Abstract
To promote the anticorrosion performance of epoxy/zinc (EP/Zn) coating, graphene oxide (GO) was directly incorporated into dual-component paint. Interestingly, it was found that the method of incorporating GO during the fabrication of the composite paints strongly influenced their performance. The samples were characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and Raman spectroscopy. The results indicated that GO could be intercalated and modified with the polyamide curing agent while preparing component B of the paint, for which the interlayer spacing of the resulting polyamide modified GO (PGO) increased, and its dispersion in organic solvent was improved. The corrosion resistance of the coatings was studied through potentiodynamic polarization testing, electrochemical impedance spectroscopy (EIS), and immersion testing. Among the three types of as-prepared coatings, i.e., neat EP/Zn coating, GO modified EP/Zn coating (GO/EP/Zn), and PGO-modified EP/Zn coating (PGO/EP/Zn), the order of the corrosion resistance of the coatings was PGO/EP/Zn > GO/EP/Zn > neat EP/Zn. This work demonstrates that although the in situ modification of GO with a curing agent is a simple method, it evidently promotes the shielding effect of the coating and enhances its corrosion resistance.
1. Introduction
Coatings are often unitized to protect metals from corrosion [1,2,3,4]. Among the various coatings, Zn-rich polymer composite paint is a widely applied type of coating due to the effect of the Zn particles, which work as anodes to provide cathodic protection for metal substrates [1,5,6,7,8]. In most of the commercial products, a heavy loading of Zn particles, up to 80% of the content, has to be used to achieve satisfying anticorrosion performance [8]. However, this high Zn content in the coating has its own disadvantages, resulting in poor mechanical coating properties, the large consumption of Zn, and the high economic cost of the paint. In order to make the best use of Zn particles in the polymer matrix in the coatings and improve their corrosion resistance, additional nanofillers have been incorporated into these coatings. For instances, various particles, such as carbon nanotubes [9], alumina [10], metal sulfides [11], and clay [7], have been added into Zn-containing polymer composite coatings.
Two-dimensional (2D) nanocarbon materials, including carbon or graphene sheets, have been directly deposited onto metal substrates as protective coatings via CVD or flame deposition [12,13,14]. They exhibit excellent corrosive and scratch resistance. The addition of graphene to polymer composite coatings is another efficient way to make use of these 2D nanocarbon materials in coating systems, where their barrier property can be fully exploited [15,16,17,18,19,20,21,22]. For Zn/polymer composite coating systems, 2D carbon sheets also promote the formation of electron conduction paths between Zn particles, thereby enhancing the cathodic protection of the coating.
Given its high aspect ratio and chemical inertness, graphene is usually modified before introducing it into resin matrices with the aim of enhancing its interactions with the matrix and/or improving its dispersibility in the coating [23,24,25,26,27,28,29,30,31]. As an oxidation product of graphene, graphene oxide (GO) can be simply defined as graphene with many organic functional groups on its surface, which can be directly prepared from natural graphite with the well-known Hummers’ method [32,33]. Due to the many functional groups on its surface, GO has good dispersion in both aqueous solutions and water-soluble polymer matrices. Extensive studies have been devoted to modifying GO to improve its dispersion in organic solvents and coatings [26,27,28,29,30,31] as well as to improve its corrosion resistance via the loading of corrosive inhibitors on its surface [34,35]. However, the addition of the modification step increases the complexity and cost of the fabrication of paints.
In this study, GO modified with the polyamide curing agent was incorporated into dual-component epoxy/Zn paints. With the in situ preintercalation of GO with polyamide in the curing agent component of the paints, the interlayer of the modified GO was increased, and its dispersion in the organic solvent was improved. The physical and chemical properties of the polyamide modified GO were comprehensively characterized. The effects of the incorporation of GO and its addition order on the anticorrosion performance of an epoxy/Zn composite coating was investigated with various electrochemical techniques.
2. Experimental Materials and Instruments
2.1. Experimental Materials
Graphene oxide (GO) was purchased from Suzhou Tanfeng Technology Co., Ltd., Suzhou, China. Epoxy resin (E44) was ordered from Xiya Reagent, Linyi, China. Zylene, n-butanol, and polyamide 651 (PA-651) were purchased from McLean Group, Shanghai, China. Carbon steel plates (Q235, size: 30 mm × 40 mm) and Zn flake powder (size: 5–15 µm) were obtained from Fuquan Metal Co., Ltd., Quanzhou, China and Xiamen Empire New Material Technology Co., Ltd., Xiamen, China.
2.2. Preparation of the PA-651-Modified GO (PGO)
Specifically, PGO could be obtained via the direct interaction between GO and PA-651. Similar to the preparation of component B during the fabrication of the dual-component epoxy paint, GO (0.03 g) was added to 1.5 g of PA-651 and mixed via magnetically stirring at 50 °C for 2 h. In order to extract the modified GO for characterizing its structure, the mixture was filtrated and then successively washed with acetone and deionized water. The product was then obtained by freeze-drying and named PGO.
2.3. Preparation of the Coating Samples
Before applying the coating, the Q235 steel sheets were successively washed with alcohol and then deionized water. After being sandblasted, the samples were ultrasonically cleaned in acetone. Two types of coatings were produced by adding GO in different orders into dual-component epoxy paint, i.e., component A (epoxy resin and Zn powder) and component B (PA-651, a curing agent). For the fabrication of the normal GO-incorporated coating, GO powder was added to component A and magnetically stirred at room temperature for 2 h. It was then mixed with component B to prepare the GO/EP/Zn coating. In contrast, the PGO/EP/Zn paint was prepared by firstly mixing GO with PA-651 at 50 °C and then magnetically stirring for 2 h to produce component B, which was subsequently mixed with component A. In all the coatings, organic solvent (xylene: n-butanol = 7:3) was utilized to dilute component A, and its mass fraction was 20 wt.% of the whole paint.
Finally, the coating was applied to the surface of the sandblasted Q235 steel sheets with a steel rod applicator and cured in an oven at 60 °C for 24 h. The thickness of the dry coating was 150 ± 10 µm. In addition, neat epoxy/Zn coating was prepared as the reference sample using the same method mentioned above.
2.4. Characterization Methods
The chemical functional groups of the sample were characterized with a Fourier transform infrared spectrometer (Thermo Scientific Nicolet iS50) over a scan range of 400 to 4000 cm−1. Both GO and PGO samples were characterized with Raman (Invia Renishaw Raman) and X-ray photoelectron spectroscopy (XPS; K-Alpha+, Thermo Scientific, East Grinstead, UK). An X-ray diffraction analyzer (XRD, Rigaku UItima IV, Nagano, Japan) was used to characterize the crystalline structure of the sample. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) tests of the samples were performed under N2 protection using a TGA/DSC 3+ thermogravimetric analyzer (Mettler Toledo International Co., Ltd., Zürich, Switzerland) at a heating rate of 5 °C min−1.
The electrochemical corrosion of the coatings was measured on a CHI 760e electrochemical workstation. A three-electrode system was constructed with a saturated calomel electrode (SCE) as the reference electrode, a platinum plate electrode as the auxiliary electrode, and 3.5 wt.% NaCl as the electrolyte solution. The potentiodynamic polarization test was carried out at a scan rate of 10 mV s−1, and the electrochemical impedance spectroscopy (EIS) tests were performed in a frequency range 10 kHz to 0.1 Hz under an amplitude voltage of 5 mV at room temperature.
3. Results and Discussion
3.1. Physical Chemical Structure of GO and PGO
As shown in Figure 1, the characteristic peaks of GO appeared at 1719 cm−1 (C=O carboxyl stretching vibration), 1619 cm−1 (C=C in aromatic ring), and 1066 cm−1 (C–O–C in epoxide), which is similar to the peaks reported in previous studies [32,35,36]. In addition, the strong and wide peak at 3000–3500 cm−1 could be ascribed to the hydroxyl groups. Two new peaks appeared at 2919 cm−1 and 2849 cm−1 in the FT-IR spectrum of PGO, and they originated from the stretching vibration of the alkyl group in polyamide 651, indicating the presence of a macromolecule chain in PGO (Figure 1). Moreover, a new peak at 1542 cm−1 (N–H stretching vibration), combined with that of the C=C in the aromatic ring (1617 cm−1), appeared along with a weakened peak at 1440 cm−1, corresponding to O=C–NH in the spectrum of PGO, identifying the presence of O=C–NH bands due to the intercalation and modification owing to PA-651. Furthermore, the weakened peak for O=C almost combined with the strong peak for the C=C bands, confirming the partial reduction of GO in the PGO product.
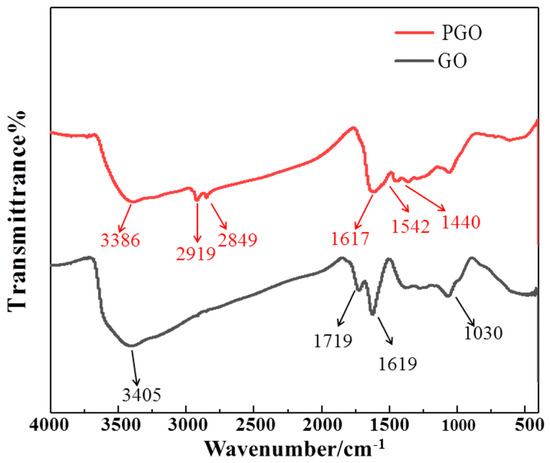
Figure 1.
FTIR spectrum of PGO and GO.
Figure 2a shows the XRD characterization results of GO and PGO. Due to the abundant oxygen-containing functional groups, the diffraction peak of GO is located at 2θ = 10.8°, which belongs to the (002) of GO, suggesting an interlayer spacing of 0.81 nm. After treatment with the curing agent, the diffraction peak of GO moved to a lower 2θ position and appeared at 2θ = 9.2°, which means that the interlayer spacing of PGO was about 0.96 nm. This indicates the increase in the interlayer spacing of GO, confirming the intercalation of PA 651 and the presence of functional groups in PGO.
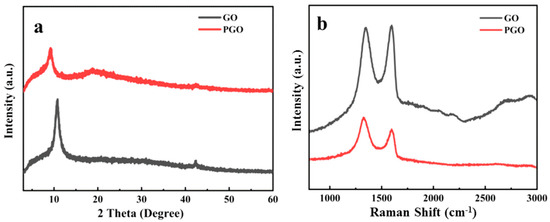
Figure 2.
XRD pattern (a) and Raman spectra (b) of GO and PGO.
Similar to other research on GO based carbon materials [2,3,4], two strong bands could be observed in the Raman spectra of both GO and PGO (Figure 2b). After the modification of GO with the polyamide, the D (1349 cm−1) and G bands (1597 cm−1) moved to 1329 cm−1 and 1596 cm−1, respectively, which indicated a certain charge transfer between the polyamide and GO. The intensity ratio between the D band and G band (ID/IG) is generally used to evaluate the quality of the carbon structure in various products (graphitization or defects). Due to the increase in isolated sp2 domains in the PA-651-modified GO, the intensity of the D band of PGO increased relative to that of pristine GO, resulting in an increase in the ID/IG from 0.98 for GO to 1.25, which demonstrated the successful reaction and modification of GO with PA-651 macromolecules.
Figure 3 shows the TGA and DSC curves of the GO samples before and after the treatment with PA-651. GO displayed a major weight loss around 200 °C (Figure 3a), which we attributed to the removal of the oxygen-containing functional groups, while the weight loss below 100 °C could be ascribed to the removal of the physically adsorbed water in the hydrophilic sample [36]. In the TGA curve of PGO, the main weight loss started from 130 °C, which is much lower than that of GO. This weight loss could have been due to the decomposition of PA-651 and confirmed the successful modification of GO with the molecules. The DSC of GO (Figure 3b) showed one strong exothermic peak around 220 °C, which was caused by the decomposition of the organic-oxygen-containing groups on the GO sheets [36,37]. For PGO, such a strong exothermic peak was absent, and the weak peak that appeared around 155 °C likely originated from the decomposition of the PA-651 macromolecules grafted onto the GO sheets. These results agree well with those of TGA.
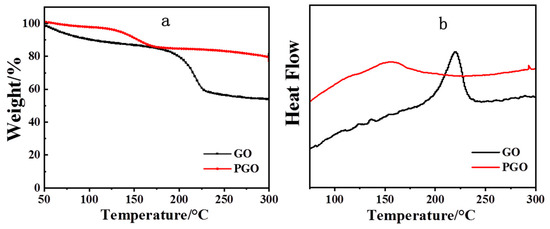
Figure 3.
TGA(a) and DSC (b) curves of GO and PGO.
The characteristic peaks in the C ls spectrum of GO according to XPS were related to C=C, C-C, C-OH, -C-O-C-, and COOH, (Figure 4a). The atomic ratio of O/C of PGO was 0.6, which is much less than that of GO (~1.3), confirming the large loss of oxygen-containing functional groups after the treatment with the curing agent. As shown in Figure 4b, two new peaks at 285.9 and 287.6 eV were detected in the C1s spectrum of PGO. They were related to C-N and HN-C=O in the PA-651 molecules grafted onto the carbon sheets, respectively. The atomic ratio of N/C of PGO was measured as 0.16. These experimental results confirmed that the polyamide macromolecules were grafted onto the surface of the GO. Additionally, the integral area ratio between the C=C and C-C peaks increased from 0.3 for GO to 1.2 for PGO, implying the occurrence of certain re-aromatization of GO in the PGO product. Furthermore, the advantage of first adding GO to PA-651 during the preparation of epoxy composite paints was demonstrated by its improved dispersion stability. As shown in Figure 5, the dispersion of PGO in n-butanol remained uniformly dark after 15 min of standing, in contrast with the almost complete precipitation of GO in the same solvent.
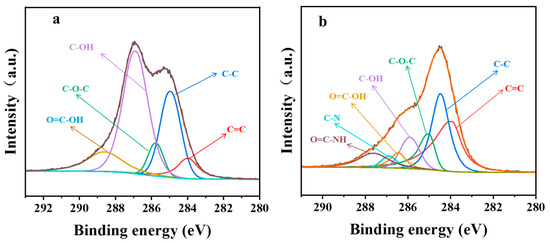
Figure 4.
C1s spectrum of (a) GO and (b) PGO.

Figure 5.
Digital photos of dispersion of GO and PGO in n-butanol: (a) just after ultrasonic irradiation and (b) after 15 min of standing.
3.2. Corrosion Resistance of the Epoxy Coatings
3.2.1. Corrosion Resistance of GO/EP/Zn Coatings
The corrosion resistance performance of the EP/Zn coating was characterized with potentiodynamic polarization tests. Figure 6 shows Tafel plots of both neat EP/Zn and GO/EP/Zn coatings in 3.5 wt.% NaCl solution. It can be seen that with the increase in GO content from 0.1 to 0.3 wt.%, the polarization curves of the coating shifted downward along with decreasing corrosion current density (Icorr). The Tafel test results, including the Icorr and corrosion potential (Ecorr), are shown in Table 1. The Icorr of neat EP/Zn coating was close to that for Zn-rich epoxy coating reported in literature [6].
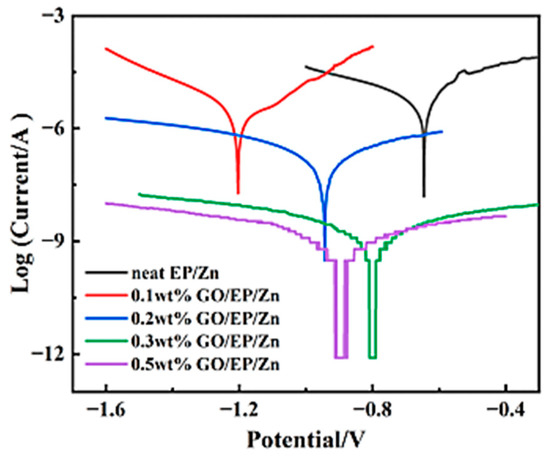
Figure 6.
Tafel curve of the coating incorporated with different contents of GO in 3.5 wt.% NaCl solution.

Table 1.
Icorr and Ecorr of GO/EP/Zn coating with different GO contents in 3.5 wt.% NaCl solution.
A lower content of GO (0.1 wt.%) was unfavorable and even deteriorated the corrosion inhibition performance of the coating, as its Icorr was larger than that of the neat EP/Zn coating. However, the Icorr of GO/EP/Zn coating decreased with the increasing GO content (Table 1). When the GO content was 0.3 wt.%, the Icorr of GO/EP/Zn was only 9.3 × 10−10 A cm−2, which indicated a nearly four orders of magnitude improvement in the corrosion resistance over that neat EP/Zn coating (6.1 × 10−6 A cm−2). However, when the GO content was further increased to 0.5 wt.%, its Icorr changed little (Table 1). Taking into account the cost of anticorrosion coatings, EP/Zn composite with 0.3 wt.% GO was determined to be the optimal coating for the surface protection of carbon steel and is called GO/EP/Zn in the following sections.
3.2.2. Corrosion Resistance of PGO/EP/Zn Coating
Interestingly, we found that the corrosion resistance of the GO-modified EP/Zn coating could be further reduced by simply changing the order of the addition of GO during the fabrication of the epoxy composite paints. Different from adding GO first in component A when manufacturing GO/EP/Zn paints, GO was added to component B (which acted as the curing agent in the epoxy system) when preparing PGO/EP/Zn paint. With this technique, the optimized GO content (0.3 wt.%) was adopted, and the as-prepared coating was named PGO/EP/Zn. Its corrosion resistance performance was also characterized with potentiodynamic polarization tests. As shown in Figure 7, its Tafel curve shifted in the positive direction along with decreasing corrosion current density in comparison with that of GO/EP/Zn. Its Icorr was 4.9 × 10−10 A cm−2, which was nearly half that of GO/EP/Zn, as shown in Table 2. The enhanced corrosion resistance could be attributed to the different reaction environments for GO for the different addition orders. During the fabrication process of PGO/EP/Zn, the plentiful amine groups in the polyamide macromolecules made component B a highly reactive solvent for GO, which contained organic functional groups, such as carboxyl, hydroxyl, and epoxy groups. With the pretreatment and mixing with component B, GO was modified by PA-651 to form PGO in component B, resulting in an increased interlayer spacing and improved dispersion stability, thus allowing the exploitation of its barrier properties to prevent corrosion in the coating. In contrast, the active groups in the epoxy oligomer in component A are not chemical active for GO, and the resultant GO/EP/Zn fabricated with this addition order exhibited inferior corrosion resistance.
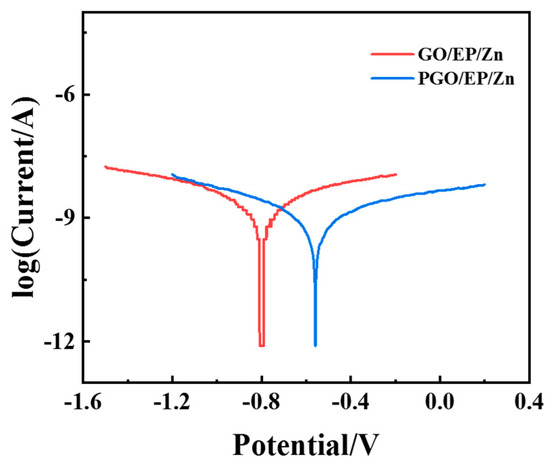
Figure 7.
Tafel curves of EP/Zn coatings modified with GO and PGO in 3.5 wt.% NaCl solution.

Table 2.
Icorr and Ecorr of neat EP/Zn, GO/EP/Zn and PGO/EP/Zn coating with different GO contents in 3.5 wt.% NaCl solution.
An immersion test was carried out to evaluate the long-term corrosion resistance performance of the three types of coatings. The impedance modulus at the lowest frequency (|Z|0.01Hz) in the Bode diagram of the coating is usually utilized as an important factor to identify the evolution of surface protection performance. As shown in Figure 8, the |Z|0.01Hz of all the three samples substantially decreased with increasing immersion time within the first tens of hours of immersion, which was due to the gradual infiltration of the corrosive species from the 3.5 wt.% NaCl electrolyte solution into the epoxy composite coating, similar to the results of other work on Zn-rich epoxy coating [22]. It was noted that the |Z|0.01Hz of neat EP/Zn decreased much faster than that of the GO/EP/Zn and PGO/EP/Zn samples, indicating the strong barrier property of GO and PGO sheets in the epoxy composite coating. In fact, the |Z|0.01Hz of neat EP/Zn was much less than those of both the GO/EP/Zn and PGO/EP/Zn samples during the whole immersion test, confirming the positive effect of the 2D GO and PGO sheet on the promotion of the corrosion resistance of the EP/Zn coating. After 2 days of immersion, the |Z|0.01Hz of all the three samples reached a relative stable state and insignificantly varied with increasing immersion time. Evidently, the stable part of the |Z|0.01Hz curve of PGO/EP/Zn was higher than that of GO/EP/Zn, indicating the superior corrosion resistance of PGO/EP/Zn compared with that of GO/EP/Zn. Moreover, after 300 h of immersion, its Tafel curve remained lower than those of both neat EP/Zn and GO/EP/Zn, as shown in Figure 9. Its Icorr was 1.2 × 10−6 A cm−2, which was nearly half that of GO/EP/Zn (4.9 × 10−5 A cm−2) and was one order magnitude less than that of neat EP/Zn (Table 3).
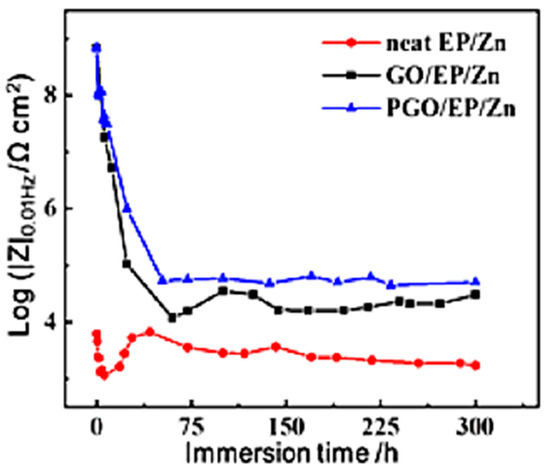
Figure 8.
The dependence of the impedance for neat EP/Zn, GO/EP/Zn, and PGO/EP/Zn coatings on immersion time in 3.5 wt.% NaCl solution.
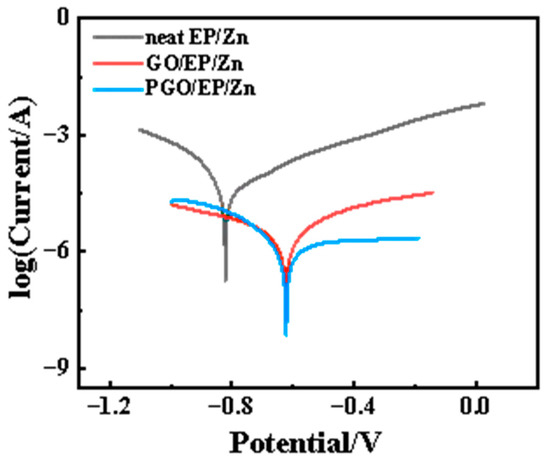
Figure 9.
Tafel curve of neat EP/Zn, GO/EP/Zn, and PGO/EP/Zn samples after 300 h of immersion in 3.5 wt.% NaCl solution.

Table 3.
Icorr and Ecorr of neat EP/Zn, GO/EP/Zn and PGO/EP/Zn coatings after 300 h of immersion in 3.5 wt.% NaCl solution.
In order to reveal the corrosion mechanism of the GO-incorporated EP/Zn coatings, Nyquist diagrams measured for the different coatings after 300 h of immersion in 3.5 wt.% NaCl solution were obtained, as shown in Figure 10. They were fitted and analyzed by the equivalent circuit, which is inset in Figure 10. In the equivalent circuit diagram, Rs refers to the solution resistance; CPE-1 and Rp represent the constant phase element value of the coating capacitor and coating pore resistance, respectively; CPE-2 and Rt represent the constant phase element and charge transfer resistance of metal-substrate double-layer capacitor, respectively [6,38]. The fitting component parameters for the EIS of GO/EP/Zn and PGO/EP/Zn in Figure 10 are shown in Table 4. As an important parameter of the fitted data in EIS, Rt is usually used to evaluate the corrosion resistance performance of a coating, where a high Rt value generally implies a low-speed electrochemical corrosion reaction. With the incorporation of GO, it was found that the Rt of both the GO/EP/Zn and PGO/EP/Zn coatings increased to varying degrees. We found that the Rt value of PGO/EP/Zn (64,520 Ω·cm−2) was more than twice that of GO/EP/Zn (31,830 Ω·cm−2), which agreed with the aforementioned Tafel results (Figure 9). This meant that the corrosion resistance of the EP/Zn coating was strongly promoted by the incorporation of 0.3 wt.% GO. In addition, a positive correlation exists between the Rp value and the physical barrier properties of a coating [1,6,38]. As shown in Table 4, the Rp value of PGO/EP/Zn was 13,115 Ω·cm−2, which was considerably more than that of GO/EP/Zn (5386 Ω·cm−2). This could be due to the grafting of PA 651 onto PGO, which led to its enhanced dispersibility in the coating and improved interaction with the matrix, therefore favoring the filling of micropores in the epoxy matrix and hindering the permeation of corrosives from the electrolyte solution.
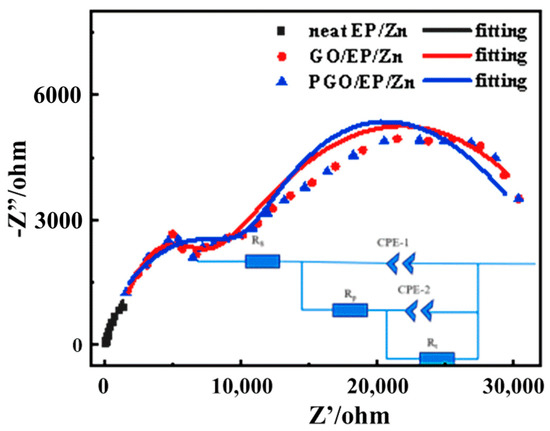
Figure 10.
Nyquist diagrams of neat EP/Zn, GO/EP/Zn, and PGO/EP/Zn samples after 300 h of immersion in 3.5 wt.% NaCl solution and the fitted data based on the inset equivalent circuit.

Table 4.
Electrochemical parameters extracted from the EIS results for the three coating samples.
The surface morphologies of the different coatings after 300 h of immersion (the circle area) to 3.5 wt.% NaCl solution are shown in Figure 11. After the long-term immersion test, the neat EP/Zn coating displayed a surface morphology different from that of the GO-incorporated epoxy composite coatings. It was seen that more than half of its exposed surface was covered with red corrosion products. The red materials were likely ferric compounds, which originated from the corrosion of the steel substrate. However, such red materials were totally absent on the surface of the GO/EP/Zn and PGO/EP/Zn coating samples, confirming the greatly enhanced corrosion resistance of the GO-incorporated epoxy composite coatings. For the GO/EP/Zn coating, some blistering appeared on its exposed surface, which was in contrast with that of the featureless surface of the PGO/EP/Zn coating. This finding also demonstrated the superior corrosion resistance of the PGO/EP/Zn coatings.
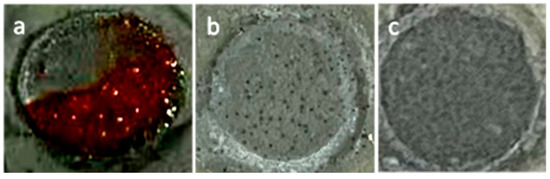
Figure 11.
Visual observations of the different coating samples exposed to 3.5 wt.% NaCl solution for 300 h of immersion: (a) neat EP/Zn, (b) GO/EP/Zn, and (c) PGO/EP/Zn samples. The diameter of the circle in the image is 1.1 cm.
4. Conclusions
In this study, GO was incorporated into epoxy/Zn composite paint to improve the corrosion resistance of the composite coating; the effect of the method of incorporating GO into the paint on this performance was studied as well. The main conclusions were drawn as follows:
- The corrosion resistance of epoxy/Zn composite paint was significantly improved by directly incorporating GO into the coating, and the optimized GO content was 0.3 wt.%.
- GO was successfully modified with PA-651 to form PGO in component B of the paint, resulting in increased interlayer spacing, higher thermal stability, as well as improved dispersion stability in the epoxy solvent.
- Due to the polyamide modification and the physical shielding of GO nanosheets, the PGO-incorporated EP/Zn coatings displayed a lower corrosion current density and a larger impedance modulus than the GO/EP/Zn coatings, when GO was added into component A of the epoxy composite paint.
- The modification of GO with the curing agent helped with filling the micropores in the epoxy coating, increasing the coating pore resistance and charge transfer resistance during the corrosion process.
Author Contributions
Conceptualization, S.H. and X.D.; data curation, L.Y., Y.L. and L.D.; investigation, S.H., L.Y., Y.L. and L.D.; methodology, G.W. and Z.Z.; supervision, X.D.; validation, G.W. and Z.Z.; writing—original draft, S.H.; writing—review and editing, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Key Project of Intelligent Manufacturing from the Educational Commission of Guangdong Province of China (project No. 2020ZDZX2048) and the Guangdong Province Science and Technology Plan (project No. 2021A0505030041).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Xusheng Du would like to acknowledge support from the Educational Commission of Guangdong Province and the Department of Science and Technology of Guangdong Province of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Wu, Y.; Ma, L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019, 169, 107694. [Google Scholar] [CrossRef]
- Wang, X.; Zou, J.; Du, X. Facile flame catalytic growing carbon coating on Cu panel with surface protection performance comparable to that of graphene film via CVD. Surf. Coat. Technol. 2022, 445, 128720. [Google Scholar] [CrossRef]
- Meng, W.; Zou, J.; Wang, X.; Zhang, P.; Du, X.-S. On the Distinctive Hardness, Anti-Corrosion Properties and Mechanisms of Flame-Deposited Carbon Coating with a Hierarchical Structure in Contrast to a Graphene Layer via Chemical Vapor Deposition. Nanomaterials 2022, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Marchebois, H.; Savall, C.; Bernard, J.; Touzain, S. Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta 2004, 49, 2945–2954. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Shirehjini, F.T.; Danaee, I.; Eskandari, H.; Zarei, D. Effect of Nano Clay on Corrosion Protection of Zinc-rich Epoxy Coatings on Steel 37. J. Mater. Sci. Technol. 2016, 32, 1152–1160. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Park, S.M.; Shon, M.Y. Effects of multi-walled carbon nanotubes on corrosion protection of zinc-rich epoxy resin coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Jalili, M.; Rostami, M.; Ramezanzadeh, B. An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 2015, 328, 95–108. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, L.; Tang, H.; Ye, H.; Li, M. Effect of Nano-SnS and Nano-MoS2 on the Corrosion Protection Performance of the Polyvinylbutyral and Zinc-Rich Polyvinylbutyral Coatings. Nanomaterials 2019, 9, 956. [Google Scholar] [CrossRef]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2018, 30, 1702944. [Google Scholar] [CrossRef]
- Zou, J.; Wang, X.; Zhang, P.; Du, X. Ultrafast flame coating of carbon and chemical vapor deposition of graphene on NiTi alloy to enhance its corrosion resistance. Diam. Relat. Mater. 2022, 128, 109231. [Google Scholar] [CrossRef]
- Wang, X.; Zou, J.; Lin, J.; Du, X. Promoting the corrosion resistance of carbon steel via facile catalytic deposition of novel hierarchical carbon film layer in an ethanol combustion flame. Diam. Relat. Mater. 2021, 120, 108714. [Google Scholar] [CrossRef]
- Halkjær, S.; Iversen, J.; Kyhl, L.; Chevallier, J.; Andreatta, F.; Yu, F.; Stoot, A.; Camilli, L.; Bøggild, P.; Hornekær, L. Low-temperature synthesis of a graphene-based, corrosion-inhibiting coating on an industrial grade alloy. Corros. Sci. 2019, 152, 1–9. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Mohamadzadeh Moghadam, M.H.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Han, D.; Ma, S.; Guo, W.; Cai, H.; Wang, X. Effect of graphene on corrosion resistance of waterborne inorganic zinc-rich coatings. J. Alloys Compd. 2019, 774, 255–264. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion Protection of Graphene-Modified Zinc-Rich Epoxy Coatings in Dilute NaCl Solution. ACS Appl. Nano Mater. 2019, 2, 180–190. [Google Scholar] [CrossRef]
- Golovin, V.A.; Tyurina, S.A. Current trends in the modification of zinc-filled polymer coatings. Int. J. Corros. Scale Inhib. 2020, 9, 372–393. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Dutta, G.K.; Karak, N. Bio-based waterborne polyester/cellulose nanofiber-reduced graphene oxide-zinc oxide nanocomposite: An approach towards sustainable mechanically robust anticorrosive coating. Cellulose 2022, 29, 1679–1703. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Long, Z.; Zhan, Y. Synergistic effect of functional carbon nanotubes and graphene oxide on the anti-corrosion performance of epoxy coating. Polym. Adv. Technol. 2017, 28, 754–762. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Qiang, Y.; Chen, Z.; Wang, L.; Gao, X.; Fang, Z. π-π interaction between fluorinated reduced graphene oxide and acridizinium ionic liquid: Synthesis and anti-corrosion application. Carbon 2020, 159, 292–302. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Jing, L.-C.; Wang, T.; Cao, W.-W.; Wen, J.-G.; Zhao, H.; Ning, Y.-J.; Yuan, X.-T.; Tian, Y.; Teng, L.-H.; Geng, H.-Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, Y.; Jing, C. Enhancing the Corrosion Resistance of Epoxy Coatings by Impregnation with a Reduced Graphene Oxide-Hydrophobic Ionic Liquid Composite. ChemElectroChem 2018, 5, 3300–3306. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Du, X.; Zhou, C.; Liu, H.Y.; Mai, Y.-W.; Wang, G.X. Facile chemical synthesis of nitrogen-doped graphene sheets and their electrochemical capacitance. J. Power Sources 2013, 241, 460–466. [Google Scholar] [CrossRef]
- Tian, Y.; Bi, Z.; Cui, G. Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings. Polymers 2021, 13, 1657. [Google Scholar] [CrossRef]
- Kasaeian, M.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-Benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM). Corros. Sci. 2018, 145, 119–134. [Google Scholar] [CrossRef]
- Nikpour, B.; Ramezanzadeh, B.; Bahlakeh, G.; Mahdavian, M. Synthesis of graphene oxide nanosheets functionalized by green corrosion inhibitive compounds to fabricate a protective system. Corros. Sci. 2017, 127, 240–259. [Google Scholar] [CrossRef]
- Du, X.S.; Yu, Z.-Z.; Dasari, A.; Ma, J.; Mo, M.; Meng, Y.Z.; Mai, Y.-W. New method to prepare graphite nanocomposites. Chem. Mater. 2008, 20, 2066. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.Y.; Mai, Y.-W. Ultrafast Synthesis of Multifunctional NDoped Graphene Foam in an Ethanol Flame. ACS Nano 2016, 10, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).