Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance
Abstract
1. Introduction
2. Experimental Materials and Instruments
2.1. Experimental Materials
2.2. Preparation of the PA-651-Modified GO (PGO)
2.3. Preparation of the Coating Samples
2.4. Characterization Methods
3. Results and Discussion
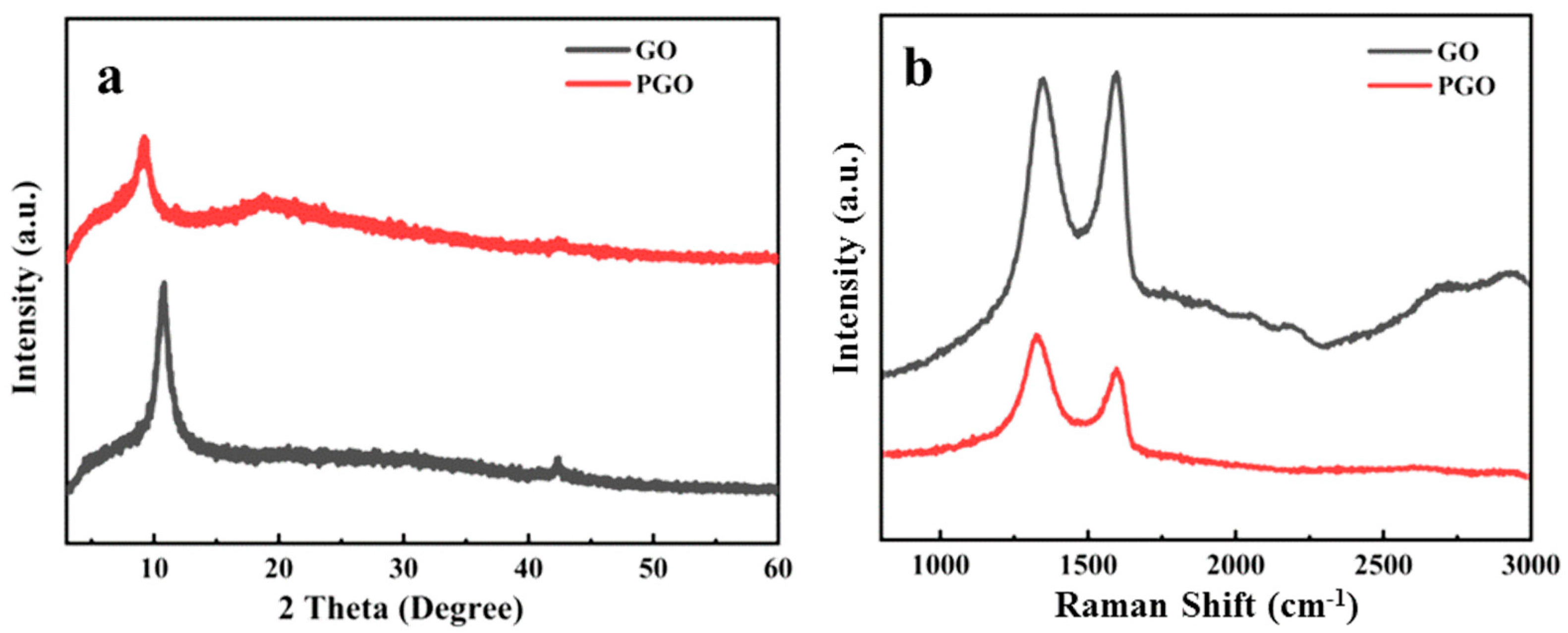
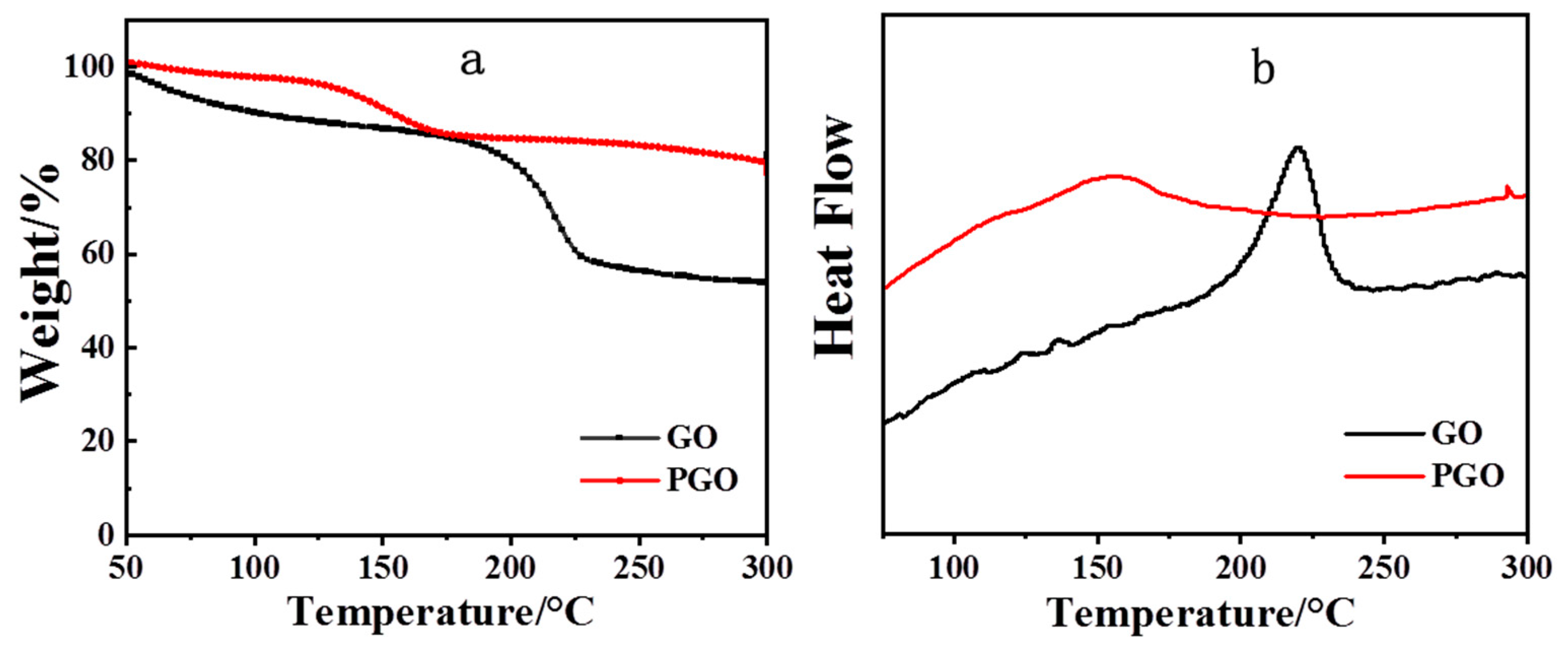
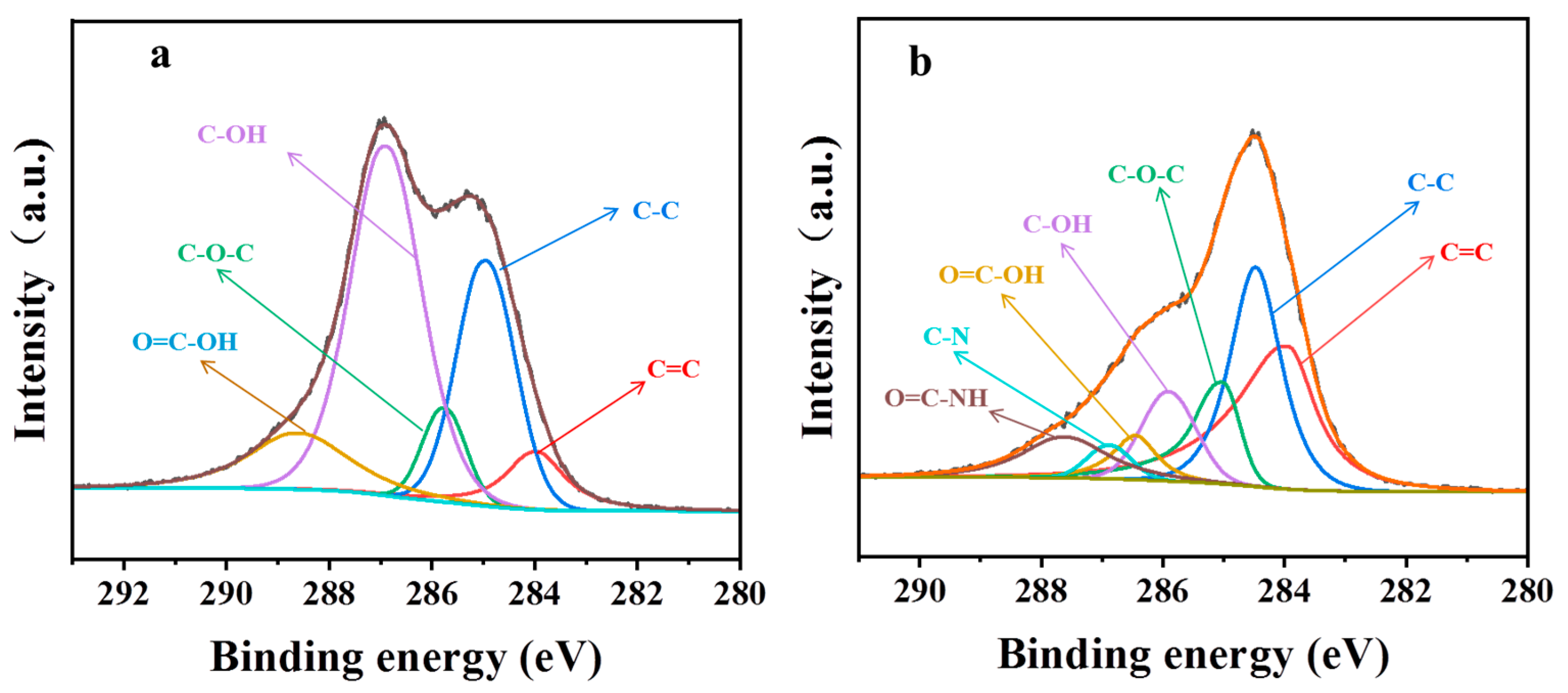
3.1. Physical Chemical Structure of GO and PGO
3.2. Corrosion Resistance of the Epoxy Coatings
3.2.1. Corrosion Resistance of GO/EP/Zn Coatings
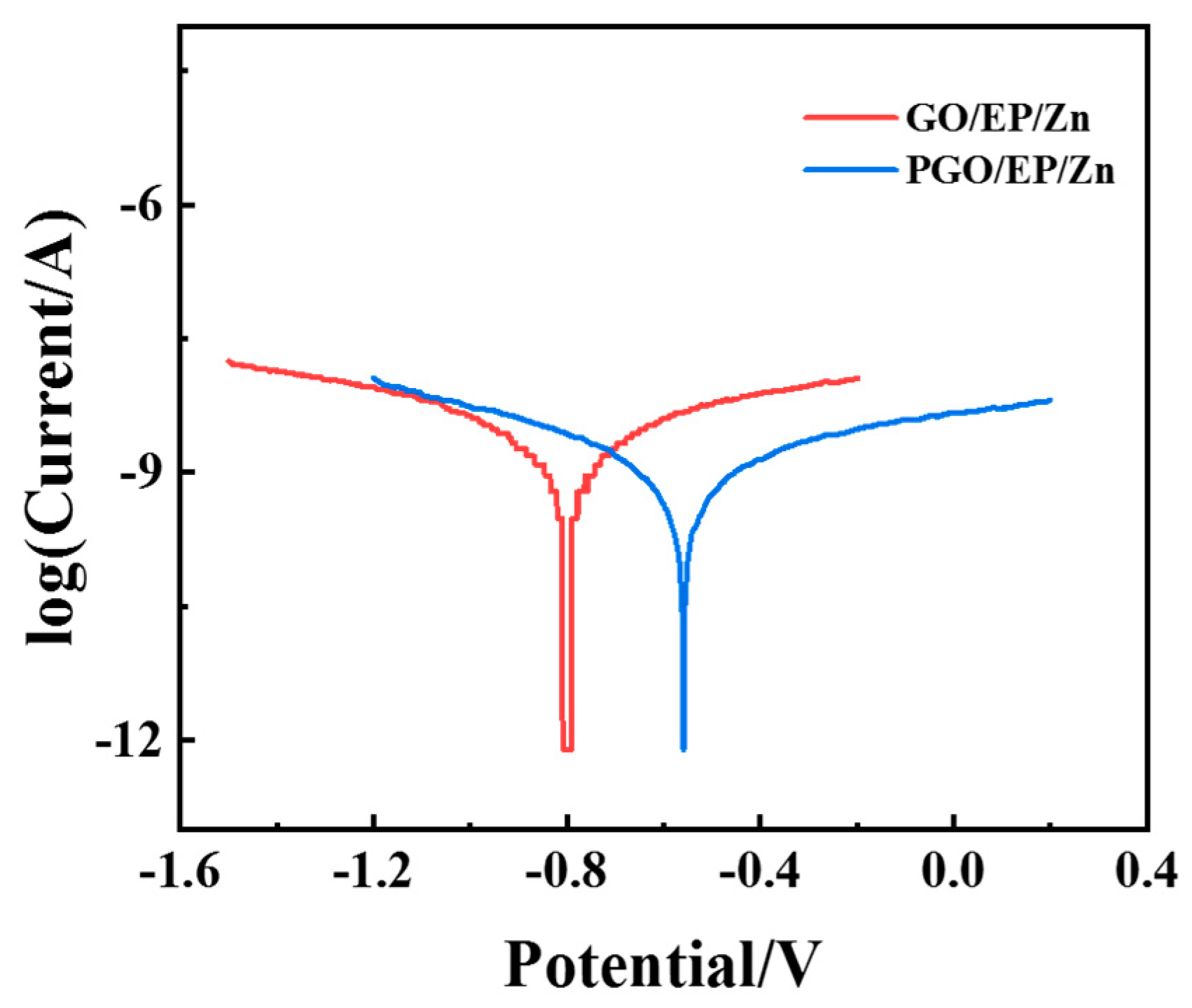
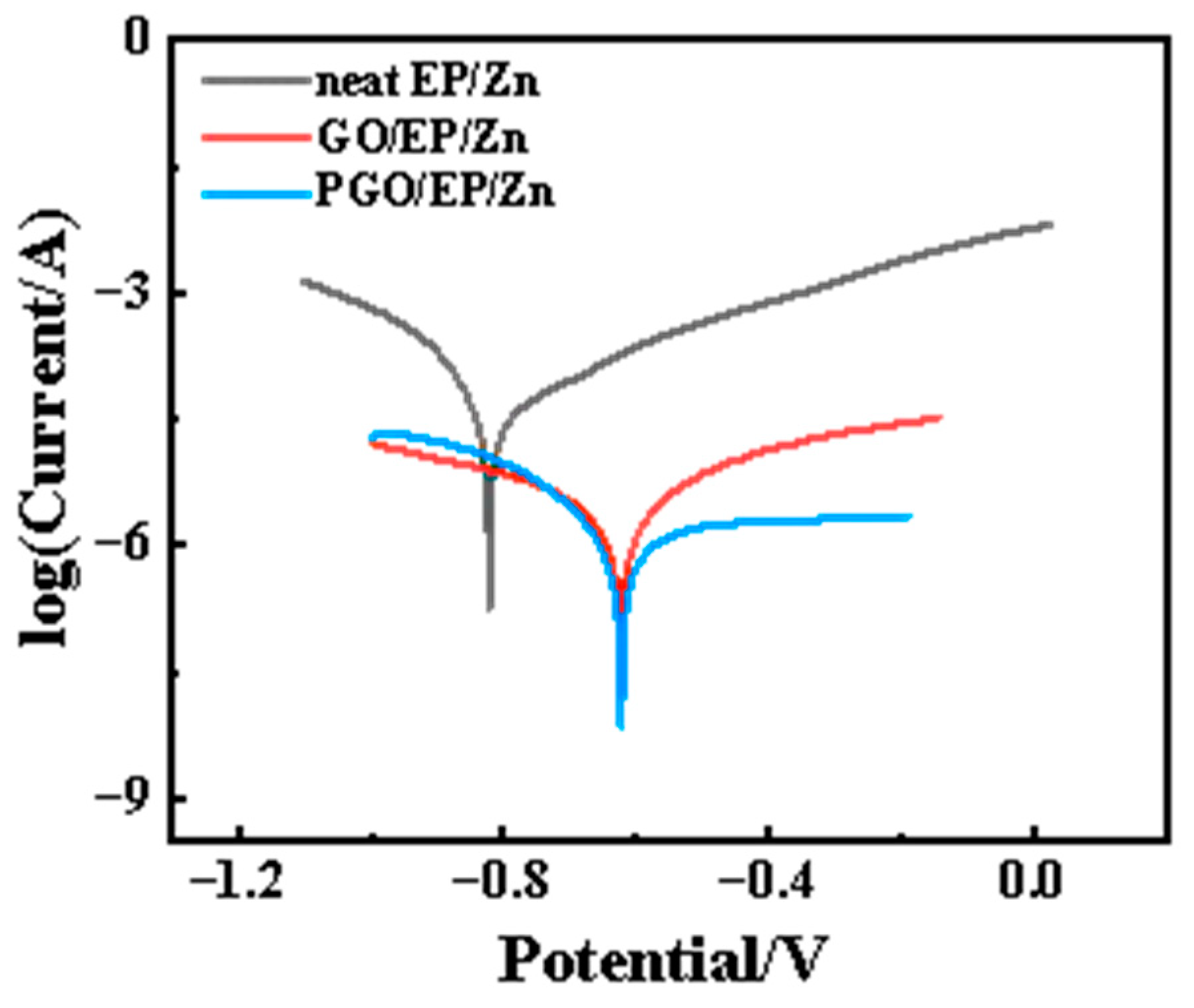
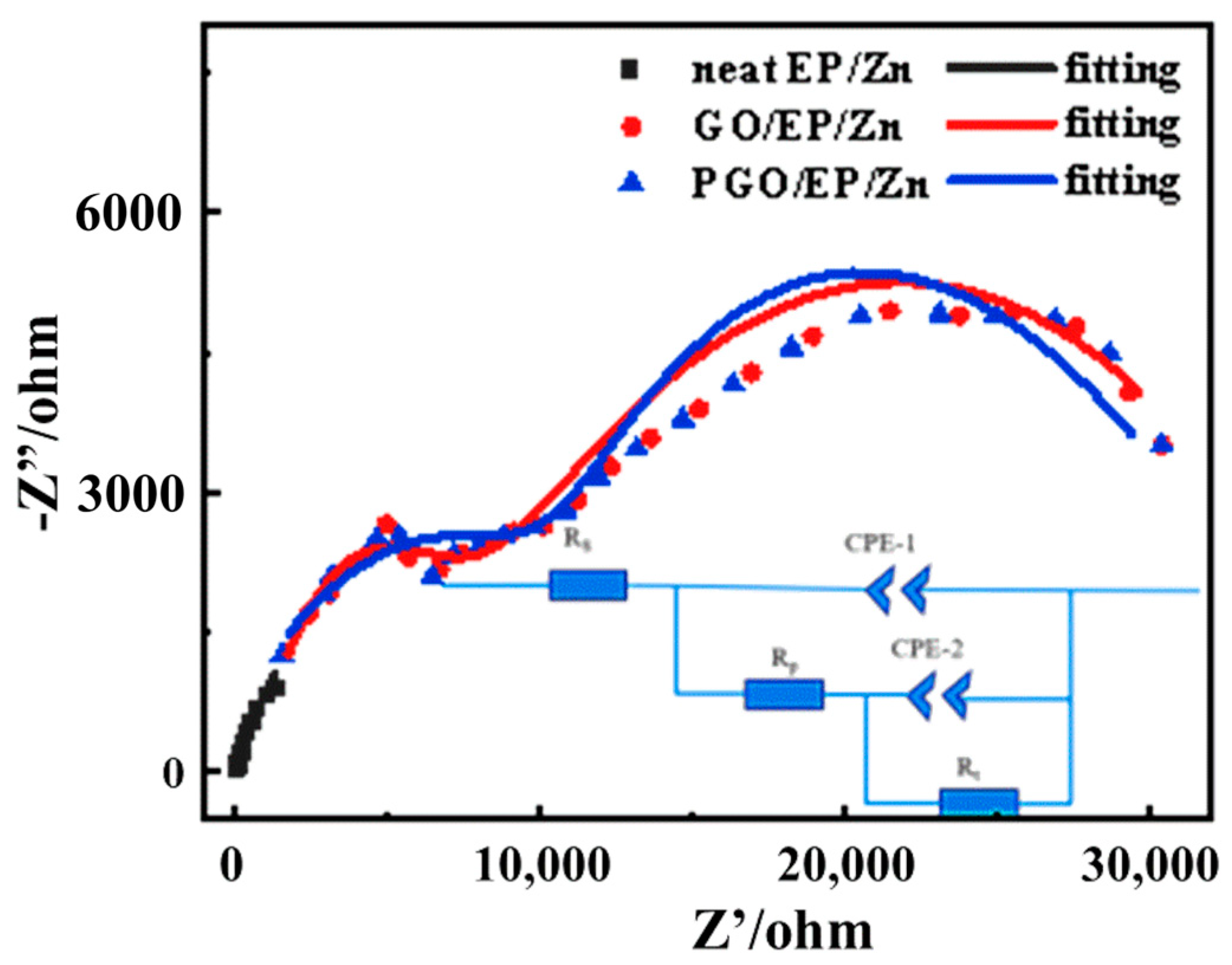
3.2.2. Corrosion Resistance of PGO/EP/Zn Coating
4. Conclusions
- The corrosion resistance of epoxy/Zn composite paint was significantly improved by directly incorporating GO into the coating, and the optimized GO content was 0.3 wt.%.
- GO was successfully modified with PA-651 to form PGO in component B of the paint, resulting in increased interlayer spacing, higher thermal stability, as well as improved dispersion stability in the epoxy solvent.
- Due to the polyamide modification and the physical shielding of GO nanosheets, the PGO-incorporated EP/Zn coatings displayed a lower corrosion current density and a larger impedance modulus than the GO/EP/Zn coatings, when GO was added into component A of the epoxy composite paint.
- The modification of GO with the curing agent helped with filling the micropores in the epoxy coating, increasing the coating pore resistance and charge transfer resistance during the corrosion process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Wu, Y.; Ma, L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019, 169, 107694. [Google Scholar] [CrossRef]
- Wang, X.; Zou, J.; Du, X. Facile flame catalytic growing carbon coating on Cu panel with surface protection performance comparable to that of graphene film via CVD. Surf. Coat. Technol. 2022, 445, 128720. [Google Scholar] [CrossRef]
- Meng, W.; Zou, J.; Wang, X.; Zhang, P.; Du, X.-S. On the Distinctive Hardness, Anti-Corrosion Properties and Mechanisms of Flame-Deposited Carbon Coating with a Hierarchical Structure in Contrast to a Graphene Layer via Chemical Vapor Deposition. Nanomaterials 2022, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Marchebois, H.; Savall, C.; Bernard, J.; Touzain, S. Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta 2004, 49, 2945–2954. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Shirehjini, F.T.; Danaee, I.; Eskandari, H.; Zarei, D. Effect of Nano Clay on Corrosion Protection of Zinc-rich Epoxy Coatings on Steel 37. J. Mater. Sci. Technol. 2016, 32, 1152–1160. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Park, S.M.; Shon, M.Y. Effects of multi-walled carbon nanotubes on corrosion protection of zinc-rich epoxy resin coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Jalili, M.; Rostami, M.; Ramezanzadeh, B. An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 2015, 328, 95–108. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, L.; Tang, H.; Ye, H.; Li, M. Effect of Nano-SnS and Nano-MoS2 on the Corrosion Protection Performance of the Polyvinylbutyral and Zinc-Rich Polyvinylbutyral Coatings. Nanomaterials 2019, 9, 956. [Google Scholar] [CrossRef]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2018, 30, 1702944. [Google Scholar] [CrossRef]
- Zou, J.; Wang, X.; Zhang, P.; Du, X. Ultrafast flame coating of carbon and chemical vapor deposition of graphene on NiTi alloy to enhance its corrosion resistance. Diam. Relat. Mater. 2022, 128, 109231. [Google Scholar] [CrossRef]
- Wang, X.; Zou, J.; Lin, J.; Du, X. Promoting the corrosion resistance of carbon steel via facile catalytic deposition of novel hierarchical carbon film layer in an ethanol combustion flame. Diam. Relat. Mater. 2021, 120, 108714. [Google Scholar] [CrossRef]
- Halkjær, S.; Iversen, J.; Kyhl, L.; Chevallier, J.; Andreatta, F.; Yu, F.; Stoot, A.; Camilli, L.; Bøggild, P.; Hornekær, L. Low-temperature synthesis of a graphene-based, corrosion-inhibiting coating on an industrial grade alloy. Corros. Sci. 2019, 152, 1–9. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Mohamadzadeh Moghadam, M.H.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Han, D.; Ma, S.; Guo, W.; Cai, H.; Wang, X. Effect of graphene on corrosion resistance of waterborne inorganic zinc-rich coatings. J. Alloys Compd. 2019, 774, 255–264. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion Protection of Graphene-Modified Zinc-Rich Epoxy Coatings in Dilute NaCl Solution. ACS Appl. Nano Mater. 2019, 2, 180–190. [Google Scholar] [CrossRef]
- Golovin, V.A.; Tyurina, S.A. Current trends in the modification of zinc-filled polymer coatings. Int. J. Corros. Scale Inhib. 2020, 9, 372–393. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Dutta, G.K.; Karak, N. Bio-based waterborne polyester/cellulose nanofiber-reduced graphene oxide-zinc oxide nanocomposite: An approach towards sustainable mechanically robust anticorrosive coating. Cellulose 2022, 29, 1679–1703. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Long, Z.; Zhan, Y. Synergistic effect of functional carbon nanotubes and graphene oxide on the anti-corrosion performance of epoxy coating. Polym. Adv. Technol. 2017, 28, 754–762. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Qiang, Y.; Chen, Z.; Wang, L.; Gao, X.; Fang, Z. π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Jing, L.-C.; Wang, T.; Cao, W.-W.; Wen, J.-G.; Zhao, H.; Ning, Y.-J.; Yuan, X.-T.; Tian, Y.; Teng, L.-H.; Geng, H.-Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, Y.; Jing, C. Enhancing the Corrosion Resistance of Epoxy Coatings by Impregnation with a Reduced Graphene Oxide-Hydrophobic Ionic Liquid Composite. ChemElectroChem 2018, 5, 3300–3306. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Du, X.; Zhou, C.; Liu, H.Y.; Mai, Y.-W.; Wang, G.X. Facile chemical synthesis of nitrogen-doped graphene sheets and their electrochemical capacitance. J. Power Sources 2013, 241, 460–466. [Google Scholar] [CrossRef]
- Tian, Y.; Bi, Z.; Cui, G. Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings. Polymers 2021, 13, 1657. [Google Scholar] [CrossRef]
- Kasaeian, M.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-Benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM). Corros. Sci. 2018, 145, 119–134. [Google Scholar] [CrossRef]
- Nikpour, B.; Ramezanzadeh, B.; Bahlakeh, G.; Mahdavian, M. Synthesis of graphene oxide nanosheets functionalized by green corrosion inhibitive compounds to fabricate a protective system. Corros. Sci. 2017, 127, 240–259. [Google Scholar] [CrossRef]
- Du, X.S.; Yu, Z.-Z.; Dasari, A.; Ma, J.; Mo, M.; Meng, Y.Z.; Mai, Y.-W. New method to prepare graphite nanocomposites. Chem. Mater. 2008, 20, 2066. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.Y.; Mai, Y.-W. Ultrafast Synthesis of Multifunctional NDoped Graphene Foam in an Ethanol Flame. ACS Nano 2016, 10, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]





| Sample | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| Neat EP/Zn | 6.1 × 10−6 | −0.65 |
| 0.1 wt.% GO | 3.3 × 10−6 | −1.21 |
| 0.2 wt.% GO | 1.0 × 10−7 | −0.94 |
| 0.3 wt.% GO | 9.3 × 10−10 | −0.80 |
| 0.5 wt.% GO | 8.8 × 10−10 | −0.90 |
| Sample | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| neat EP/Zn | 6.1 × 10−6 | −0.65 |
| GO/EP/Zn | 9.3 × 10−10 | −0.80 |
| PGO/EP/Zn | 4.9 × 10−10 | −0.56 |
| Sample | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| neat EP/Zn | 4.9 × 10−5 | −0.82 |
| GO/EP/Zn | 2.2 × 10−6 | −0.63 |
| PGO/EP/Zn | 1.2 × 10−6 | −0.62 |
| Sample | CPE1 (S·cm−2·sn) | Rp (Ω·cm−2) | CPE2 (S·cm−2·sn) | Rt (Ω·cm−2) |
|---|---|---|---|---|
| Neat EP/Zn | 6.84 × 10−4 | - | - | 8370 |
| GO | 1.43 × 10−7 | 5386 | 1.43 × 10−5 | 31,830 |
| PGO | 1.64 × 10−7 | 13,115 | 1.30 × 10−5 | 64,520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Wei, G.; Zhang, Z.; Yang, L.; Lin, Y.; Du, L.; Du, X. Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance. Polymers 2023, 15, 1873. https://doi.org/10.3390/polym15081873
He S, Wei G, Zhang Z, Yang L, Lin Y, Du L, Du X. Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance. Polymers. 2023; 15(8):1873. https://doi.org/10.3390/polym15081873
Chicago/Turabian StyleHe, Shengjun, Guangxiong Wei, Zhengnan Zhang, Lifeng Yang, Yuebin Lin, Longji Du, and Xusheng Du. 2023. "Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance" Polymers 15, no. 8: 1873. https://doi.org/10.3390/polym15081873
APA StyleHe, S., Wei, G., Zhang, Z., Yang, L., Lin, Y., Du, L., & Du, X. (2023). Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance. Polymers, 15(8), 1873. https://doi.org/10.3390/polym15081873






