Clove Essential Oil Pickering Emulsions Stabilized with Lactoferrin/Fucoidan Complexes: Stability and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LF–FD Complexes
2.3. Characterization of LF–FD Complexes
2.3.1. Determination of Turbidity
2.3.2. Particle Size, Polydispersity Index (PdI) and Zeta Potential
2.3.3. Analysis of LF–FD Complexes Micromorphology
2.3.4. Determination of Contact Angles
2.3.5. Analysis of Fourier Transform Infrared (FTIR) Spectroscopy
2.3.6. Analysis of Differential Scanning Calorimetry (DSC)
2.4. Preparation of Pickering Emulsions
2.5. Characterization of the Pickering Emulsions
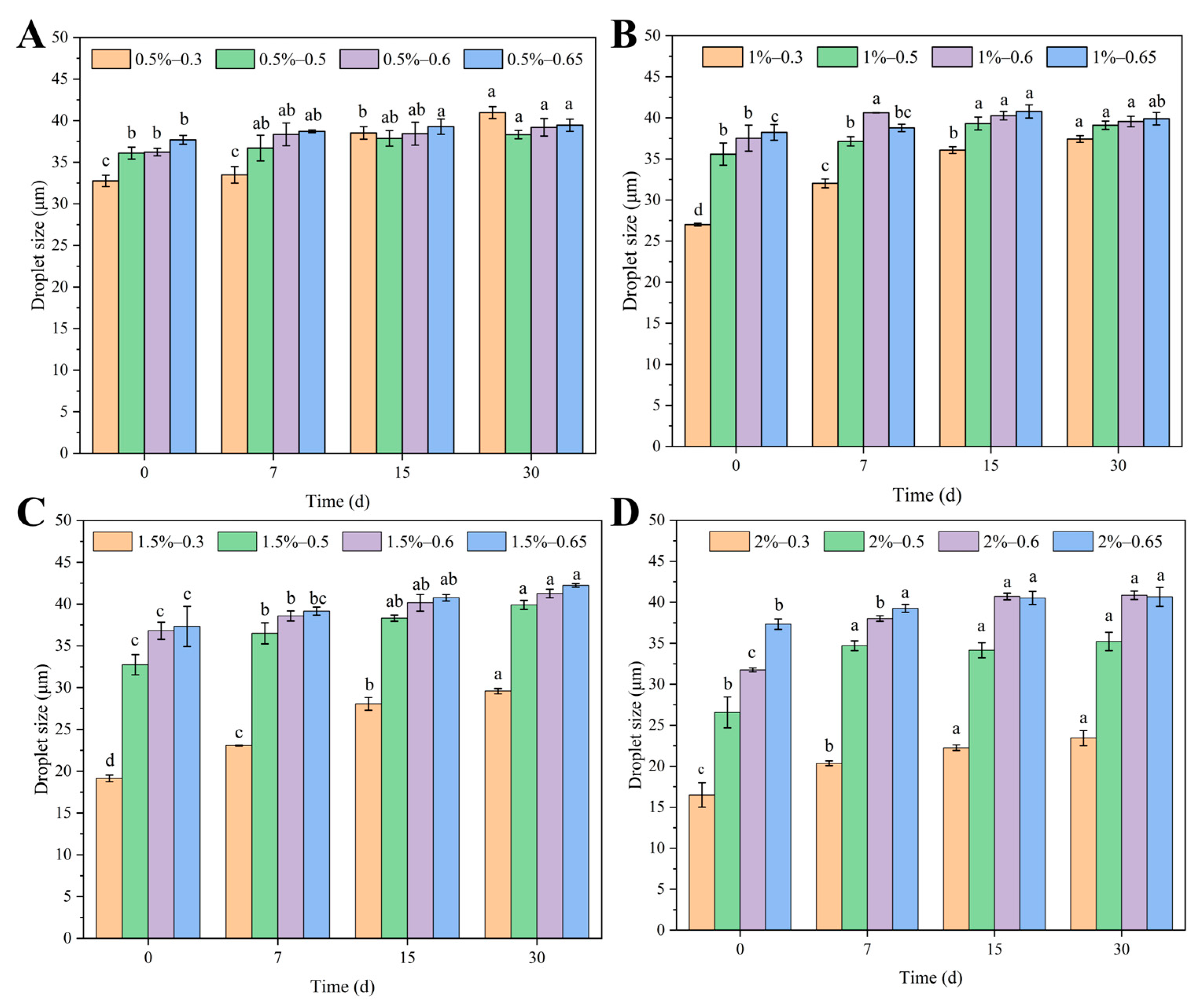
2.5.1. Type of Emulsion, Appearance, and Droplet Size
2.5.2. Visual Assessment of Morphology
2.6. The Stability of Pickering Emulsions
2.6.1. Storage Stability
2.6.2. Centrifugal Stability
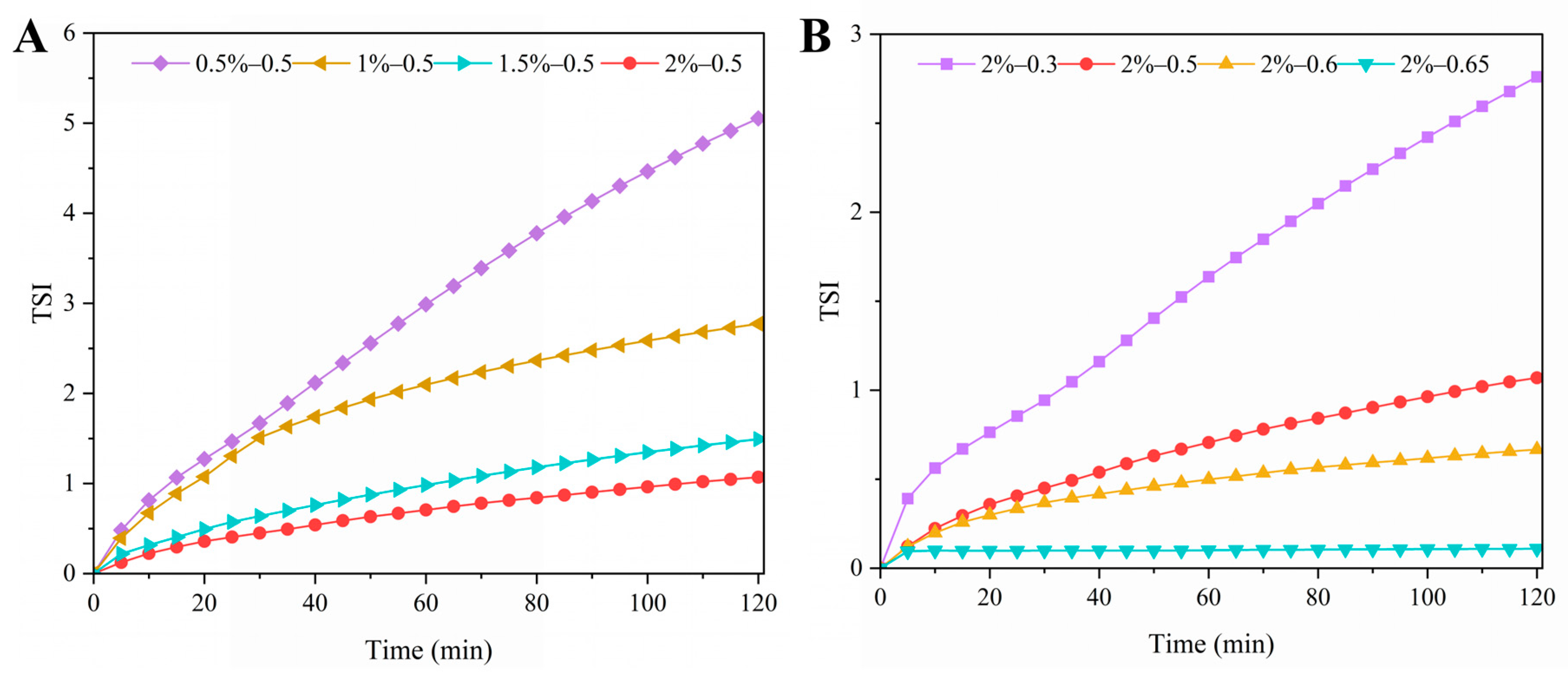
2.6.3. Turbiscan Stability Index (TSI) and Difference in Backscattered Light (ΔBS)
2.7. Rheological Properties of Pickering Emulsions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the LF–FD Complexes
3.1.1. Effect of pH on LF–FD Interactions
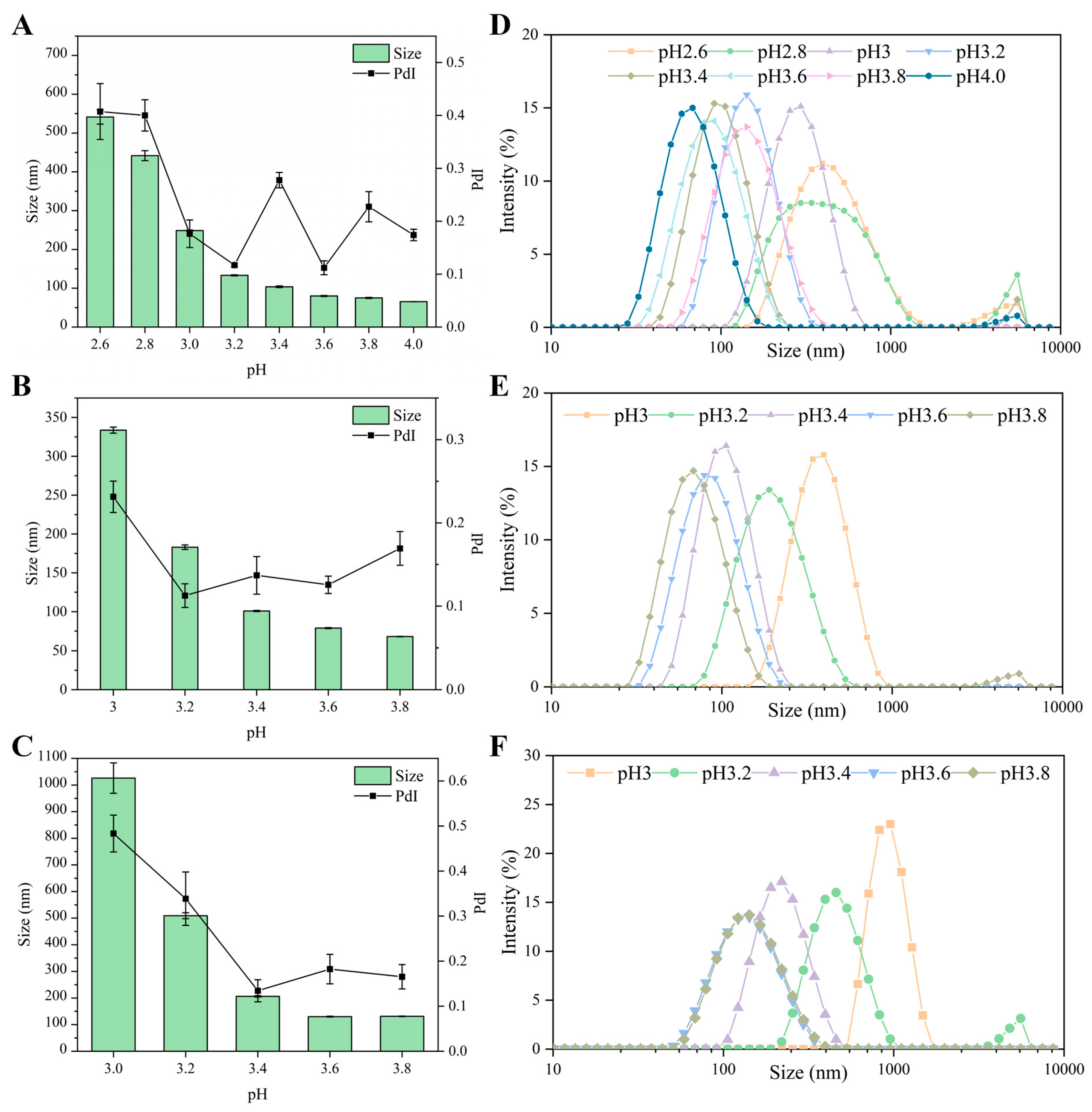
3.1.2. Effect of pH on Particle Size
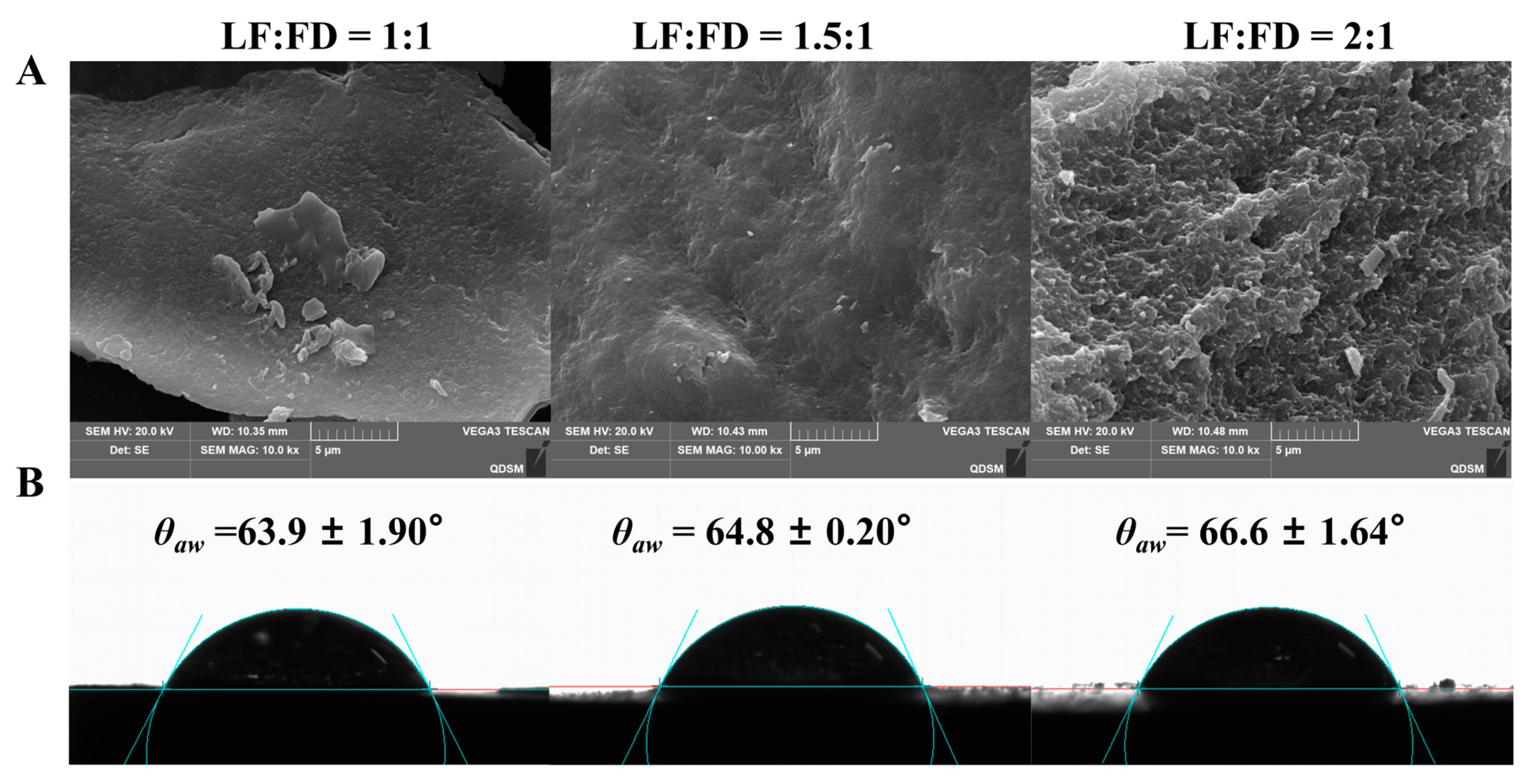
3.1.3. The Micromorphology Characters of LF–FD Complexes
3.1.4. Contact Angle Analysis of the LF–FD Complexes
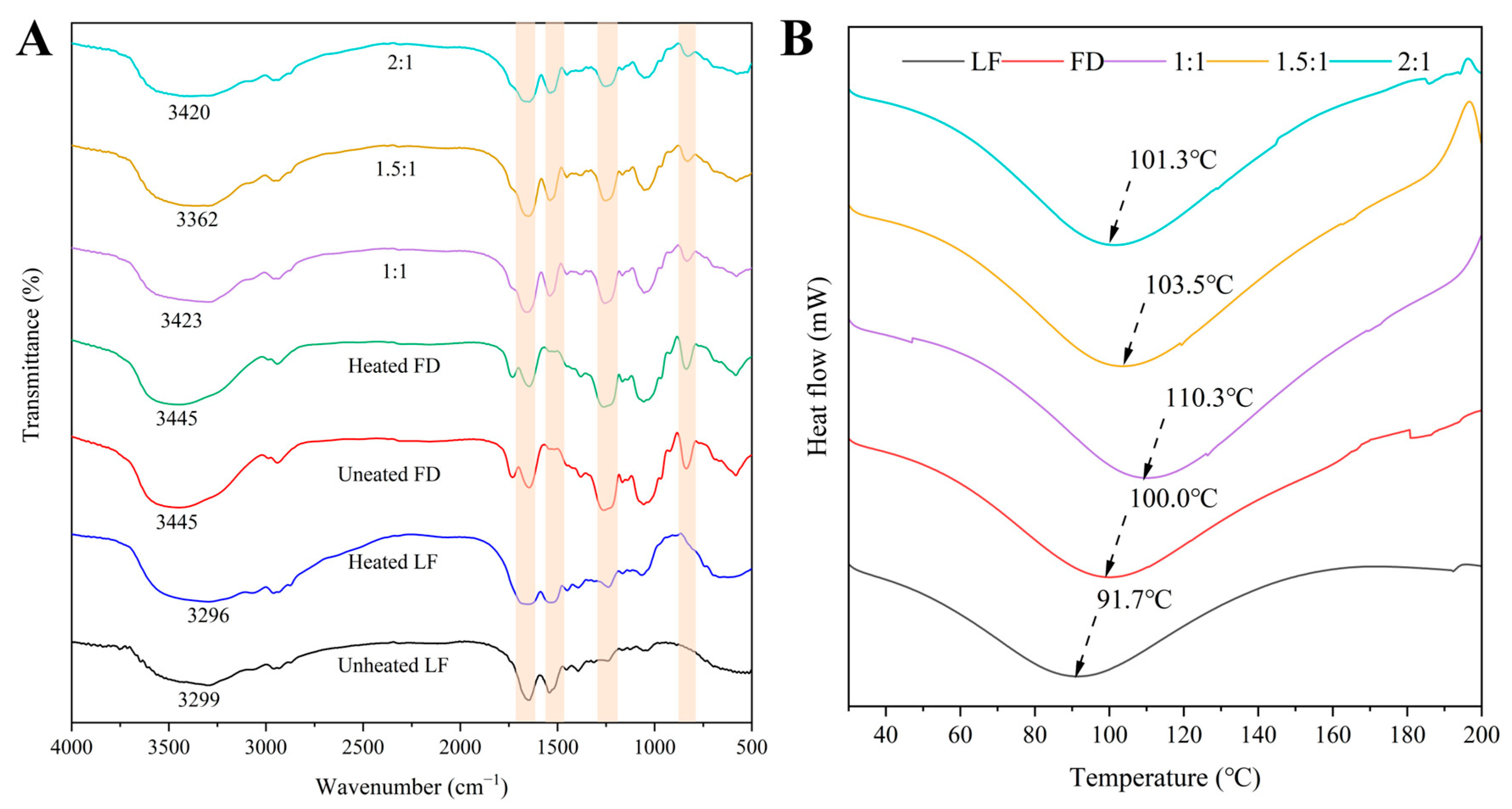
3.1.5. The FTIR Spectra of the LF–FD Complexes
3.1.6. The DSC Analysis of the LF–FD Complexes
3.2. Characterization of Pickering Emulsions
3.2.1. Type of Emulsion
3.2.2. Storage Stability
3.2.3. Centrifugal Stability and Heating Stability
3.2.4. Microstructure Observation
3.2.5. TSI
3.2.6. ΔBS
3.2.7. Rheological Properties of Pickering Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Tsabet, È.; Fradette, L. Study of the properties of oil, particles, and water on particle adsorption dynamics at an oil/water interface using the colloidal probe technique. Chem. Eng. Res. Des. 2016, 109, 307–316. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Li, Y.; Huang, Q. Assembly of Pickering emulsions using milled starch particles with different amylose/amylopectin ratios. Food Hydrocoll. 2018, 84, 47–57. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Cunha, R.L. One-step ultrasound producing O/W emulsions stabilized by chitosan particles. Food Res. Int. 2018, 107, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X. In vitro digestion of Pickering emulsions stabilized by soft whey protein microgel particles: Influence of thermal treatment. Soft Matter 2016, 12, 3558–3569. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.; Xue, C.; Tang, Q.; Zhang, T.; Chang, Y.; Wang, Y. Fucoxanthin-loaded nanoparticles composed of gliadin and chondroitin sulfate: Synthesis, characterization and stability. Food Chem. 2022, 379, 132163. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.C.; Wang, Q. Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, T.; Li, X.; Tang, C. Novel nanoparticles from insoluble soybean polysaccharides of Okara as unique Pickering stabilizers for oil-in-water emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Lu, W.; Ma, Y.; Wang, X.; An, X.; Fan, Z. The preparation of lactoferrin/magnesium silicate lithium injectable hydrogel and application in promoting wound healing. Int. J. Biol. Macromol. 2022, 220, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Zhang, L.; Ren, C.; Xu, X.; Du, M.; Zhou, J.; Zhu, B.; Wu, C. Lactoferrin network with MC3T3-E1 cell proliferation, auxiliary mineralization, antibacterial functions: A multifunctional coating for biofunctionalization of implant surfaces. Colloids Surf. B Biointerfaces 2022, 216, 112598. [Google Scholar] [CrossRef]
- Odatsu, T.; Kuroshima, S.; Shinohara, A.; Valanezhad, A.; Sawase, T. Lactoferrin with Zn-ion protects and recovers fibroblast from H2O2-induced oxidative damage. Int. J. Biol. Macromol. 2021, 190, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Atallah, M.A.; Sallam, M.A.; Abdelmoneem, M.A.; Teleb, M.; Elkhodairy, K.A.; Bekhit, A.A.; Khafaga, A.F.; Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green self-assembled lactoferrin carboxymethyl cellulose nanogels for synergistic chemo/herbal breast cancer therapy. Colloids Surf. B Biointerfaces 2022, 217, 112657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.R.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y. Anti-inflammatory effect of fucoidan isolated from fermented Sargassum fusiforme in in vitro and in vivo models. Int. J. Biol. Macromol. 2022, 222, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.C.L.; Dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Pawar, J.S.; Ghosh, I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021, 181, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, T.; Jiao, W.; Zhang, Y.; Liu, W.; Zhang, Y.; Huang, L.; Lv, S. Inhibited digestion of lactoferrin-lactose complexes: Preparation, structural characterization and digestion behaviors. LWT 2022, 172, 114141. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering emulsions stabilized by ovotransferrin–gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Rahman, M.N.; Sarkar, M.T.A.; Elseifi, M.A.; Mayeux, C.; Cooper, S.B. Effects of emulsion types, application rates, and crumb rubber on the laboratory performance of chip seal. Constr. Build. Mater. 2020, 260, 119787. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Hernández-Marín, N.Y.; Lobato-Calleros, C.; Vernon-Carter, E.J. Stability and rheology of water-in-oil-in-water multiple emulsions made with protein-polysaccharide soluble complexes. J. Food Eng. 2013, 119, 181–187. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of beta-carotene by complex coacervation using amaranth carboxymethyl starch and lactoferrin for application in gummy candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Espinosa-Andrews, H.; Morales-Hernandez, N.; Lobato-Calleros, C.; Vernon-Carter, E.J. Mesquite gum/chitosan insoluble complexes: Relationship between the water state and viscoelastic properties. J. Disper. Sci. Technol. 2019, 40, 1345–1352. [Google Scholar] [CrossRef]
- Niu, F.; Hu, D.; Gu, F.; Du, Y.; Zhang, B.; Ma, S.; Pan, W. Preparation of ultra-long stable ovalbumin/sodium carboxymethylcellulose nanoparticle and loading properties of curcumin. Carbohydr. Polym. 2021, 271, 118451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Low, N.H.; Nickerson, M.T. Effect of pH, salt, and biopolymer ratio on the formation of pea protein isolate-gum arabic complexes. J. Agric. Food Chem. 2009, 57, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yang, Z.; Xing, J.; Guo, X.; Zhu, K. Fabrication and stabilization mechanisms of Pickering emulsions based on gliadin/arabinoxylan complexes. Food Chem. 2022, 393, 133458. [Google Scholar] [CrossRef] [PubMed]
- de Folter, J.; van Ruijven, M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef]
- Lin, T.; Dadmohammadi, Y.; Davachi, S.M.; Torabi, H.; Li, P.; Pomon, B.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Improvement of lactoferrin thermal stability by complex coacervation using soy soluble polysaccharides. Food Hydrocoll. 2022, 131, 107736. [Google Scholar] [CrossRef]
- Cai, L.; Zou, S.; Liang, D.; Luan, L. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, H.; Shang, W.; Wang, H.; Tan, M. Fabrication and characterization of Pickering emulsion gels stabilized by gliadin/starch complex for the delivery of astaxanthin. Food Hydrocoll. 2023, 137, 108388. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhu, J.; Huang, Q. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Xia, T.; Gao, Y.; Liu, Y.; Wei, Z.; Xue, C. Lactoferrin particles assembled via transglutaminase-induced crosslinking: Utilization in oleogel-based Pickering emulsions with improved curcumin bioaccessibility. Food Chem. 2022, 374, 131779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Z.; Huang, X.N.; Wu, Z.L.; Yin, S.W.; Zhu, J.H.; Tang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Synthesis, characterization and application of antibacterial lactoferrin nanoparticles. Curr. Res. Food Sci. 2022, 5, 642–652. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Tong, M.; Lu, Y.; Ouyang, X.; Ling, J. Encapsulation of curcumin using fucoidan stabilized zein nanoparticles: Preparation, characterization, and in vitro release performance. J. Mol. Liq. 2021, 329, 115586. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of Physicochemical and Conformational Properties of Kidney Bean Vicilin (Phaseolin) by Glycation with Glucose: Implications for Structure-Function Relationships of Legume Vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, C.; Li, Y.; Qin, X.; Zhang, H.; Hu, Y.; Liu, Z.; Guo, X.; Liu, G. Improving the physicochemical stability of Pickering emulsion stabilized by glycosylated whey protein isolate/cyanidin-3-glucoside to deliver curcumin. Int. J. Biol. Macromol. 2023, 229, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Z.; Xue, C. Pickering emulsions stabilized by zein–gallic acid composite nanoparticles: Impact of covalent or non-covalent interactions on storage stability, lipid oxidation and digestibility. Food Chem. 2023, 408, 135254. [Google Scholar] [CrossRef] [PubMed]
- Kamwilaisak, K.; Rittiwut, K.; Jutakridsada, P.; Iamamorphanth, W.; Pimsawat, N.; Knijnenburg, J.T.N.; Theerakulpisut, S. Rheology, stability, antioxidant properties, and curcumin release of oil-in-water Pickering emulsions stabilized by rice starch nanoparticles. Int. J. Biol. Macromol. 2022, 214, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, C.; Tang, X. In situ rapid conjugation of chitosan-gum Arabic coacervated complex with cinnamaldehyde in cinnamon essential oil to stabilize high internal phase Pickering emulsion. Food Hydrocoll. 2023, 134, 108103. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Li, H.; Zhang, H.; Chi, Y.; Xia, N.; Li, Z.; Jiang, L.; Zhang, X.; Rayan, A.M. Fabrication and digestive characteristics of high internal phase Pickering emulsions stabilized by ovalbumin-pectin complexes for improving the stability and bioaccessibility of curcumin. Food Chem. 2022, 389, 133055. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, R.E.; García-Solís, S.E.; Monroy-Rodríguez, I.; Cornejo-Mazón, M.; Calderón-Domínguez, G.; Alamilla-Beltrán, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.F. Preparation and characterization of canola oil-in-water Pickering emulsions stabilized by barley starch nanocrystals. J. Food Eng. 2022, 326, 111037. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Li, S.; Wang, W. Fabrication and characterization of acid soluble collagen stabilized Pickering emulsions. Food Hydrocoll. 2020, 106, 105875. [Google Scholar] [CrossRef]
- Jiao, B.; Shi, A.M.; Liu, H.Z.; Sheng, X.J.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Effect of electrostatically charged and neutral polysaccharides on the rheological characteristics of peanut protein isolate after high-pressure homogenization. Food Hydrocoll. 2018, 77, 329–335. [Google Scholar] [CrossRef]
- Miao, C.; Mirvakili, M.; Hamad, W.Y. A rheological investigation of oil-in-water Pickering emulsions stabilized by cellulose nanocrystals. J. Colloid Interface Sci. 2022, 608, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Han, C.; Liu, E.; Li, X.; Meng, X.; Liu, B. Pickering emulsions stabilized by pea protein isolate-chitosan nanoparticles: Fabrication, characterization and delivery EPA for digestion in vitro and in vivo. Food Chem. 2022, 378, 132090. [Google Scholar] [CrossRef] [PubMed]
- Azfaralariff, A.; Farahfaiqah, F.; Joe, L.S.; Fazry, S.; Mohamed, M.; Nazar, M.F.; Lazim, A.M. Sago starch nanocrystal-stabilized Pickering emulsions: Stability and rheological behavior. Int. J. Biol. Macromol. 2021, 182, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, Y.; Zhu, Q.; Zhang, X.; Wang, Y.; Tao, L.; Yu, L. Structure and properties of Pickering emulsions stabilized solely with novel buckwheat protein colloidal particles. Int. J. Biol. Macromol. 2023, 226, 61–67. [Google Scholar] [CrossRef] [PubMed]





| Samples | Emulsion Stability Index (%) | |||
|---|---|---|---|---|
| 0 d | 7 d | 15 d | 30 d | |
| 0.5%–0.3 | 68.28 ± 2.48 a | 62.92 ± 2.32 b | 60.97 ± 1.88 b | 60.97 ± 1.88 b |
| 0.5%–0.5 | 76.67 ± 2.18 a | 73.33 ± 1.65 b | 70.48 ± 0.82 bc | 68.10 ± 0.82 c |
| 0.5%–0.6 | 91.27 ± 2.76 a | 85.00 ± 1.44 a | 83.00 ± 2.47 a | 83.00 ± 2.47 a |
| 0.5%–0.65 | 92.03 ± 1.49 a | 91.51 ± 0.58 a | 88.53 ± 1.98 b | 88.51 ± 0.48 b |
| 1%–0.3 | 86.93 ± 3.61 a | 75.49 ± 1.70 b | 66.02 ± 1.49 c | 64.08 ± 2.21 c |
| 1%–0.5 | 88.38 ± 2.73 a | 77.68 ± 1.54 b | 75.74 ± 1.26 b | 62.16 ± 2.38 b |
| 1%–0.6 | 92.69 ± 2.33 a | 88.23 ± 0.35 b | 86.27 ± 1.76 b | 76.99 ± 1.60 c |
| 1%–0.65 | 95.51 ± 1.43 a | 89.01 ± 1.66 b | 87.51 ± 1.34 b | 87.51 ± 0.64 b |
| 1.5%–0.3 | 88.35 ± 2.95 a | 71.85 ± 1.51 b | 69.40 ± 2.82 b | 69.40 ± 2.82 b |
| 1.5%–0.5 | 97.44 ± 0.89 a | 90.26 ± 0.76 b | 88.72 ± 0.74 bc | 88.72 ± 0.74 c |
| 1.5%–0.6 | 100.00 ± 0.00 a | 92.93 ± 1.75 b | 91.92 ± 0.87 b | 91.92 ± 0.87 b |
| 1.5%–0.65 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 93.63 ± 0.85 b | 93.63 ± 0.85 b |
| 2%–0.3 | 99.05 ± 1.65 a | 78.63 ± 1.89 b | 77.68 ± 1.54 b | 75.73 ± 2.21 b |
| 2%–0.5 | 97.58 ± 4.18 a | 88.44 ± 3.79 b | 87.39 ± 3.28 b | 87.39 ± 3.28 b |
| 2%–0.6 | 100.00 ± 0.00 a | 92.16 ± 1.70 b | 92.16 ± 1.70 b | 89.22 ± 1.70 b |
| 2%–0.65 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 99.61 ± 0.68 a |
| Samples | K (Pa·sn) | n | R2 |
|---|---|---|---|
| 0.5%–0.5 | 0.535 ± 0.004 | 0.413 ± 0.003 | 0.995 |
| 1%–0.5 | 1.053 ± 0.013 | 0.351 ± 0.008 | 0.996 |
| 1.5%–0.5 | 1.778 ± 0.018 | 0.367 ± 0.004 | 0.998 |
| 2%–0.5 | 2.843 ± 0.040 | 0.262 ± 0.116 | 0.995 |
| 2%–0.3 | 0.556 ± 0.004 | 0.405 ± 0.003 | 0.999 |
| 2%–0.6 | 6.543 ± 0.036 | 0.167 ± 0.004 | 0.998 |
| 2%–0.65 | 7.340 ± 0.026 | 0.133 ± 0.003 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Wei, Z.; Xu, Y.; Xue, C. Clove Essential Oil Pickering Emulsions Stabilized with Lactoferrin/Fucoidan Complexes: Stability and Rheological Properties. Polymers 2023, 15, 1820. https://doi.org/10.3390/polym15081820
Xi X, Wei Z, Xu Y, Xue C. Clove Essential Oil Pickering Emulsions Stabilized with Lactoferrin/Fucoidan Complexes: Stability and Rheological Properties. Polymers. 2023; 15(8):1820. https://doi.org/10.3390/polym15081820
Chicago/Turabian StyleXi, Xiaohong, Zihao Wei, Yanan Xu, and Changhu Xue. 2023. "Clove Essential Oil Pickering Emulsions Stabilized with Lactoferrin/Fucoidan Complexes: Stability and Rheological Properties" Polymers 15, no. 8: 1820. https://doi.org/10.3390/polym15081820