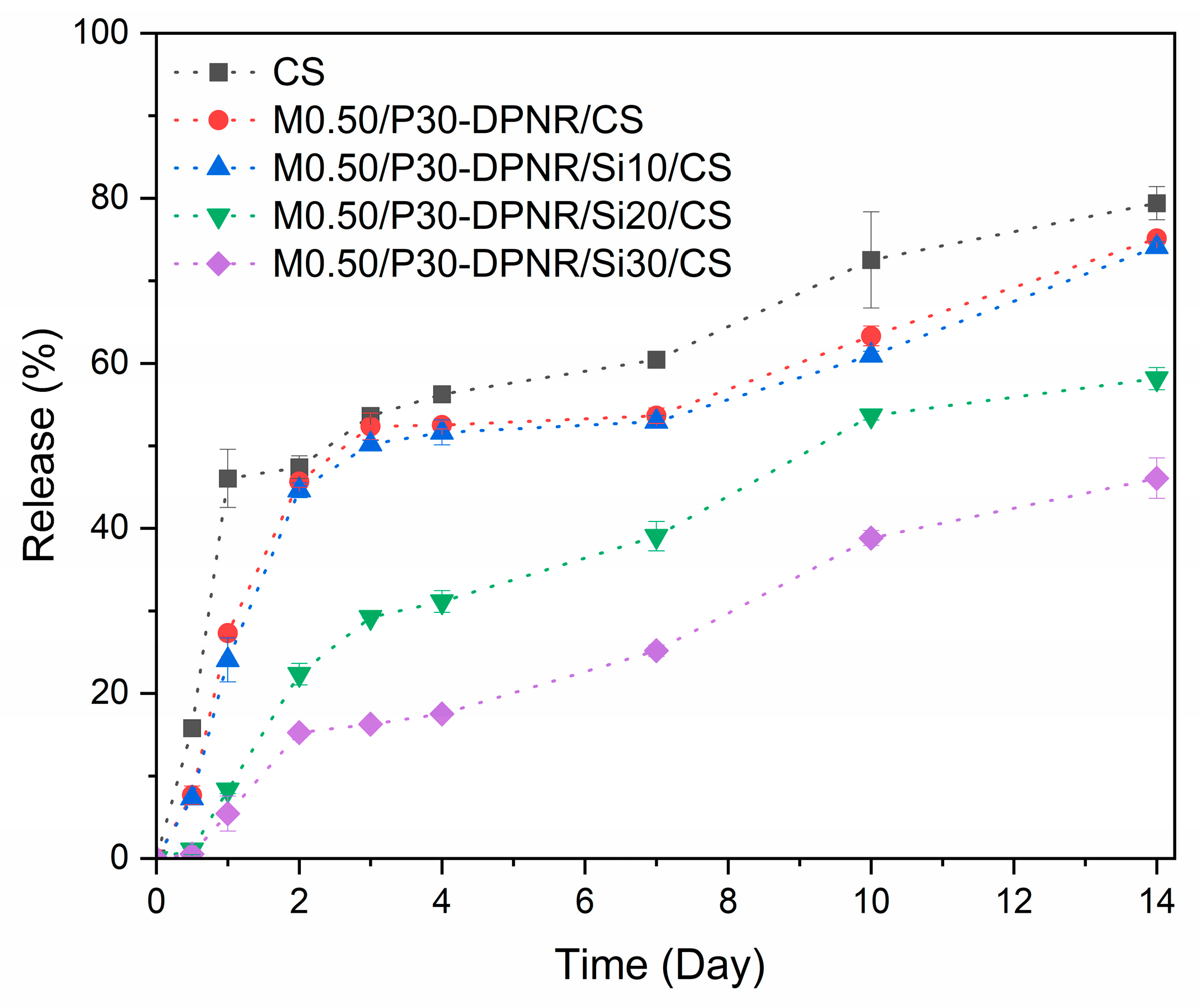
3.3.3. Loading Percentage and Release Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhou, Z.-H.; Li, L.; Luo, Y.-L.; Xu, F.; Chen, Y. Dual Stimuli-Responsive Supramolecular Self-Assemblies Based on the Host–Guest Interaction between β-Cyclodextrin and Azobenzene for Cellular Drug Release. Mol. Pharm. 2020, 17, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Hua, M.; Yu, H.; Wei, S.; Wang, A.; Zhou, J. Photothermal-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles Based on Base-Pairing Rules. ACS Biomater. Sci. Eng. 2019, 5, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wang, T.; Li, X.; Li, X. Polylactic Acid–Graphene Oxide-based Materials for Loading and Sustained Release of Poorly Soluble Pesticides. Langmuir 2020, 36, 12336–12345. [Google Scholar] [CrossRef]
- Eddarai, E.M.; El Mouzahim, M.; Boussen, R.; Bellaouchou, A.; Guenbour, A.; Zarrouk, A. Chitosan-kaolinite clay composite as durable coating material for slow release NPK fertilizer. Int. J. Biol. Macromol. 2022, 195, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, B.; Tong, Z.; Yang, Y. Chitosan and Graphene Oxide Nanocomposites as Coatings for Controlled-Release Fertilizer. Water Air Soil Pollut. 2019, 230, 146. [Google Scholar] [CrossRef]
- Tian, H.; Liu, Z.; Zhang, M.; Guo, Y.; Zheng, L.; Li, Y.C. Biobased Polyurethane, Epoxy Resin, and Polyolefin Wax Composite Coating for Controlled-Release Fertilizer. ACS Appl. Mater. Interfaces 2019, 11, 5380–5392. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Américo-Pinheiro, J.H.P.; Vo, D.-V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Panrat, K.; Boonme, P.; Taweepreda, W.; Pichayakorn, W. Formulations of Natural Rubber Latex as Film Former for Pharmaceutical Coating. Procedia Chem. 2012, 4, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Song, S.; Yang, Z.; Liu, Y.; He, Z.; Zhou, C.; Du, L.; Sun, D.; Li, P. Hydrophobic modification of castor oil-based polyurethane coated fertilizer to improve the controlled release of nutrient with polysiloxane and halloysite. Prog. Org. Coat. 2022, 165, 106756. [Google Scholar] [CrossRef]
- Cui, Y.; Xiang, Y.; Xu, Y.; Wei, J.; Zhang, Z.; Li, L.; Li, J. Poly-acrylic acid grafted natural rubber for multi-coated slow release compound fertilizer: Preparation, properties and slow-release characteristics. Int. J. Biol. Macromol. 2020, 146, 540–548. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, W.K.; Song, X.; Gao, Z.; Wen, Y.; Ong, C.N.; Loh, C.S.; Swarup, S.; Li, J. Converting Okara to Superabsorbent Hydrogels as Soil Supplements for Enhancing the Growth of Choy Sum (Brassica sp.) under Water-Limited Conditions. ACS Sustain. Chem. Eng. 2020, 8, 9425–9433. [Google Scholar] [CrossRef]
- Inphonlek, S.; Bureewong, N.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites. Polymers 2022, 14, 4602. [Google Scholar] [CrossRef]
- Sahakaro, K.; Beraheng, S. Reinforcement of maleated natural rubber by precipitated silica. J. Appl. Polym. Sci. 2008, 109, 3839–3848. [Google Scholar] [CrossRef]
- Panploo, K.; Chalermsinsuwan, B.; Poompradub, S. Effect of amine types and temperature of a natural rubber based composite material on the carbon dioxide capture. Chem. Eng. J. 2020, 402, 125332. [Google Scholar] [CrossRef]
- Oliveira, N.C.; Paula, A.J.; Maia, M.T.; Noronha, V.T.; Alves, A.C.; Freire, R.M.; Souza Filho, A.G.; Ferreira, O.P.; Faria, A.F.; do Nascimento, R.M. Silica Nanoparticles Modified with Fluorescent Dyes as Probes for in Situ Characterization of Natural Rubber Coatings. ACS Appl. Nano Mater. 2022, 5, 14492–14506. [Google Scholar] [CrossRef]
- Boonying, P.; Boonpavanitchakul, K.; Kangwansupamonkon, W. Green Bio-composite Coating Film from Lignin/Pre-vulcanized Natural Rubber Latex for Controlled-release Urea Fertilizer. J. Polym. Environ. 2023, 31, 1642–1655. [Google Scholar] [CrossRef]
- Boonying, P.; Boonpavanitchakul, K.; Amnuaypanich, S.; Kangwansupamonkon, W. Natural rubber-lignin composites modified with natural rubber-graft-polyacrylamide as an effective coating for slow-release fertilizers. Ind. Crops Prod. 2023, 191, 116018. [Google Scholar] [CrossRef]
- Pipertzis, A.; Kafetzi, M.; Giaouzi, D.; Pispas, S.; Floudas, G.A. Grafted Copolymer Electrolytes Based on the Poly(acrylic acid-co-oligo ethylene glycol acrylate) (P(AA-co-OEGA)) Ion-Conducting and Mechanically Robust Block. ACS Appl. Polym. Mater. 2022, 4, 7070–7080. [Google Scholar] [CrossRef]
- Kawahara, S.; Klinklai, W.; Kuroda, H.; Isono, Y. Removal of proteins from natural rubber with urea. Polym. Adv. Technol. 2004, 15, 181–184. [Google Scholar] [CrossRef]
- Rimdusit, N.; Jubsilp, C.; Mora, P.; Hemvichian, K.; Thuy, T.T.; Karagiannidis, P.; Rimdusit, S. Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers 2021, 13, 3447. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jia, C.; Wang, D.; Deng, J.; Xu, G.; Wu, C.; Dong, M.; Guo, Z. Synthesis and characterization of porous tree gum grafted copolymer derived from Prunus cerasifera gum polysaccharide. Int. J. Biol. Macromol. 2019, 133, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Kochthongrasamee, T.; Prasassarakich, P.; Kiatkamjornwong, S. Effects of redox initiator on graft copolymerization of methyl methacrylate onto natural rubber. J. Appl. Polym. Sci. 2006, 101, 2587–2601. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, T.; Yan, J.; Li, X.; Xie, Y.; Chen, H. Fabrication and characterization of matrine-loaded konjac glucomannan/fish gelatin composite hydrogel as antimicrobial wound dressing. Food Hydrocoll. 2020, 104, 105702. [Google Scholar] [CrossRef]
- Riyajan, S.-A. Environmentally Friendly Novel Maleated Poly(vinyl alcohol) Grafted 1, 4-Butanediol Modified with Biopolymer for Encapsulation of Capsaicin. J. Polym. Environ. 2019, 27, 2637–2649. [Google Scholar] [CrossRef]
- Sringam, J.; Pankongadisak, P.; Trongsatitkul, T.; Suppakarn, N. Improving Mechanical Properties of Starch-Based Hydrogels Using Double Network Strategy. Polymers 2022, 14, 3552. [Google Scholar] [CrossRef]
- Perez Bravo, J.J.; Francois, N. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef]
- Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. [Google Scholar] [CrossRef]
- Wang, X.; Lü, S.; Gao, C.; Xu, X.; Wei, Y.; Bai, X.; Feng, C.; Gao, N.; Liu, M.; Wu, L. Biomass-based multifunctional fertilizer system featuring controlled-release nutrient, water-retention and amelioration of soil. RSC Adv. 2014, 4, 18382. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-poly(acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Wongthong, P.; Nakason, C.; Pan, Q.; Rempel, G.L.; Kiatkamjornwong, S. Styrene-assisted grafting of maleic anhydride onto deproteinized natural rubber. Eur. Polym. J. 2014, 59, 144–155. [Google Scholar] [CrossRef]
- Silakul, P.; Magaraphan, R. A polymer electrolyte by ozonolysis of poly(3-(trimethoxysilyl) propyl methacrylate) grafted on natural rubber latex in colloid state and its application. Iran. Polym. J. 2019, 28, 455–470. [Google Scholar] [CrossRef]
- Lamb, D.J.; Anstey, J.F.; Fellows, C.M.; Monteiro, M.J.; Gilbert, R.G. Modification of natural and artificial polymer colloids by “topology-controlled” emulsion polymerization. Biomacromolecules 2001, 2, 518–525. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Enhanced Swelling and Responsive Properties of Pineapple Peel Carboxymethyl Cellulose-g-poly(acrylic acid-co-acrylamide) Superabsorbent Hydrogel by the Introduction of Carclazyte. J. Agric. Food Chem. 2017, 65, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Galvin, C.J.; Genzer, J. Swelling of Hydrophilic Polymer Brushes by Water and Alcohol Vapors. Macromolecules 2016, 49, 4316–4329. [Google Scholar] [CrossRef]
- Nun-anan, P.; Wisunthorn, S.; Pichaiyut, S.; Nathaworn, C.D.; Nakason, C. Influence of nonrubber components on properties of unvulcanized natural rubber. Polym. Adv. Technol. 2020, 31, 44–59. [Google Scholar] [CrossRef]
- Zheng, M.; Lian, F.; Zhu, Y.; Zhang, Y.; Liu, B.; Zhang, L.; Zheng, B. pH-responsive poly (xanthan gum-g-acrylamide-g-acrylic acid) hydrogel: Preparation, characterization, and application. Carbohydr. Polym. 2019, 210, 38–46. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Zhang, Y.; Mu, C.; Zhou, J.; Zhang, W.; Shi, B. Nonswelling Silica–Poly(acrylic acid) Composite for Efficient and Simultaneous Removal of Cationic Dye, Heavy Metal, and Surfactant-Stabilized Emulsion from Wastewater. Ind. Eng. Chem. Res. 2020, 59, 3383–3393. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, X.; Wang, X.; Gu, A.; Zhang, L.; Chen, X.; Li, J.; Yu, Z. Chitosan-g-Poly(acrylic acid) Copolymer and Its Sodium Salt as Stabilized Aqueous Binders for Silicon Anodes in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16274–16283. [Google Scholar] [CrossRef]
- Abdallah, M.; Martin, M.; El Tahchi, M.R.; Balme, S.; Faour, W.H.; Varga, B.; Cloitre, T.; Páll, O.; Cuisinier, F.J.G.; Gergely, C.; et al. Influence of Hydrolyzed Polyacrylamide Hydrogel Stiffness on Podocyte Morphology, Phenotype, and Mechanical Properties. ACS Appl. Mater. Interfaces 2019, 11, 32623–32632. [Google Scholar] [CrossRef] [PubMed]
- Nghiem Thi, T.; Dao Van, H.; Cao Hong, H.; Nguyen Thi Ha, H.; Nurul Hayati, Y.; Kawahara, S. Preparation and properties of colloidal silica-filled natural rubber grafted with poly(methyl methacrylate). Polym. Bull. 2022, 79, 6011–6027. [Google Scholar] [CrossRef]
- Nuntahirun, P.; Yamamoto, O.; Paoprasert, P. Temperature-responsive N-isopropylacrylamide-grafted natural rubber. Polym. Bull. 2018, 75, 1387–1401. [Google Scholar] [CrossRef]
- Nghiem, T.; Nguyen, L.; Phan, N.; Yusof, N.; Kawahara, S. Formation of an in situ nanosilica nanomatrix via graft copolymerization of vinyltriethoxysilane onto natural rubber. Polym. Adv. Technol. 2020, 31, 482–491. [Google Scholar] [CrossRef]
- Poompradub, S.; Thirakulrati, M.; Prasassarakich, P. In situ generated silica in natural rubber latex via the sol–gel technique and properties of the silica rubber composites. Mater. Chem. Phys. 2014, 144, 122–131. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sukhlaaied, W. Fabrication and Properties of a Novel Porous Material from Biopolymer and Natural Rubber for Organic Compound Absorption. J. Polym. Environ. 2019, 27, 1918–1936. [Google Scholar] [CrossRef]
- Yin, C.; Lan, J.; Wang, X.; Zhang, Y.; Ran, R.; Shi, L.-Y. Shape-Stable Hydrated Salts/Polyacrylamide Phase-Change Organohydrogels for Smart Temperature Management. ACS Appl. Mater. Interfaces 2021, 13, 21810–21821. [Google Scholar] [CrossRef]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Kamel, E.M.; Elzanaty, A.M.; Mahmoud, A.M.; Abdel-Gawad, O.F. Eco-friendly and biodegradable sodium alginate/quaternized chitosan hydrogel for controlled release of urea and its antimicrobial activity. Carbohydr. Polym. 2022, 291, 119555. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Luo, Y.; Bai, B.; Cao, F. pH-Responsive Eco-Friendly Chitosan-Chlorella Hydrogel Beads for Water Retention and Controlled Release of Humic Acid. Water 2022, 14, 1190. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P. Superabsorbent polymers in agriculture and other applications: A review. Polym.-Plast. Technol. Mater. 2019, 59, 341–356. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Pimenta, I.F.; Figueiredo, L.R.F.; Santos, A.M.C.; Oliveira, J.E.; Medeiros, E.S. Development of controlled release fertilizer systems for KCl using glycerol-based polymers. J. Appl. Polym. Sci. 2022, 139, 51747. [Google Scholar] [CrossRef]
- Ureña-Amate, M.D.; Socias-Viciana, M.D.; Urbano-Juan, M.D.; García-Alcaraz, M.D. Effects of pH and Crosslinking Agent in the Evaluation of Hydrogels as Potential Nitrate-Controlled Release Systems. Polymers 2023, 15, 1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Tong, Z.; Gao, B.; Gao, N.; Shen, T.; Wan, Y.; Yu, Z.; Liu, L.; Ma, X.; et al. Self-Assembly of Hydrophobic and Self-Healing Bionanocomposite-Coated Controlled-Release Fertilizers. ACS Appl. Mater. Interfaces 2020, 12, 27598–27606. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Review on Magnetic Natural Polymer Constructed Hydrogels as Vehicles for Drug Delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]




| Ingredients | Contents (phr) | |||
|---|---|---|---|---|
| M0.50/P30-DPNR | M0.50/P30-DPNR /Si10 | M0.50/P30-DPNR /Si20 | M0.50/P30-DPNR /Si30 | |
| DPNR | 100 | 100 | 100 | 100 |
| Terric16A | 5 | 5 | 5 | 5 |
| CHP | 1 | 1 | 1 | 1 |
| TEPA | 1 | 1 | 1 | 1 |
| Acrylic acid | 15 | 15 | 15 | 15 |
| Acrylamide | 15 | 15 | 15 | 15 |
| MBA * | 0.50 | 0.50 | 0.50 | 0.50 |
| Silica | 0 | 10 | 20 | 30 |
| Samples | T10 | Tmax | Residue at 600 °C |
|---|---|---|---|
| (°C) | (°C) | (%) | |
| MBA0.50/P30-DPNR | 280.3 | 378.5 | 4.52 |
| MBA0.50/P30-DPNR/Si10 | 281.5 | 381.5 | 10.47 |
| MBA0.50/P30-DPNR/Si20 | 284.7 | 383.3 | 13.68 |
| MBA0.50/P30-DPNR/Si30 | 289.0 | 384.2 | 26.03 |
| Samples | Compressive Strength at 80% Strain | Compressive Modulus at 5–10% Strain |
|---|---|---|
| (MPa) | (MPa) | |
| MBA0.50/P30-DPNR | 10.71 ± 0.94 | 0.71 ± 0.13 |
| MBA0.50/P30-DPNR/Si10 | 12.74 ± 0.93 | 0.84 ± 0.20 |
| MBA0.50/P30-DPNR/Si20 | 17.29 ± 1.99 | 1.10 ± 0.13 |
| MBA0.50/P30-DPNR/Si30 | 14.27 ± 1.64 | 0.98 ± 0.23 |
| Samples | R2 | k (d−1) | n | |||
|---|---|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |||
| CS | 0.6419 | 0.7126 | 0.7361 | 0.7541 | 30.64 | 0.5427 |
| M0.50/P30-DPNR/CS | 0.8218 | 0.8621 | 0.9098 | 0.9020 | 21.35 | 0.8096 |
| M0.50/P30-DPNR/Si10/CS | 0.8422 | 0.8776 | 0.9233 | 0.9239 | 20.08 | 0.8321 |
| M0.50/P30-DPNR/Si20/CS | 0.9298 | 0.9417 | 0.9647 | 0.9702 | 9.57 | 0.9519 |
| M0.50/P30-DPNR/Si30/CS | 0.8480 | 0.8541 | 0.9144 | 0.9437 | 5.54 | 0.9841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inphonlek, S.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer. Polymers 2023, 15, 1770. https://doi.org/10.3390/polym15071770
Inphonlek S, Jarukumjorn K, Chumsamrong P, Ruksakulpiwat C, Ruksakulpiwat Y. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer. Polymers. 2023; 15(7):1770. https://doi.org/10.3390/polym15071770
Chicago/Turabian StyleInphonlek, Supharat, Kasama Jarukumjorn, Pranee Chumsamrong, Chaiwat Ruksakulpiwat, and Yupaporn Ruksakulpiwat. 2023. "Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer" Polymers 15, no. 7: 1770. https://doi.org/10.3390/polym15071770