Templating Effect of Water-Soluble Anionic Phthalocyaninate on the Electropolymerization of 3,4-Ethylenedioxythiophene
Abstract
:1. Introduction
2. Materials and Methods
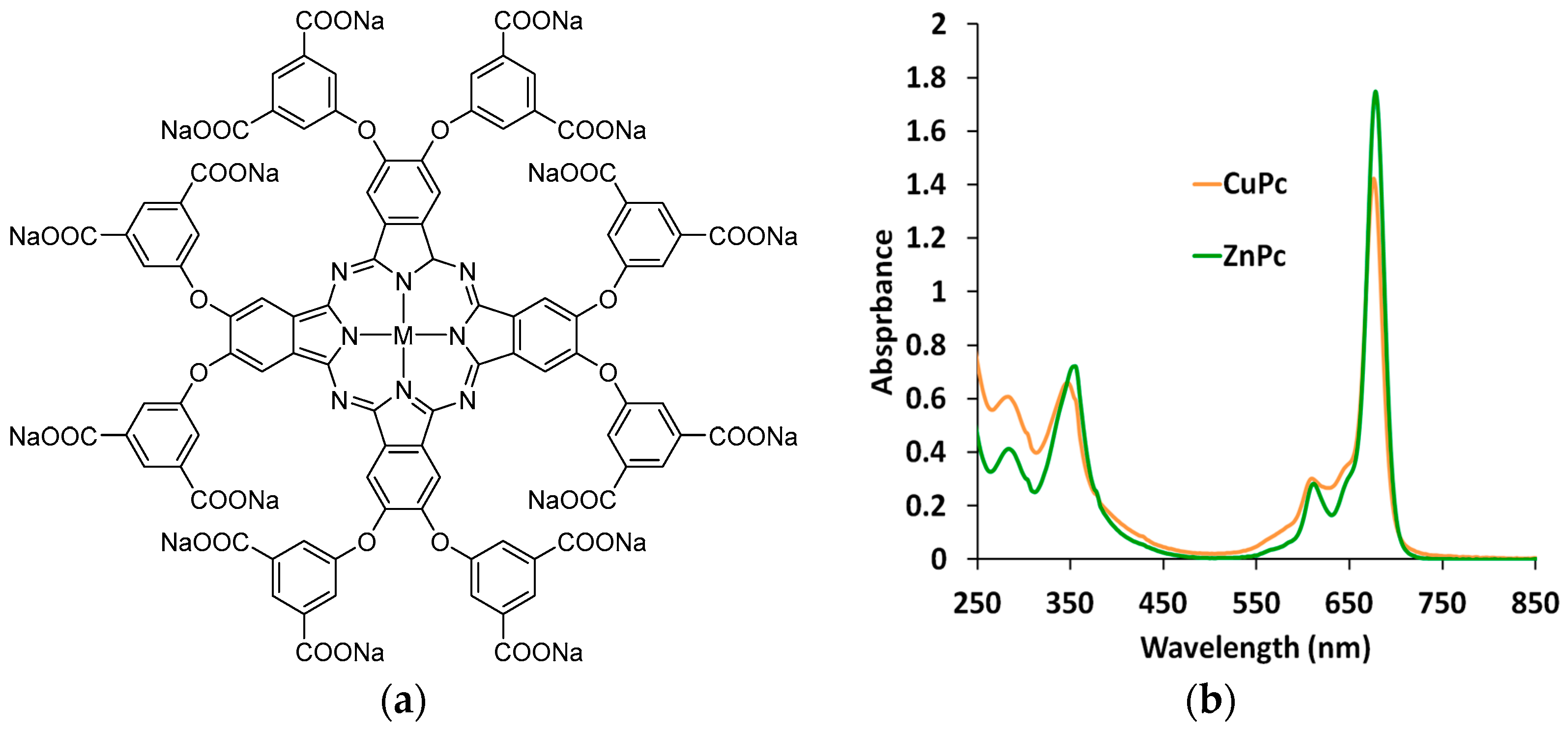
2.1. Synthesis of Copper (Zinc) Octa(3,5-Pentoxycarbonylphenoxy)Phthalocyaninates
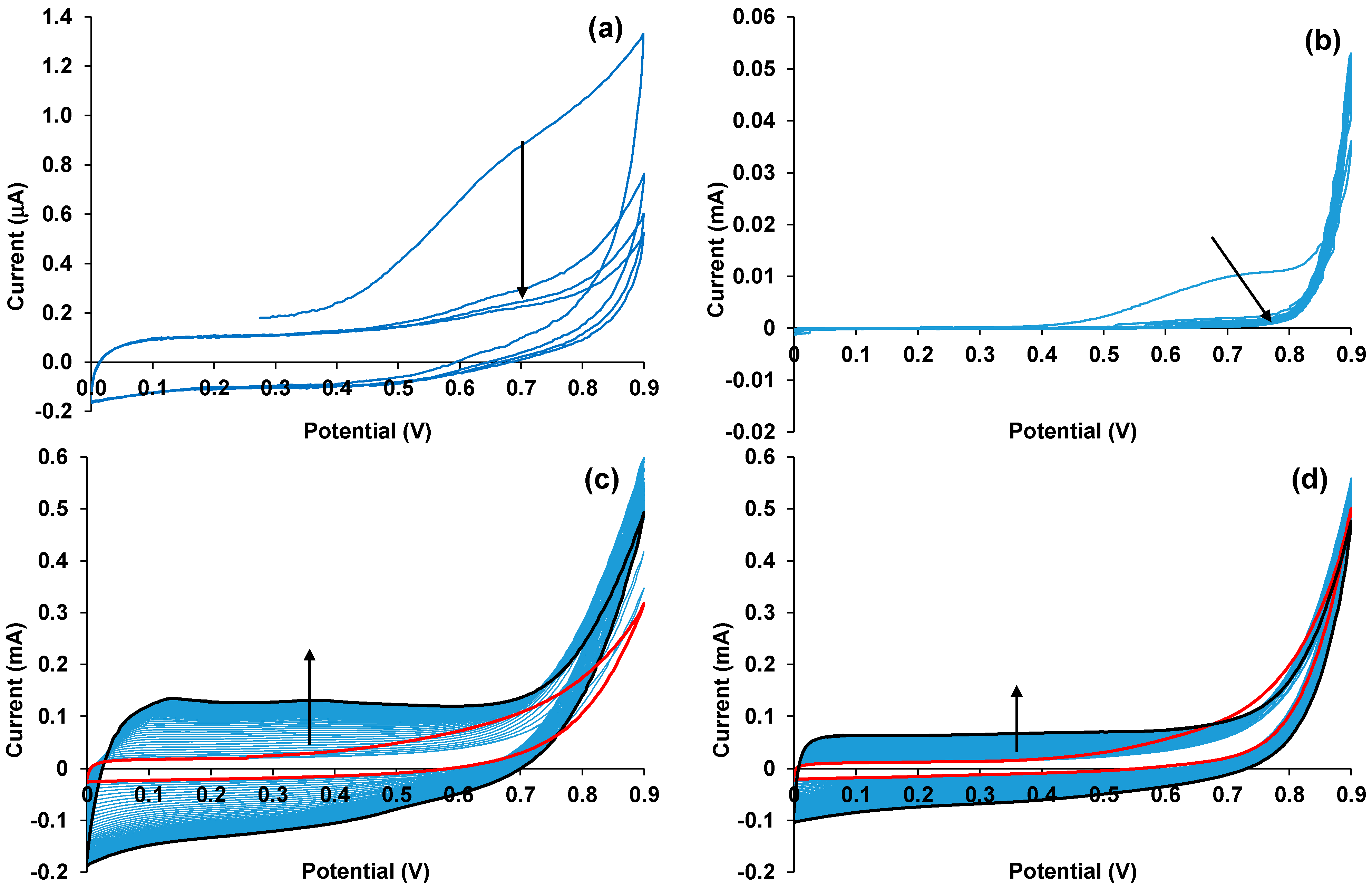
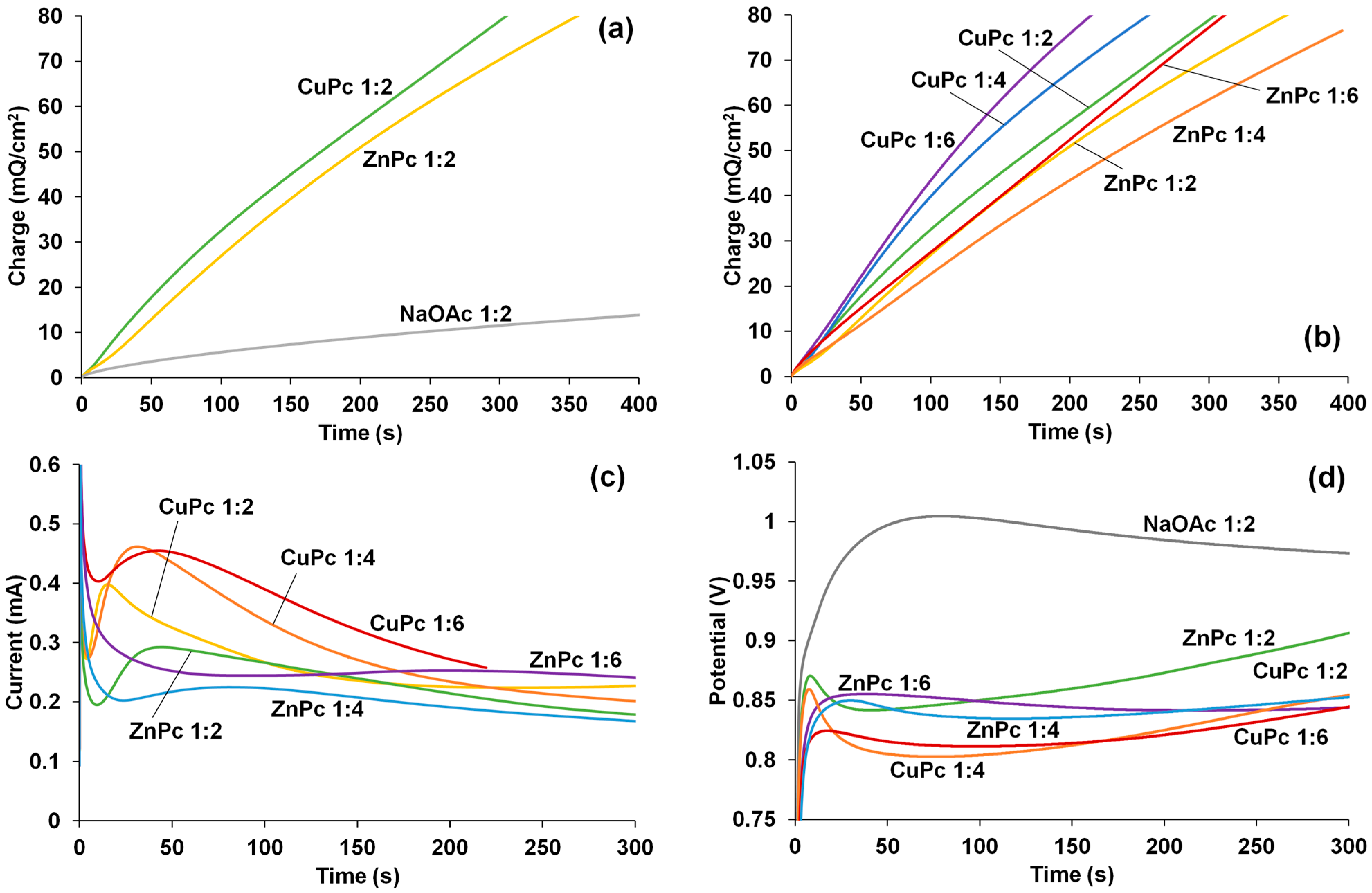
2.2. EDOT Electropolymerization in the Presence of CuPc and ZnPc
3. Results and Discussion
3.1. Spectroscopy of Metal Phthalocyaninates Aqueous Solutions
3.2. Electrochemical Synthesis of PEDOT–MPc Composites
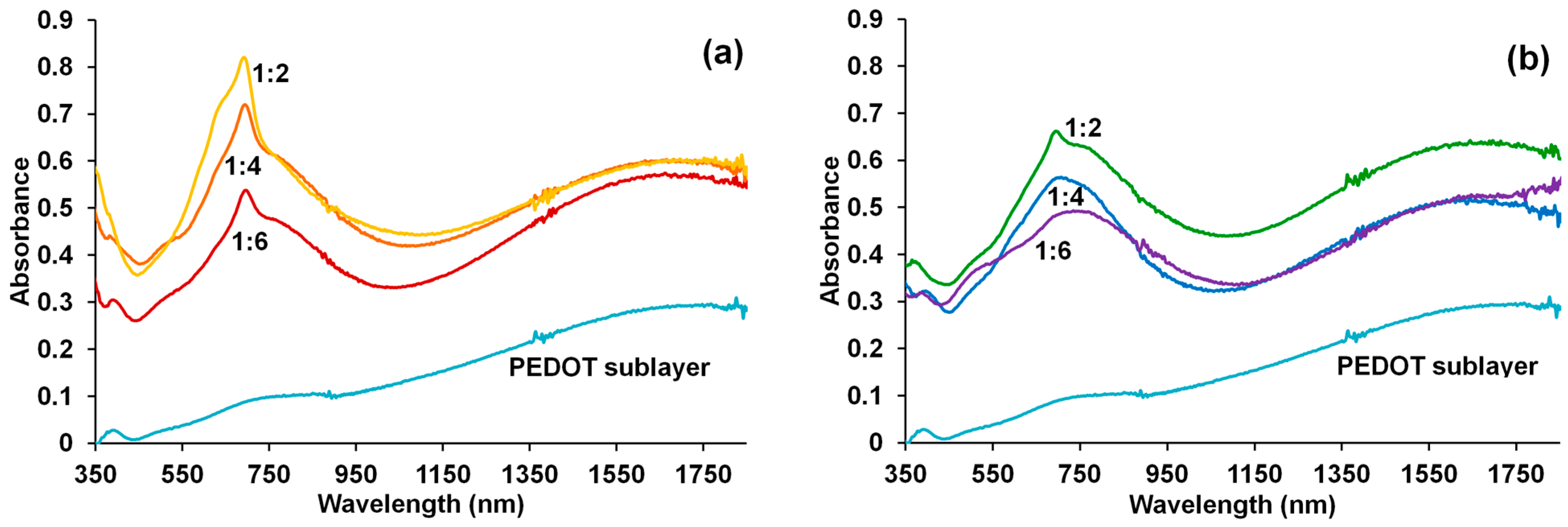
3.3. Electron Spectroscopy in the UV–Visible and Near-IR Regions
3.4. Raman Spectroscopy
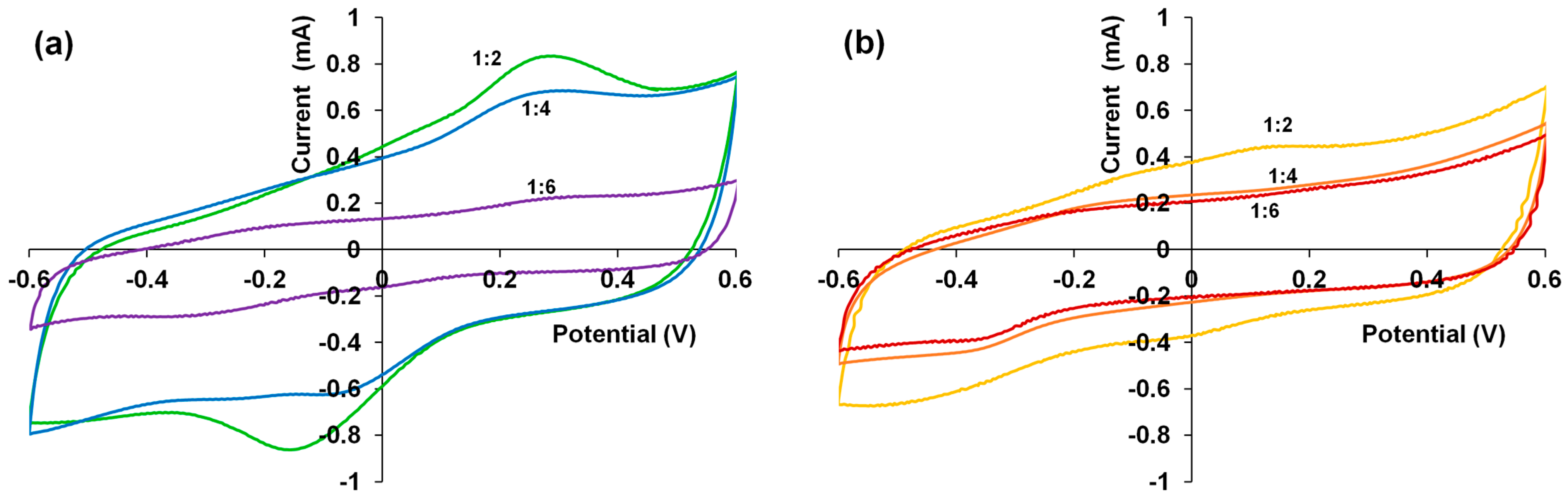
3.5. Cyclic Voltammetry
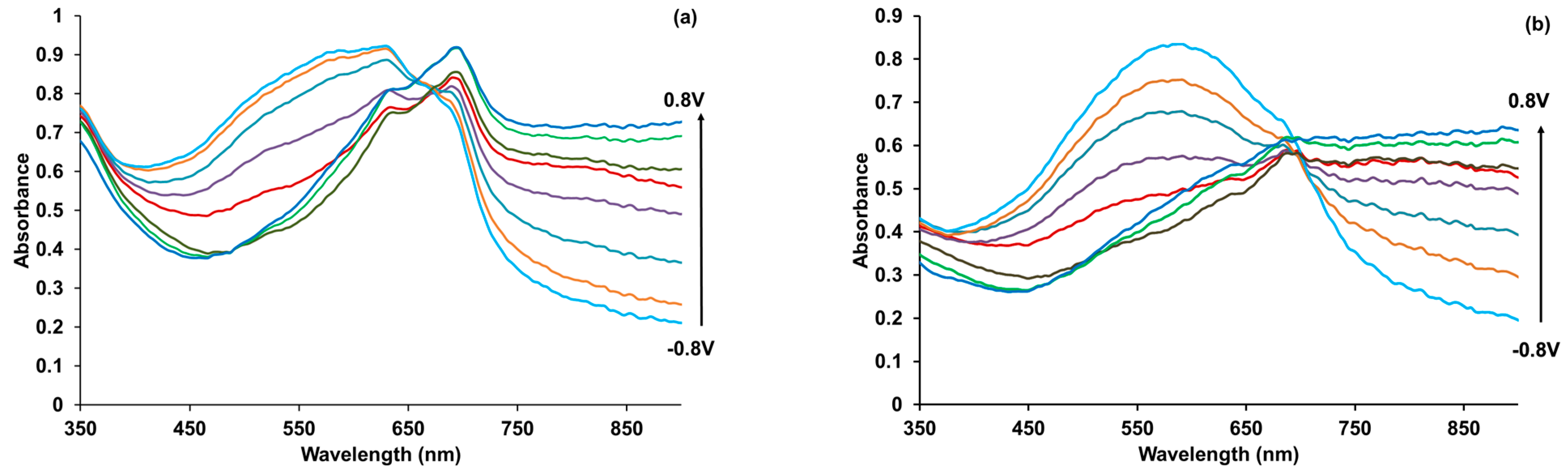
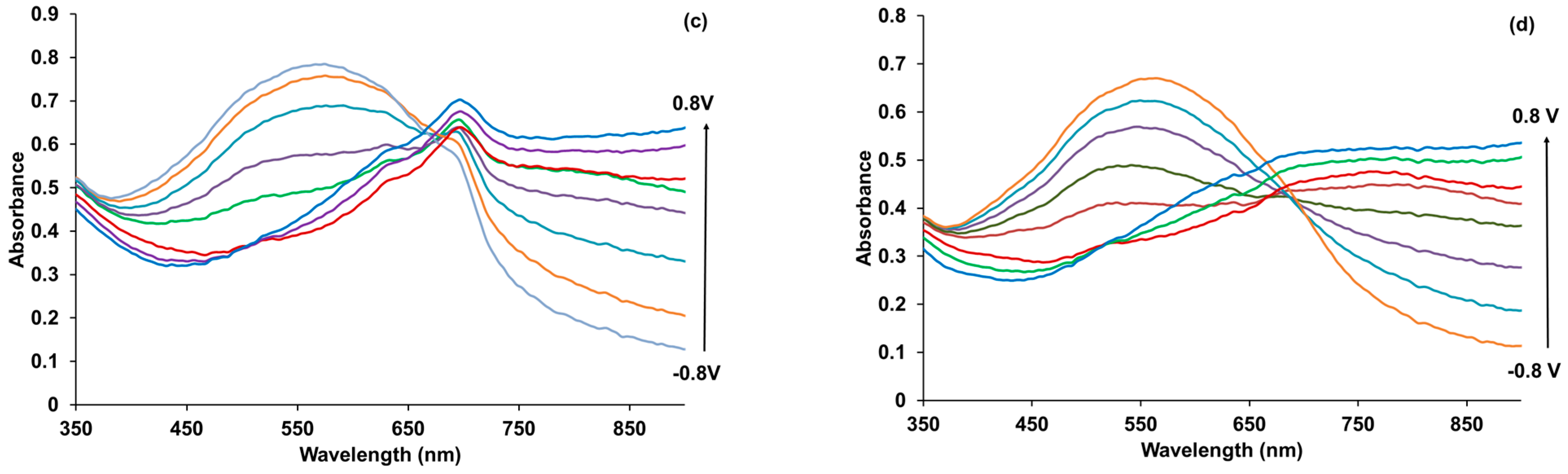
3.6. Spectroelectrochemical Studies
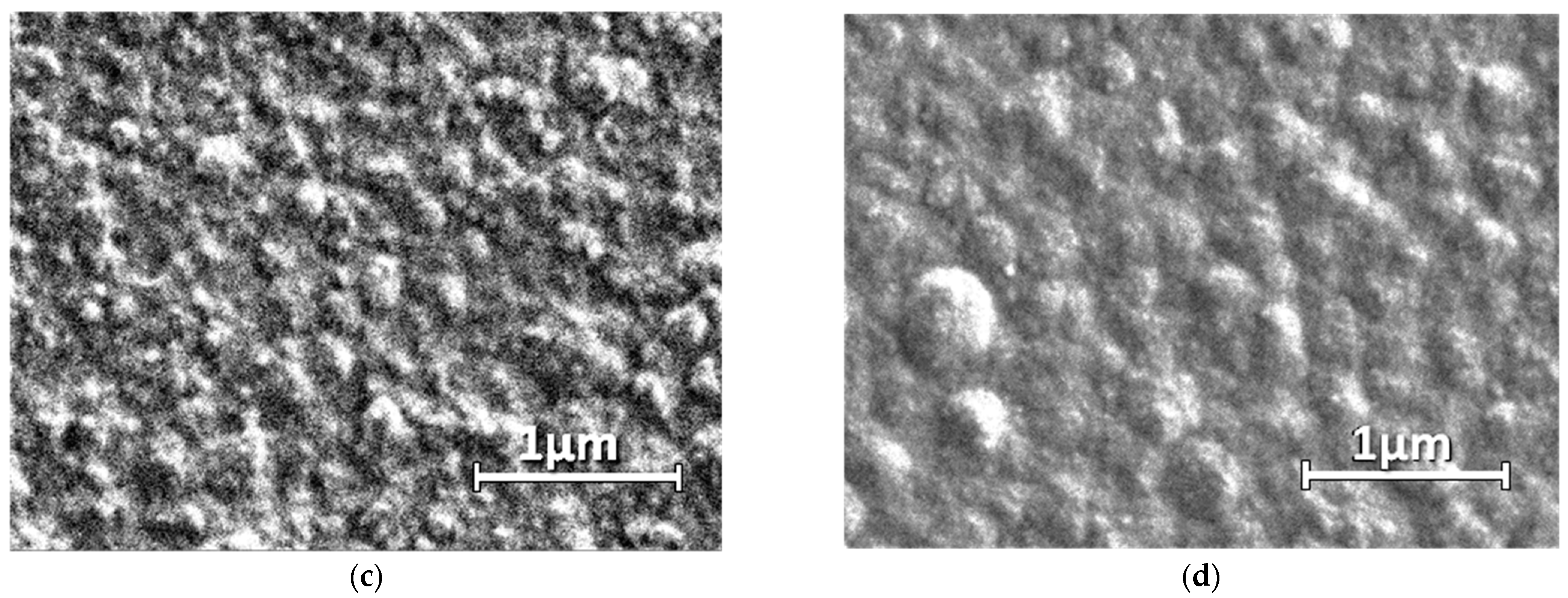
3.7. The Morphology of PEDOT–MPc Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, N.; Petsagkourakis, I.; Chen, S.; Berggren, M.; Crispin, X.; Jonsson, M.P.; Zozoulenko, I. Electric Transport Properties in PEDOT Thin Films. In Conjugated Polymers; CRC Press: Boca Raton, FL, USA, 2019; pp. 45–128. [Google Scholar]
- Gribkova, O.L.; Iakobson, O.D.; Nekrasov, A.A.; Cabanova, V.A.; Tverskoy, V.A.; Tameev, A.R.; Vannikov, A.V. Ultraviolet-Visible-Near Infrared and Raman spectroelectrochemistry of poly(3,4-ethylenedioxythiophene) complexes with sulfonated polyelectrolytes. The role of inter- and intra-molecular interactions in polyelectrolyte. Electrochim. Acta 2016, 222, 409–420. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Kabanova, V.A.; Yagodin, A.V.; Averin, A.A.; Nekrasov, A.A. Water-Soluble Phthalocyanine with Ionogenic Groups as a Molecular Template for Electropolymerization of 3,4-Ethylenedioxythiophene. Russ. J. Electrochem. 2022, 58, 957–967. [Google Scholar] [CrossRef]
- Lukyanov, D.A.; Vereshchagin, A.A.; Soloviova, A.V.; Grigorova, O.V.; Vlasov, P.S.; Levin, O.V. Sulfonated Polycatechol Immobilized in a Conductive Polymer for Enhanced Energy Storage. ACS Appl. Energy Mater. 2021, 4, 5070–5078. [Google Scholar] [CrossRef]
- Yamato, H.; Kai, K.; Ohwa, M.; Wernet, W.; Matsumura, M. Mechanical, electrochemical and optical properties of poly(3,4-ethylenedioxythiophene)/sulfated poly(β-hydroxyethers) composite films. Electrochim. Acta 1997, 42, 2517–2523. [Google Scholar] [CrossRef]
- Baigorria, E.; Durantini, J.E.; Martínez, S.R.; Milanesio, M.E.; Palacios, Y.B.; Durantini, A.M. Potentiation Effect of Iodine Species on the Antimicrobial Capability of Surfaces Coated with Electroactive Phthalocyanines. ACS Appl. Bio Mater. 2021, 4, 8559–8570. [Google Scholar] [CrossRef]
- Göktuğ, Ö.; Soganci, T.; Ak, M.; Şener, M.K. Efficient synthesis of EDOT modified ABBB-type unsymmetrical zinc phthalocyanine: Optoelectrochromic and glucose sensing properties of its copolymerized film. New J. Chem. 2017, 41, 14080–14087. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, X.; Tao, Y.; Zhang, C.; Cheng, H. Preparation, characterizations and electrochromic properties of copolymers containing 5, 10, 15, 20-tetra(thienyl) porphyrin and thiophene derivatives. Synth. Met. 2019, 258, 116202. [Google Scholar] [CrossRef]
- Solis, C.; Baigorria, E.; Milanesio, M.E.; Morales, G.; Durantini, E.N.; Otero, L.; Gervaldo, M. Electrochemical polymerization of EDOT modified Phthalocyanines and their applications as electrochromic materials with green coloration, and strong absorption in the Near-IR. Electrochim. Acta 2016, 213, 594–605. [Google Scholar] [CrossRef]
- Yıldız, H.K.; Korkut, S.E.; Koca, A.; Kasım Şener, M. 3,4-Ethylenedioxythiophene substituted phthalocyanines. Synth. Met. 2011, 161, 1946–1952. [Google Scholar] [CrossRef]
- Pari, M.; Reddy, K.R.V. A Facile Cobalt (II) Tetra Amino Phthalocyanine Ingrained Poloy Aniline (PANI) Nano-fiber Film Layer Based Electrode Material for Amperometric Determination of Thiocyanate. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3511–3520. [Google Scholar] [CrossRef]
- Zhihua, L.; Xucheng, Z.; Jiyong, S.; Xiaobo, Z.; Xiaowei, H.; Tahir, H.E.; Holmes, M. Fast response ammonia sensor based on porous thin film of polyaniline/sulfonated nickel phthalocyanine composites. Sens. Actuators B Chem. 2016, 226, 553–562. [Google Scholar] [CrossRef]
- Milczarek, G. Self-doped polyaniline films prepared by electropolymerization in the presence of sulfonated nickel phthalocyanine. Thin Solid Film. 2009, 517, 6100–6104. [Google Scholar] [CrossRef]
- Sizun, T.; Patois, T.; Bouvet, M.; Lakard, B. Microstructured electrodeposited polypyrrole-phthalocyanine hybrid material, from morphology to ammonia sensing. J. Mater. Chem. 2012, 22, 25246–25253. [Google Scholar] [CrossRef]
- Patois, T.; Sanchez, J.-B.; Berger, F.; Fievet, P.; Segut, O.; Moutarlier, V.; Bouvet, M.; Lakard, B. Elaboration of ammonia gas sensors based on electrodeposited polypyrrole—Cobalt phthalocyanine hybrid films. Talanta 2013, 117, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, G.; Hong, Y.; Chen, C.; He, W.; Wang, S.; Chen, Y.; Wang, C.; Sun, Y.; Wong, C. A Catalytic and Interfacing PEDOT:PSS/CuPc Polymerized on Cloth Fiber to Electro-Metalize Stretchable Copper Conductive Pattern. Adv. Mater. Interfaces 2022, 9, 2101462. [Google Scholar] [CrossRef]
- Nabid, M.R.; Asadi, S.; Shamsianpour, M.; Sedghi, R.; Osati, S.; Safari, N. Oxidative polymerization of 3,4-ethylenedioxythiophene using transition-metal tetrasulfonated phthalocyanine. React. Funct. Polym. 2010, 70, 75–80. [Google Scholar] [CrossRef]
- Andriianova, A.N.; Biglova, Y.N.; Mustafin, A.G. Effect of metal phthalocyanines on the synthesis and physicochemical properties of polyaniline. Mendeleev Commun. 2020, 30, 624–626. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, S.; Yin, Q.; Jiang, B.; Wang, Y.; Du, K.; Yin, Q. Polyaniline doped with copper phthalocyanine disulfonic acid and their unique thermoelectric performance. Polymer 2022, 261, 125337. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhou, X.; Zhang, L.; Xu, B. Facile phthalocyanine doping into PEDOT leads to highly efficient and stable inverted metal halide perovskite solar cells. J. Mater. Chem. A 2018, 6, 12515–12522. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Hu, L.; Yin, Q.; Du, K.; Yin, Q.; Huang, L. Enhanced thermoelectric properties of poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate)/copper phthalocyanine disulfonic acid composite films. J. Appl. Polym. Sci. 2021, 138, 50883. [Google Scholar] [CrossRef]
- Ouyang, M.; Hu, X.; Shao, X.; Chen, L.; Li, W.; Bai, R.; Zhang, L.; Lv, X.; Tameev, A.; Zhang, C. In situ preparation and determination of electrochemical and electrochromic properties of copper phthalocyanine-polyaniline nanocomposite films. RSC Adv. 2019, 9, 34382–34388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Jensen, T.J.; Fronczek, F.R.; Hammer, R.P.; Smith, K.M.; Vicente, M.G.H. Synthesis and Cellular Studies of Nonaggregated Water-Soluble Phthalocyanines. J. Med. Chem. 2005, 48, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Bi, Q.; Tameev, A.; Zhang, Y.; Qian, L.; Ouyang, M.; Zhang, C. A new green-to-transmissive polymer with electroactive poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) as an interface layer for achieving high-performance electrochromic device. J. Polym. Sci. 2020, 58, 937–947. [Google Scholar] [CrossRef]
- Martynov, A.G.; Mack, J.; May, A.K.; Nyokong, T.; Gorbunova, Y.G.; Tsivadze, A.Y. Methodological Survey of Simplified TD-DFT Methods for Fast and Accurate Interpretation of UV–Vis–NIR Spectra of Phthalocyanines. ACS Omega 2019, 4, 7265–7284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, S.; Ağar, E. Synthesis, spectroscopic properties, thermal properties and aggregation behaviors of macrogol-substituted phthalocyanines. J. Mol. Struct. 2019, 1187, 121–131. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Yang, J.; Fu, Q.; Zhao, B.-Z.; Tian, L.; Yu, H.-L. Synthesis, crystal structure and characterization of a new zinc phthalocyanine complex. J. Mol. Struct. 2007, 827, 149–154. [Google Scholar] [CrossRef]
- Yoon, S.M.; Song, H.J.; Hwang, I.C.; Kim, K.S.; Choi, H.C. Single crystal structure of copper hexadecafluorophthalocyanine (F 16CuPc) ribbon. Chem. Commun. 2010, 46, 231–233. [Google Scholar] [CrossRef] [Green Version]
- Gribkova, O.L.; Iakobson, O.D.; Nekrasov, A.A.; Cabanova, V.A.; Tverskoy, V.A.; Vannikov, A.V. The influence of polyacid nature on poly(3,4-ethylenedioxythiophene) electrosynthesis and its spectroelectrochemical properties. J. Solid State Electrochem. 2016, 20, 2991–3001. [Google Scholar] [CrossRef]
- Kabanova, V.; Gribkova, O.; Nekrasov, A. Poly(3,4-ethylenedioxythiophene) Electrosynthesis in the Presence of Mixtures of Flexible-Chain and Rigid-Chain Polyelectrolytes. Polymers 2021, 13, 3866. [Google Scholar] [CrossRef]
- Zozoulenko, I.; Singh, A.; Singh, S.K.; Gueskine, V.; Crispin, X.; Berggren, M. Polarons, Bipolarons, And Absorption Spectroscopy of PEDOT. ACS Appl. Polym. Mater. 2019, 1, 83–94. [Google Scholar] [CrossRef]
- Garreau, S.; Duvail, J.L.; Louarn, G. Spectroelectrochemical studies of poly(3,4-ethylenedioxythiophene) in aqueous medium. Synth. Met. 2001, 125, 325–329. [Google Scholar] [CrossRef]
- Łapkowski, M.; Proń, A. Electrochemical oxidation of poly(3,4-ethylenedioxythiophene)—“In situ” conductivity and spectroscopic investigations. Synth. Met. 2000, 110, 79–83. [Google Scholar] [CrossRef]
- Garreau, S.; Louarn, G.; Froyer, G.; Lapkowski, M.; Chauvet, O. Spectroelectrochemical studies of the C14-alkyl derivative of poly(3,4-ethylenedioxythiophene) (PEDT). Electrochim. Acta 2001, 46, 1207–1214. [Google Scholar] [CrossRef]
- Lokesh, K.S.; Adriaens, A. Electropolymerization of palladium tetraaminephthalocyanine: Characterization and supercapacitance behavior. Dye. Pigment. 2015, 112, 192–200. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhang, Y.; Jiang, J. Theoretical investigation of the molecular, electronic structures and vibrational spectra of a series of first transition metal phthalocyanines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1232–1246. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribkova, O.; Kabanova, V.; Yagodin, A.; Averin, A.; Teplonogova, M.; Martynov, A.; Nekrasov, A. Templating Effect of Water-Soluble Anionic Phthalocyaninate on the Electropolymerization of 3,4-Ethylenedioxythiophene. Polymers 2023, 15, 1854. https://doi.org/10.3390/polym15081854
Gribkova O, Kabanova V, Yagodin A, Averin A, Teplonogova M, Martynov A, Nekrasov A. Templating Effect of Water-Soluble Anionic Phthalocyaninate on the Electropolymerization of 3,4-Ethylenedioxythiophene. Polymers. 2023; 15(8):1854. https://doi.org/10.3390/polym15081854
Chicago/Turabian StyleGribkova, Oxana, Varvara Kabanova, Alexey Yagodin, Aleksey Averin, Maria Teplonogova, Alexander Martynov, and Alexander Nekrasov. 2023. "Templating Effect of Water-Soluble Anionic Phthalocyaninate on the Electropolymerization of 3,4-Ethylenedioxythiophene" Polymers 15, no. 8: 1854. https://doi.org/10.3390/polym15081854





