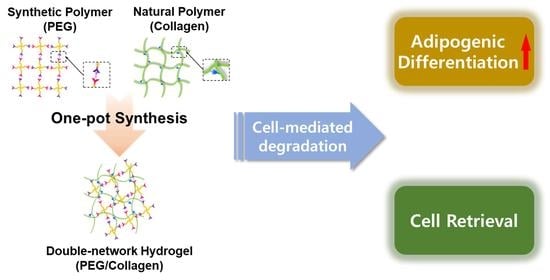
One-Pot Synthesis of Double-Network PEG/Collagen Hydrogel for Enhanced Adipogenic Differentiation and Retrieval of Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
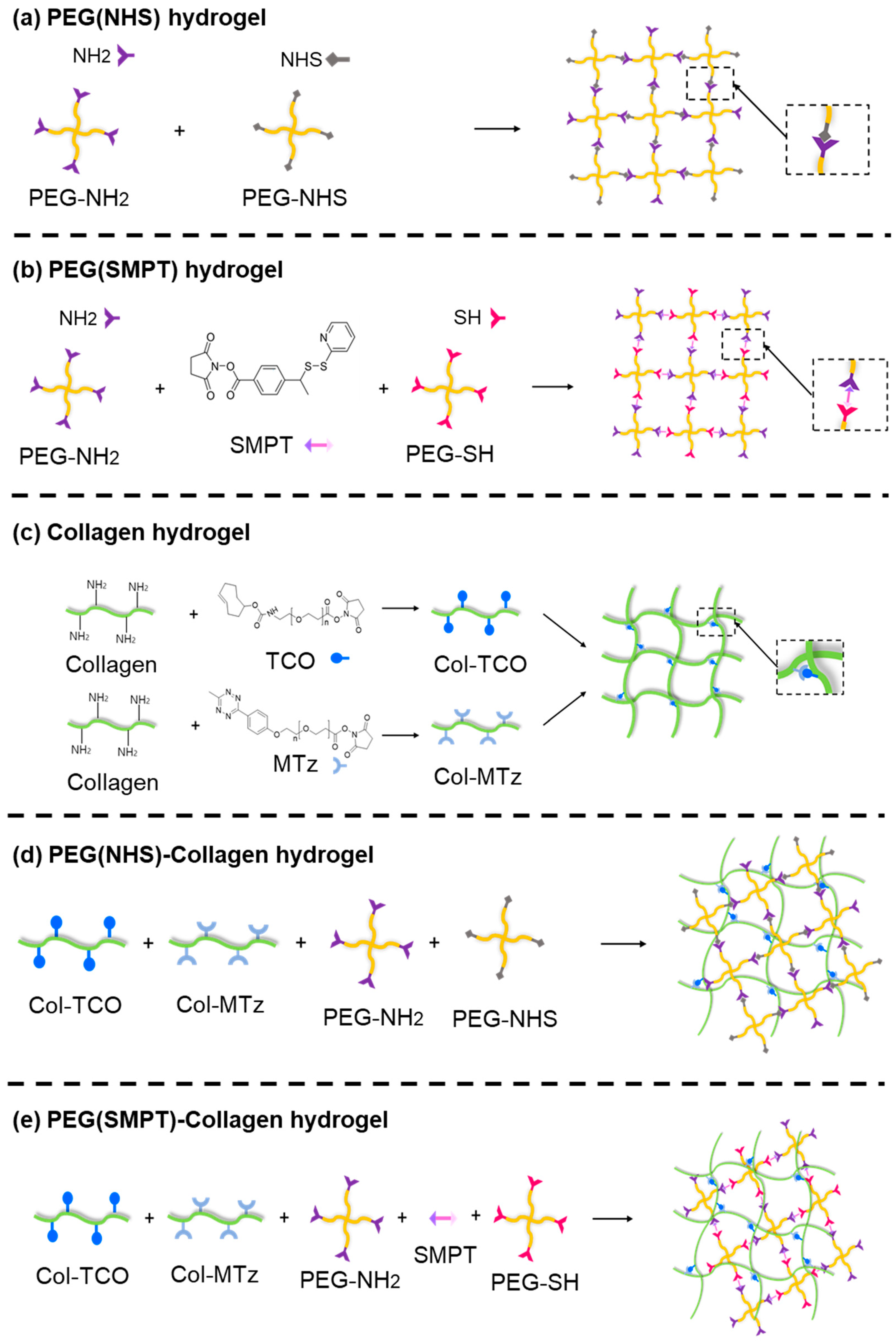
2.2. Synthesis of Single-Crosslinked Hydrogels
2.2.1. PEG (NHS) Hydrogel
2.2.2. PEG (SMPT) Hydrogel
2.2.3. Collagen Hydrogel
2.3. One-Pot Synthesis of Double-Network Hydrogel
2.3.1. PEG (NHS)-Collagen Hydrogel
2.3.2. PEG (SMPT)-Collagen Hydrogel
2.4. Rheological Characterization of Hydrogel
2.5. Cell Culture and Encapsulation
2.6. Analysis of Cell Morphology and Cell Viability
2.7. Oil Red O Staining
2.8. QPCR Analysis
2.9. Cell Retrieval
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Hydrogels
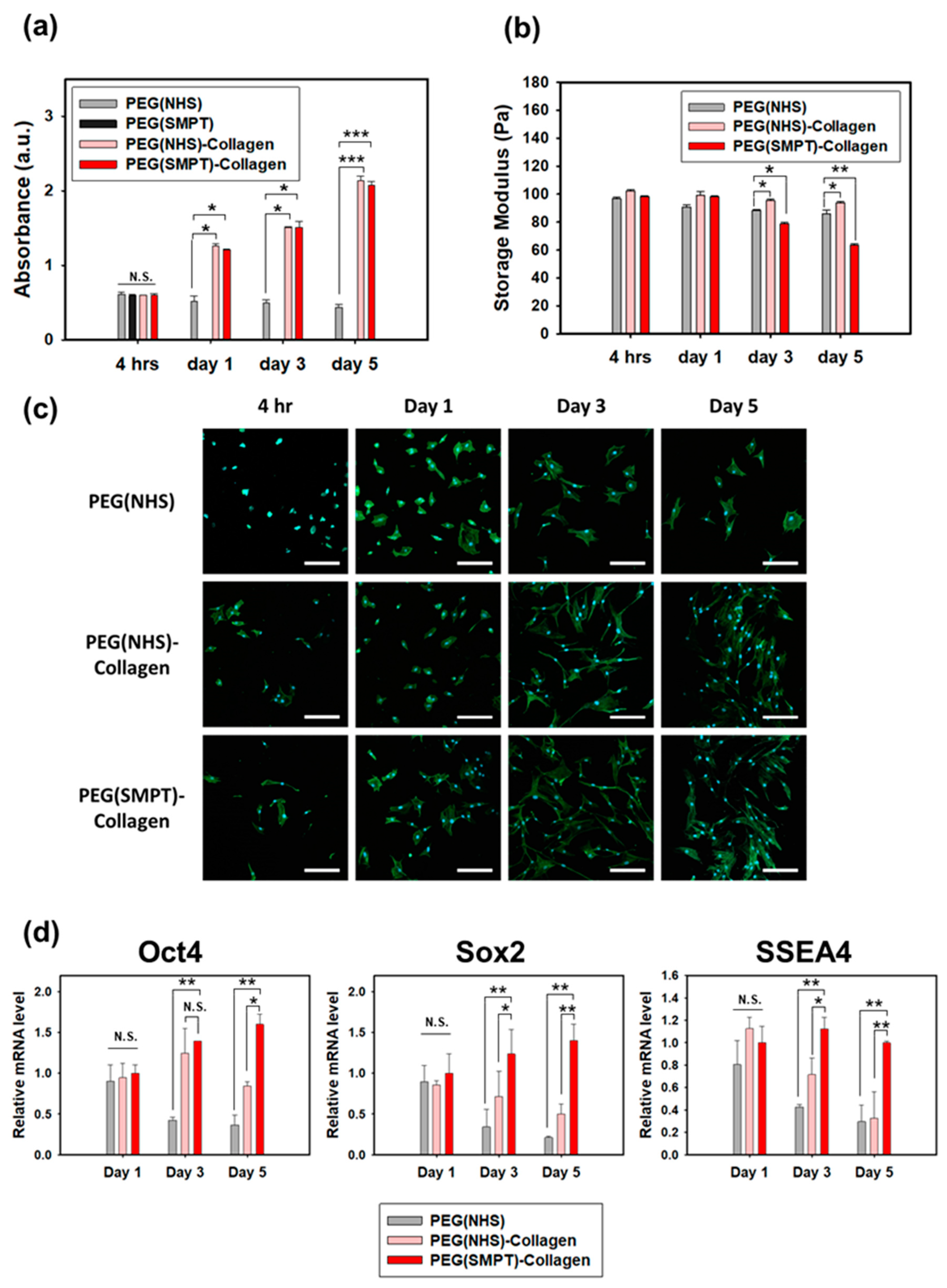
3.2. Rheological Properties of Hydrogels
3.3. ADSC Culture within the Hydrogels
3.4. Adipogenic Differentiation in the Hydrogel
3.5. Cell Retrieval
4. Further Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safina, I.; Embree, M.C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater. 2022, 143, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Butler, P.E.; Seifalian, A.M. Adipose-derived stem cells for clinical applications: A review. Cell Prolif. 2010, 44, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Katz, A.J.; Bunnell, B. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, B.; Al-Ghadban, S.; Ives, C.; L’Ecuyer, M.; Monjure, T.; Romero-Lopez, M.; Li, Z.; Goodman, S.; Lin, H.; Tuan, R.; et al. Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors. Biomolecules 2020, 10, 1070. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef] [Green Version]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [Green Version]
- Cushing, M.; Anseth, K. Hydrogel cell cultures. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Marchant, R. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Kratochvil, M.J.; Seymour, A.J.; Li, T.L.; Paşca, S.P.; Kuo, C.J.; Heilshorn, S.C. Engineered materials for organoid systems. Nat. Rev. Mater. 2019, 4, 606–622. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Abdeen, A.A.; Zhang, D.; Kilian, K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef]
- Shen, W.; Zheng, J.; Zhou, Z.; Zhang, D. Approaches for the synthesis of o-nitrobenzyl and coumarin linkers for use in photocleavable biomaterials and bioconjugates and their biomedical applications. Acta Biomater. 2020, 115, 75–91. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Li, Y.; Zhou, Z.; Cheng, Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials 2017, 112, 133–140. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, X. Injectable and degradable pH-responsive hydrogels via spontaneous amino–yne click reaction. ACS Appl. Mater. Interfaces 2018, 10, 361–370. [Google Scholar] [CrossRef]
- Altinbasak, I.; Arslan, M.; Sanyal, R.; Sanyal, A. Pyridyl disulfide-based thiol–disulfide exchange reaction: Shaping the design of redox-responsive polymeric materials. Polym. Chem. 2020, 11, 7603–7624. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kalkhof, S.; Sinz, A. Chances and pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Anal. Bioanal. Chem. 2008, 392, 305–312. [Google Scholar] [CrossRef]
- Abello, N.; Kerstjens, H.A.M.; Postma, D.S.; Bischoff, R. Selective Acylation of Primary Amines in Peptides and Proteins. J. Proteome Res. 2007, 6, 4770–4776. [Google Scholar] [CrossRef]
- Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Camci-Unal, G.; Patel, A.; Khademhosseini, A.; Kanamori, T. Activated-Ester-Type Photocleavable Crosslinker for Preparation of Photodegradable Hydrogels Using a Two-Component Mixing Reaction. Adv. Healthc. Mater. 2015, 4, 246–254. [Google Scholar] [CrossRef]
- Hafeez, S.; Ooi, H.W.; Suylen, D.; Duimel, H.; Hackeng, T.M.; van Blitterswijk, C.; Baker, M.B. Desymmetrization via activated esters enables rapid synthesis of multifunctional benzene-1, 3, 5-tricarboxamides and creation of supramolecular hydrogelators. J. Am. Chem. Soc. 2022, 144, 4057–4070. [Google Scholar] [CrossRef]
- Stenzel, M. Bioconjugation using thiols: Old chemistry rediscovered to connect polymers with nature’s building blocks. ACS Macro Lett. 2013, 2, 14–18. [Google Scholar] [CrossRef]
- Carlson, J.C.T.; Mikula, H.; Weissleder, R. Unraveling tetrazine-triggered bioorthogonal elimination enables chemical tools for ultrafast release and universal cleavage. J. Am. Chem. Soc. 2018, 140, 3603–3612. [Google Scholar] [CrossRef] [Green Version]
- Bakirdogen, G.; Kahveci, E.L.S.; Kahveci, M.U. Fast and efficient preparation of three-arm star block copolymers via tetrazine ligation. Eur. Polym. J. 2020, 140, 110027. [Google Scholar] [CrossRef]
- Braun, K.; Wiessler, M.; Pipkorn, R.; Ehemann, V.; Bäuerle, T.; Fleischhacker, H.; Müller, G.; Lorenz, P.; Waldeck, W. A cyclic-RGD-bioshuttle functionalized with TMZ by DARinv “Click Chemistry” targeted to αvβ3 integrin for therapy. Int. J. Med. Sci. 2010, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Denk, C.; Svatunek, D.; Filip, T.; Wanek, T.; Lumpi, D.; Fröhlich, J.; Kuntner, P.-D.D.C.; Mikula, H. Development of a 18F-Labeled Tetrazine with Favorable Pharmacokinetics for Bioorthogonal PET Imaging. Angew. Chem. Int. Ed. 2014, 53, 9655–9659. [Google Scholar] [CrossRef]
- Johann, K.; Svatunek, D.; Seidl, C.; Rizzelli, S.; Bauer, T.A.; Braun, L.; Koynov, K.; Mikula, H.; Barz, M. Tetrazine- and trans-cyclooctene-functionalised polypept(o)ides for fast bioorthogonal tetrazine ligation. Polym. Chem. 2020, 11, 4396–4407. [Google Scholar] [CrossRef]
- Biswas, N.; Waring, A.J.; Walther, F.J.; Dluhy, R.A. Structure and conformation of the disulfide bond in dimeric lung surfactant peptides SP-B1–25 and SP-B8–25. Biochim. Biophys. Acta BBA–Biomembr. 2007, 1768, 1070–1082. [Google Scholar] [CrossRef] [Green Version]
- Suys, O.; Derenne, A.; Goormaghtigh, E. ATR-FTIR Biosensors for Antibody Detection and Analysis. Int. J. Mol. Sci. 2022, 23, 11895. [Google Scholar] [CrossRef]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Novel Thiol-Ene Hybrid Coating for Metal Protection. Coatings 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Sihota, P.; Yadav, R.N.; Dhiman, V.; Bhadada, S.K.; Mehandia, V.; Kumar, N. Investigation of diabetic patient’s fingernail quality to monitor type 2 diabetes induced tissue damage. Sci. Rep. 2019, 9, 3193. [Google Scholar] [CrossRef]
- Sevier, C.; Kaiser, C. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 2002, 3, 836–847. [Google Scholar] [CrossRef]
- Dmitrenko, O.; Thorpe, C.; Bach, R. Mechanism of SN2 disulfide bond cleavage by phosphorus nucleophiles: Implications for biochemical disulfide reducing agents. J. Org. Chem. 2007, 72, 8298–8307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldshmid, R.; Seliktar, D. Hydrogel Modulus Affects Proliferation Rate and Pluripotency of Human Mesenchymal Stem Cells Grown in Three-Dimensional Culture. ACS Biomater. Sci. Eng. 2017, 3, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.M.; Kyburz, K.A.; Anseth, K.S. Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad. Sci. USA 2015, 112, E3757–E3764. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Panopoulos, A.; Herrerías, A.; Bissig, K.-D.; Lutz, M.; Berggren, W.T.; Verma, I.M.; Belmonte, J.C.I. A High Proliferation Rate Is Required for Cell Reprogramming and Maintenance of Human Embryonic Stem Cell Identity. Curr. Biol. 2011, 21, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Cao, L.; Li, S.; Yu, L.; Ding, J. Interplay of matrix stiffness and cell–cell contact in regulating differentiation of stem cells. ACS Appl. Mater. Interfaces 2016, 8, 21903–21913. [Google Scholar] [CrossRef]
- Su, T.; Xu, M.; Lu, F.; Chang, Q. Adipogenesis or osteogenesis: Destiny decision made by mechanical properties of biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef]
- Das, R.K.; Gocheva, V.; Hammink, R.; Zouani, O.F.; Rowan, A.E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016, 15, 318–325. [Google Scholar] [CrossRef]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [Green Version]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta BBA–Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Yagyu, H.; Chen, G.; Yokoyama, M.; Hirata, K.; Augustus, A.; Kako, Y.; Seo, T.; Hu, Y.; Lutz, E.P.; Merkel, M.; et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Investig. 2003, 111, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Tenenbaum, A. Adiponectin: A manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc. Diabetol. 2014, 13, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urs, S.; Harrington, A.; Liaw, L.; Small, D. Selective expression of an aP2/Fatty Acid Binding Protein4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006, 15, 647–653. [Google Scholar] [CrossRef]
- Chakrabarti, P. Promoting Adipose Specificity: The Adiponectin Promoter. Endocrinology 2010, 151, 2408–2410. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]




| Gene | Primer Sequences | |
|---|---|---|
| Β-actin | Sense | ACTACCTTCAACTCCATC |
| Antisense | TGATCTTGATCTTCATTGTG | |
| Oct4 | Sense | ACATCAAAGCTCTGCAGAAA |
| Antisense | CTGAATACCTTCCCAAATAGAAC | |
| Sox2 | Sense | TGCGAGCGCTGCACAT |
| Antisense | GCAGCGTGTACTTATCCTTCTTCA | |
| SSEA4 | Sense | TGGACGGGCACAACTTCATC |
| Antisense | GGGCAGGTTCTTGGCACTCT | |
| Adiponectin | Sense | TGGTGAGAAGGGTGAGAA |
| Antisense | AGATCTTGGTAAAGCGAATG | |
| aP2 | Sense | TGCAGCTTCCTTCTCACCTTGA |
| Antisense | TCCTGGCCCAGTATGAAGGAAATC | |
| PPAR-γ | Sense | ATGACAGCGACTTGGCAA |
| Antisense | AATGTTGGCAGTGGCTCA | |
| Non-Degradable | Degradable | ||
|---|---|---|---|
| Single crosslink | Natural | Collagen | |
| Synthetic | PEG (NHS) | PEG (SMPT) | |
| Double crosslink | Hybrid | PEG (NHS)-Collagen | PEG (SMPT)-Collagen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Hong, H.J.; Ahn, S.; Kim, D.; Kang, S.H.; Cho, K.; Koh, W.-G. One-Pot Synthesis of Double-Network PEG/Collagen Hydrogel for Enhanced Adipogenic Differentiation and Retrieval of Adipose-Derived Stem Cells. Polymers 2023, 15, 1777. https://doi.org/10.3390/polym15071777
Lee H, Hong HJ, Ahn S, Kim D, Kang SH, Cho K, Koh W-G. One-Pot Synthesis of Double-Network PEG/Collagen Hydrogel for Enhanced Adipogenic Differentiation and Retrieval of Adipose-Derived Stem Cells. Polymers. 2023; 15(7):1777. https://doi.org/10.3390/polym15071777
Chicago/Turabian StyleLee, Hwajung, Hye Jin Hong, Sujeong Ahn, Dohyun Kim, Shin Hyuk Kang, Kanghee Cho, and Won-Gun Koh. 2023. "One-Pot Synthesis of Double-Network PEG/Collagen Hydrogel for Enhanced Adipogenic Differentiation and Retrieval of Adipose-Derived Stem Cells" Polymers 15, no. 7: 1777. https://doi.org/10.3390/polym15071777