Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
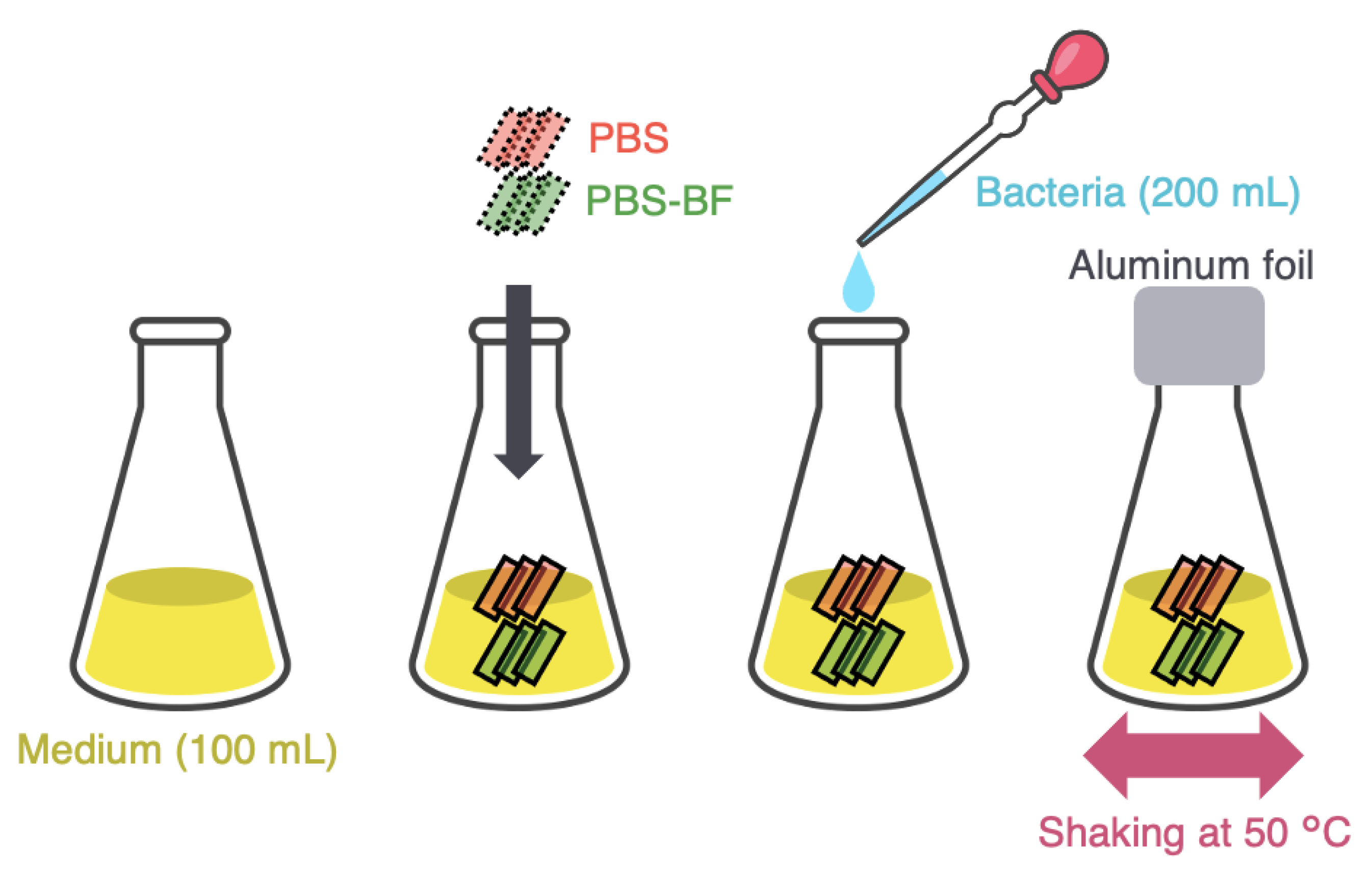
2.2. Microbial Degradation Test
2.3. Tensile Test
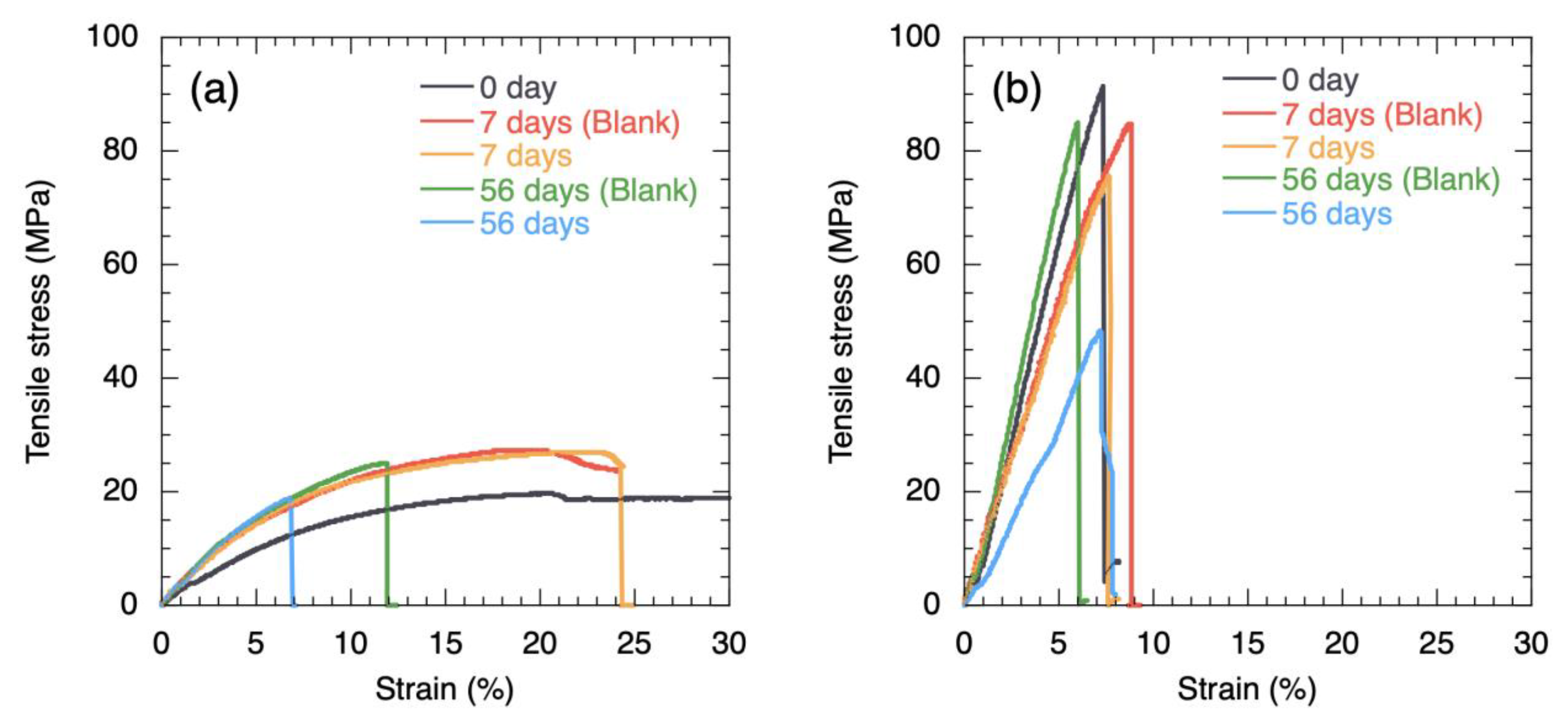
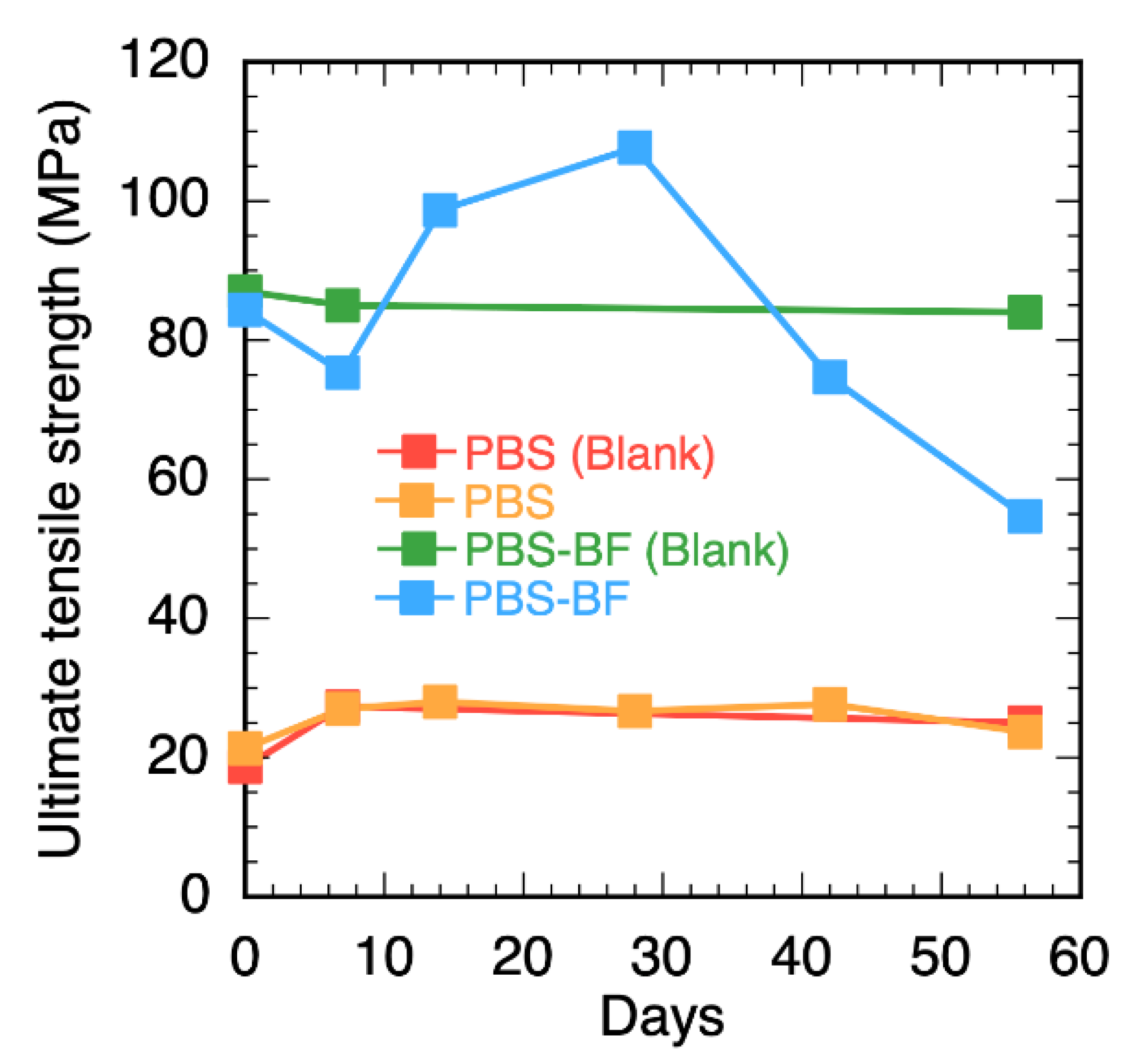
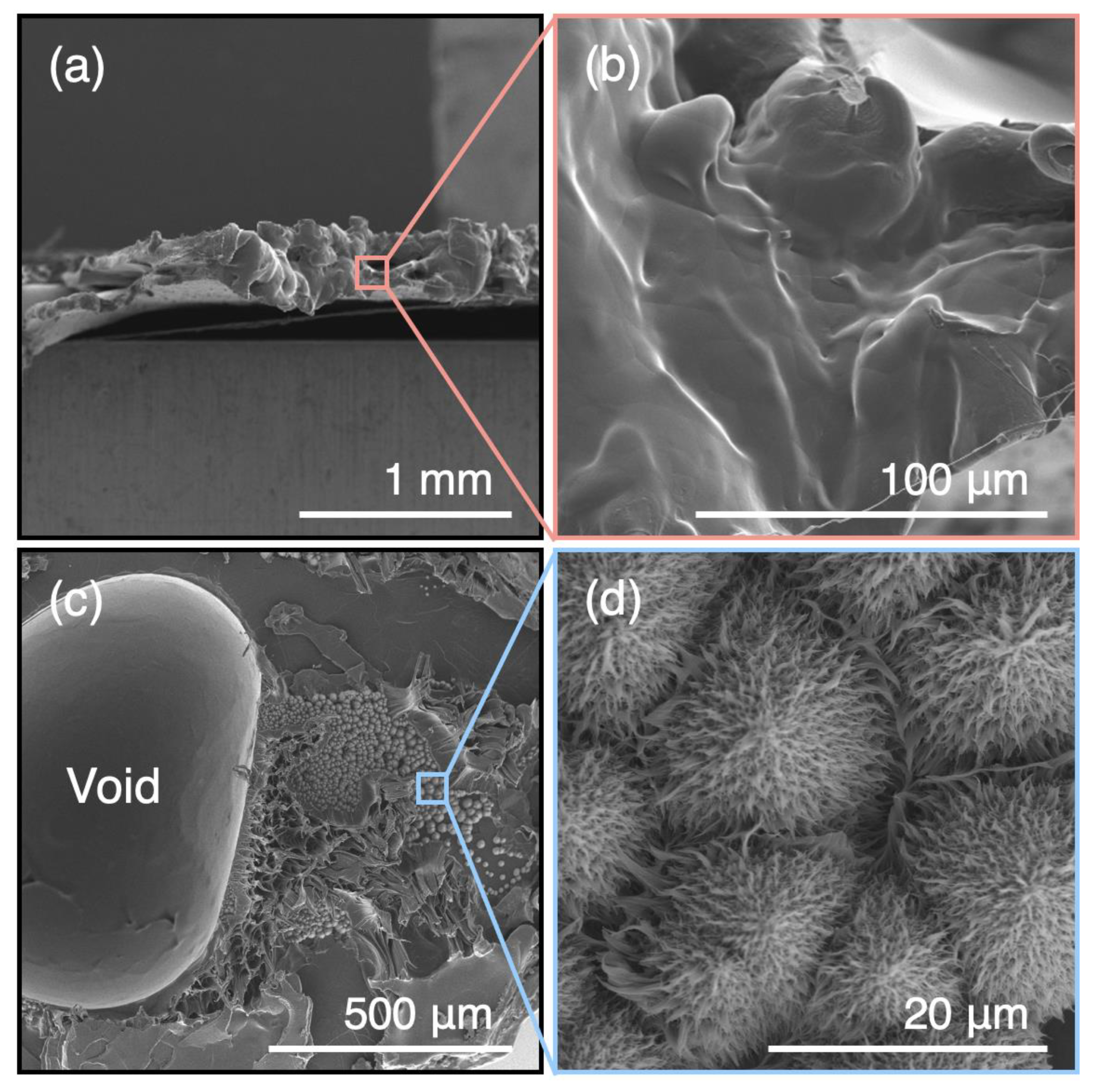
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Phil. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, B.-H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Moussa, H. Life Cycle Assessment of a Hybrid Poly Butylene Succinate Composite. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [Green Version]
- Zini, E.; Scandola, M. Green Composites: An Overview. Polym. Compos. 2011, 32, 1905–1915. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Progress Report on Natural Fiber Reinforced Composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Shaiju, P.; Dorian, B.-B.; Senthamaraikannan, R.; Padamati, R.B. Biodegradation of Poly (Butylene Succinate) (PBS)/Stearate Modified Magnesium-Aluminium Layered Double Hydroxide Composites under Marine Conditions Prepared via Melt Compounding. Molecules 2020, 25, 5766. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Thermo-Mechanical Properties and Creep Modelling of Wine Lees Filled Polyamide 11 (PA11) and Polybutylene Succinate (PBS) Bio-Composites. Compos. Sci. Technol. 2020, 188, 107974. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, L.; Lin, P.; Yu, Q.; Han, Q.; Huang, S.; Zhang, B.; Yin, X.; Wu, J.; Yang, F.; et al. Intense Shear Induced Caterpillar-like Continuous Hierarchical Fiber Enhanced Poly(Butylene Succinate) Biocomposite towards Strong Mechanical Performance. Compos B Eng. 2020, 200, 108273. [Google Scholar] [CrossRef]
- Xie, Y.; Kurita, H.; Ishigami, R.; Narita, F. Assessing the Flexural Properties of Epoxy Composites with Extremely Low Addition of Cellulose Nanofiber Content. Appl. Sci. 2020, 10, 1159. [Google Scholar] [CrossRef] [Green Version]
- Kurita, H.; Ishigami, R.; Wu, C.; Narita, F. Mechanical Properties of Mechanically-Defibrated Cellulose Nanofiber Reinforced Epoxy Resin Matrix Composites. J. Compos. Mater. 2021, 55, 455–464. [Google Scholar] [CrossRef]
- Wu, C.; Egawa, S.; Kanno, T.; Kurita, H.; Wang, Z.; Iida, E.; Narita, F. Nanocellulose Reinforced Silkworm Silk Fibers for Application to Biodegradable Polymers. Mater. Des. 2021, 202, 109537. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Biocomposites Based on Poly(Butylene Succinate) and Curaua: Mechanical and Morphological Properties. Polym. Test. 2015, 45, 168–173. [Google Scholar] [CrossRef]
- Lopresto, V.; Leone, C.; De Iorio, I. Mechanical Characterisation of Basalt Fibre Reinforced Plastic. Compos. B Eng. 2011, 42, 717–723. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A Review on Basalt Fibre and Its Composites. Compos. B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Borhan, T.M. Properties of Glass Concrete Reinforced with Short Basalt Fibre. Mater. Des. 2012, 42, 265–271. [Google Scholar] [CrossRef]
- Li, Y.; Sang, L.; Wei, Z.; Ding, C.; Chang, Y.; Chen, G.; Zhang, W.; Liang, J. Mechanical Properties and Crystallization Behavior of Poly(Butylene Succinate) Composites Reinforced with Basalt Fiber. J. Therm. Anal. Calorim. 2015, 122, 261–270. [Google Scholar] [CrossRef]
- Shen, Y.; Gallet-Pandellé, A.; Kurita, H.; Narita, F. Fabrication, Tensile Properties, and Photodecomposition of Basalt Fiber-Reinforced Cellulose Acetate Matrix Composites. Polymers 2021, 13, 3944. [Google Scholar] [CrossRef]
- Gao, L.; Adesina, A.; Das, S. Properties of Eco-Friendly Basalt Fibre Reinforced Concrete Designed by Taguchi Method. Constr. Build. Mater. 2021, 302, 124161. [Google Scholar] [CrossRef]
- Jarerat, A.; Tokiwa, Y. Degradation of Poly(Tetramethylene Succinate) by Thermophilic Actinomycetes. Biotechnol. Lett. 2001, 23, 647–651. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic Degradation of Biodegradable Polyesters under Simulated Environmental Conditions. J. Appl. Polym. Sci. 2015, 132, 42189. [Google Scholar] [CrossRef]
- Cho, K.; Lee, J.; Kwon, K. Hydrolytic Degradation Behavior of Poly(Butylene Succinate)s with Different Crystalline Morphologies. J. Appl. Polym. Sci. 2001, 79, 1025–1033. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Wongwaiwattanakul, C.; Chaiwutthinan, P.; Prasassarakich, P. Biodegradable Poly(Lactic Acid)/Poly(Butylene Succinate)/Wood Flour Composites: Physical and Morphological Properties. Polym. Compos. 2017, 38, 2841–2851. [Google Scholar] [CrossRef]
- Sirichalarmkul, A.; Kaewpirom, S. Enhanced Biodegradation and Processability of Biodegradable Package from Poly(Lactic Acid)/Poly(Butylene Succinate)/Rice-husk Green Composites. J. Appl. Polym. Sci. 2021, 138, 50652. [Google Scholar] [CrossRef]
- Cousin, P.; Hassan, M.; Vijay, P.; Robert, M.; Benmokrane, B. Chemical Resistance of Carbon, Basalt, and Glass Fibers Used in FRP Reinforcing Bars. J. Compos. Mater. 2019, 53, 3651–3670. [Google Scholar] [CrossRef]
- Bhat, T.; Fortomaris, D.; Kandare, E.; Mouritz, A.P. Properties of Thermally Recycled Basalt Fibres and Basalt Fibre Composites. J. Mater. Sci. 2018, 53, 1933–1944. [Google Scholar] [CrossRef]
- Persico, L.; Giacalone, G.; Cristalli, B.; Tufano, C.; Saccorotti, E.; Casalone, P.; Mattiazzo, G. Recycling Process of a Basalt Fiber-Epoxy Laminate by Solvolysis: Mechanical and Optical Tests. Fibers 2022, 10, 55. [Google Scholar] [CrossRef]


| Composite Materials | Test Duration (Days) | UTS (MPa) | Source | ||
|---|---|---|---|---|---|
| PBS [Before Test] | Composite [Before Test] | Composite [After Test] | |||
| PBS–BF | 56 | 20 | 86 | 54 | This study |
| PLA/PBS–wood flour | 90 | 40 | 50 | 45 | [25] |
| PLA/PBS–rice husks | 180 | 35 | 22 | 12 | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rova, L.; Kurita, H.; Kudo, S.; Hatayama, S.; Kanno, T.; Gallet--Pandellé, A.; Narita, F. Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation. Polymers 2023, 15, 1796. https://doi.org/10.3390/polym15071796
Rova L, Kurita H, Kudo S, Hatayama S, Kanno T, Gallet--Pandellé A, Narita F. Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation. Polymers. 2023; 15(7):1796. https://doi.org/10.3390/polym15071796
Chicago/Turabian StyleRova, Lovisa, Hiroki Kurita, Shinji Kudo, Sho Hatayama, Teruyoshi Kanno, Alia Gallet--Pandellé, and Fumio Narita. 2023. "Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation" Polymers 15, no. 7: 1796. https://doi.org/10.3390/polym15071796
APA StyleRova, L., Kurita, H., Kudo, S., Hatayama, S., Kanno, T., Gallet--Pandellé, A., & Narita, F. (2023). Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation. Polymers, 15(7), 1796. https://doi.org/10.3390/polym15071796










