Bio-Based Photoreversible Networks Containing Coumarin Groups for Future Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
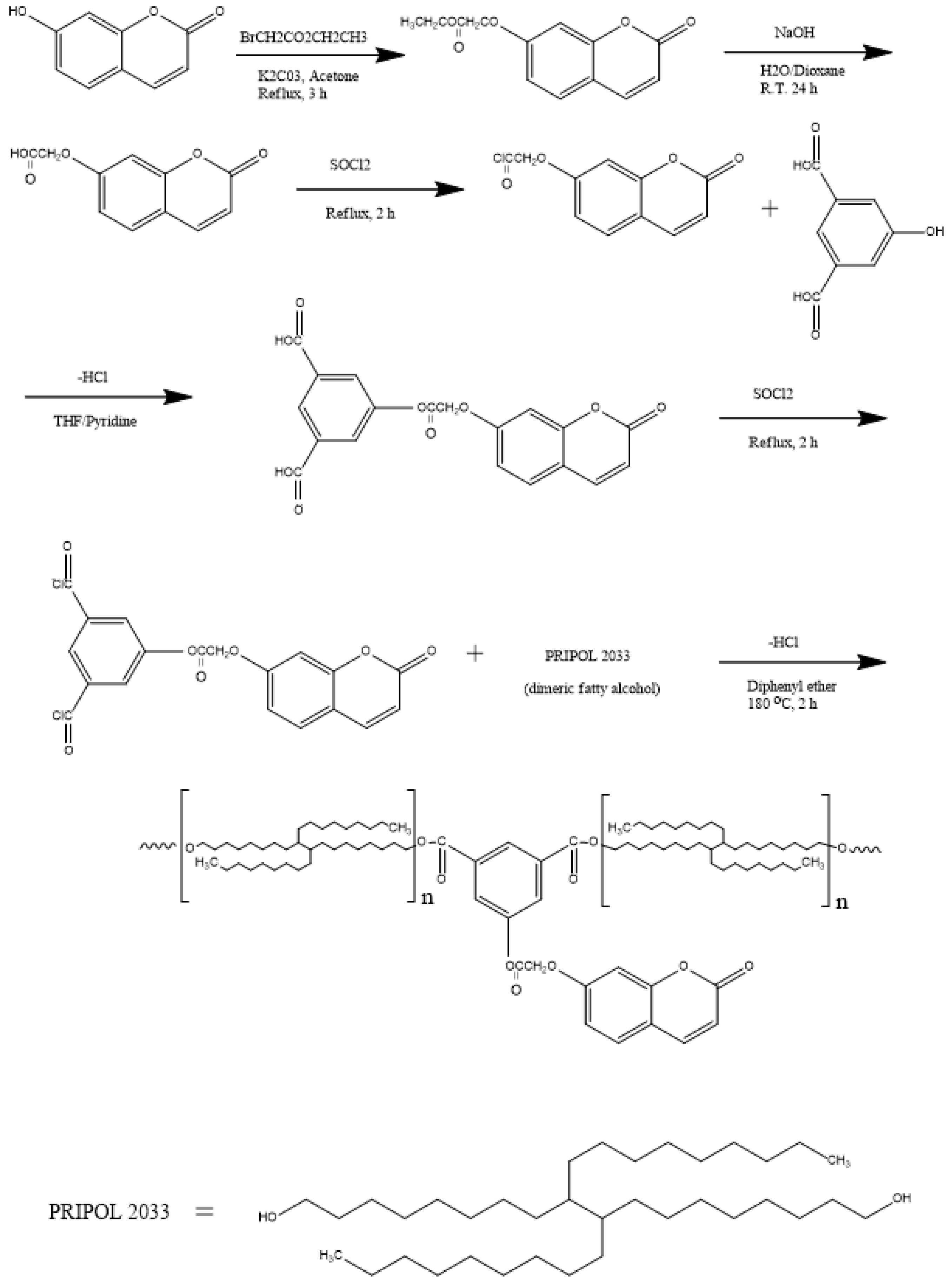
2.2. Synthesis of 7-Carboxymethoxycoumarin
2.3. Synthesis of 7-(3,5-Dicarboxyphenyl) Carbonylmethoxycoumarin (ICM)
2.4. Condensation Reaction
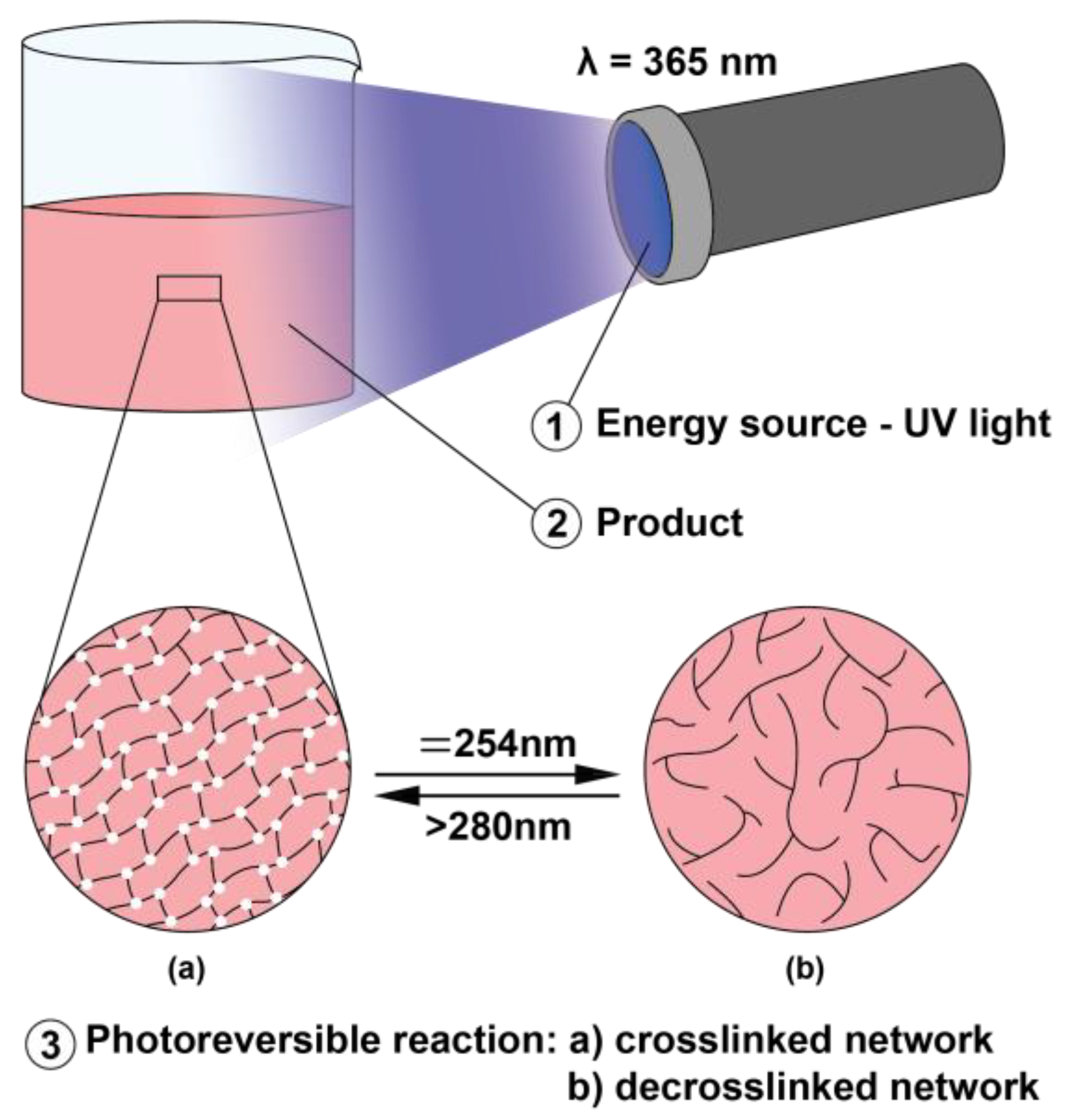
2.5. Photoreversible Reaction
2.6. Characterization Methods
3. Results and Discussion
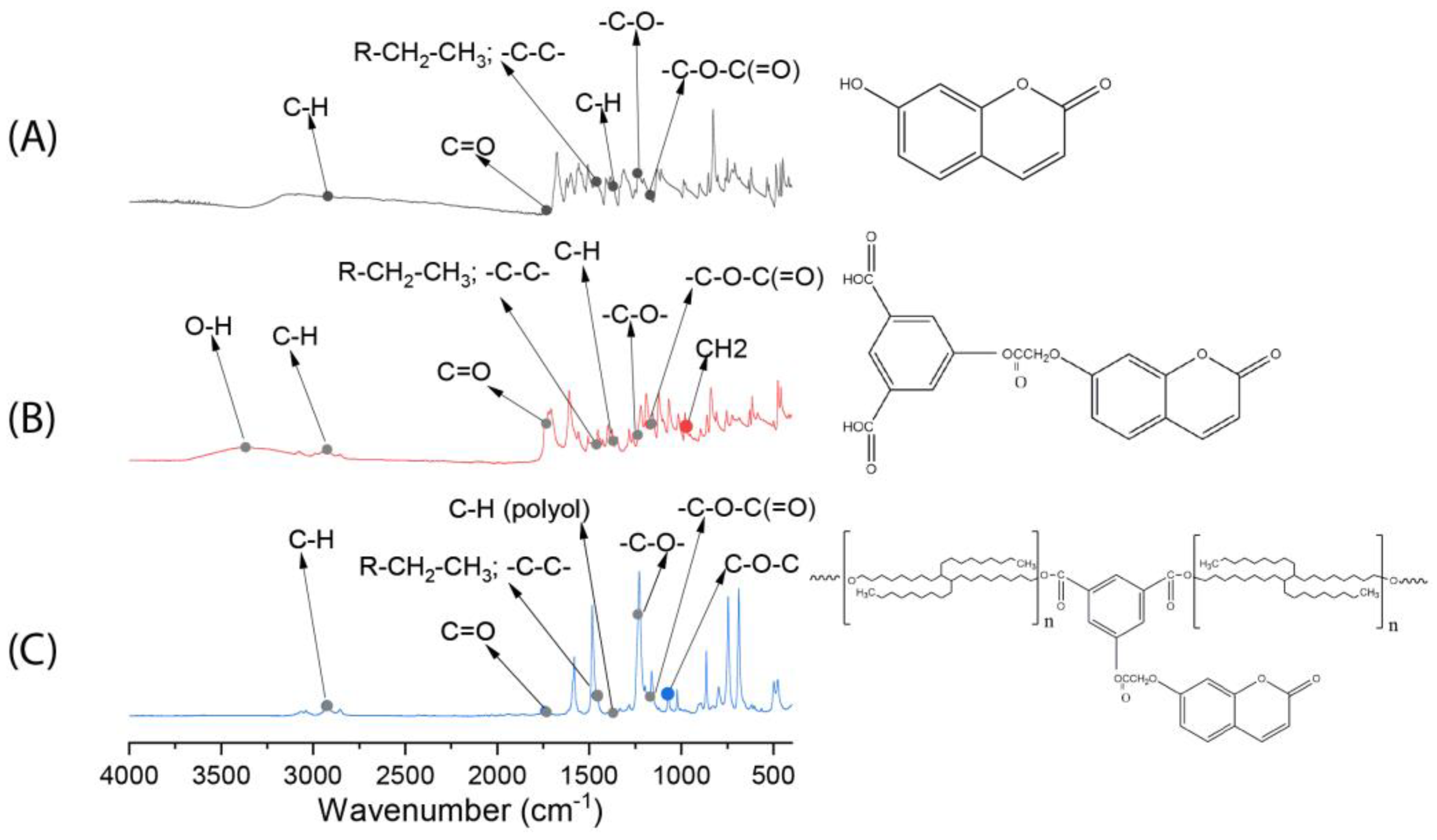
3.1. FTIR Spectroscopy
3.2. NMR Spectroscopy
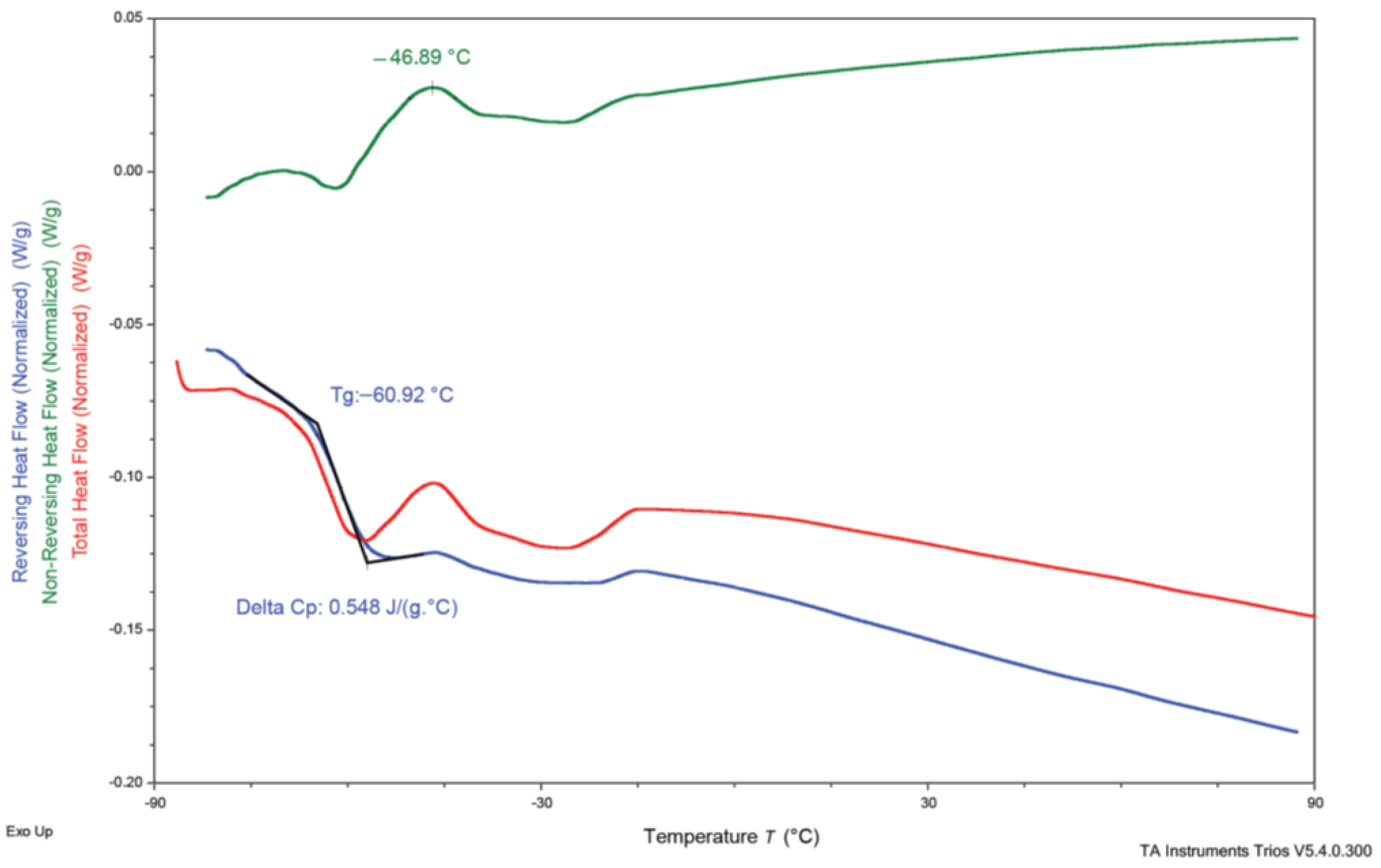
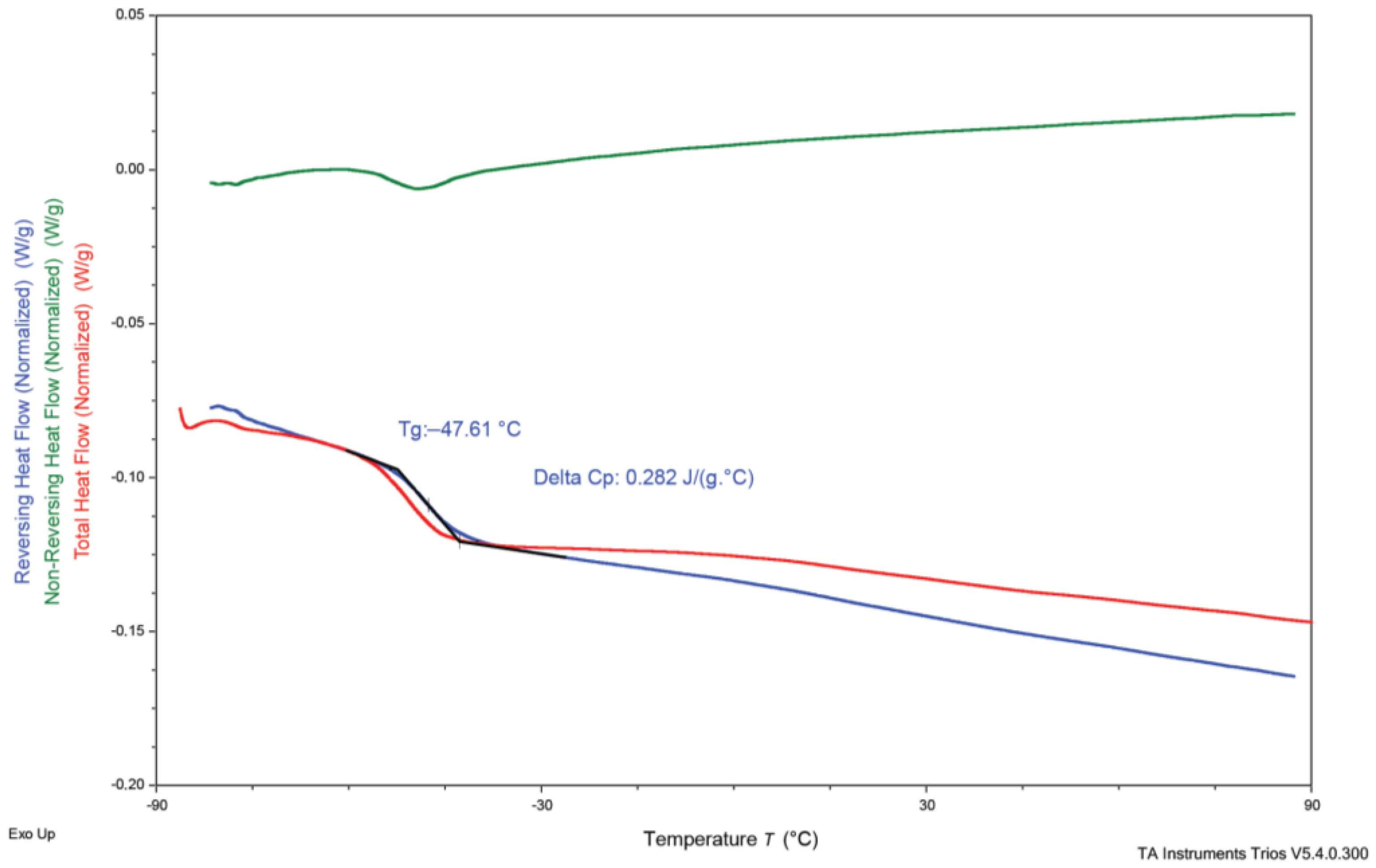
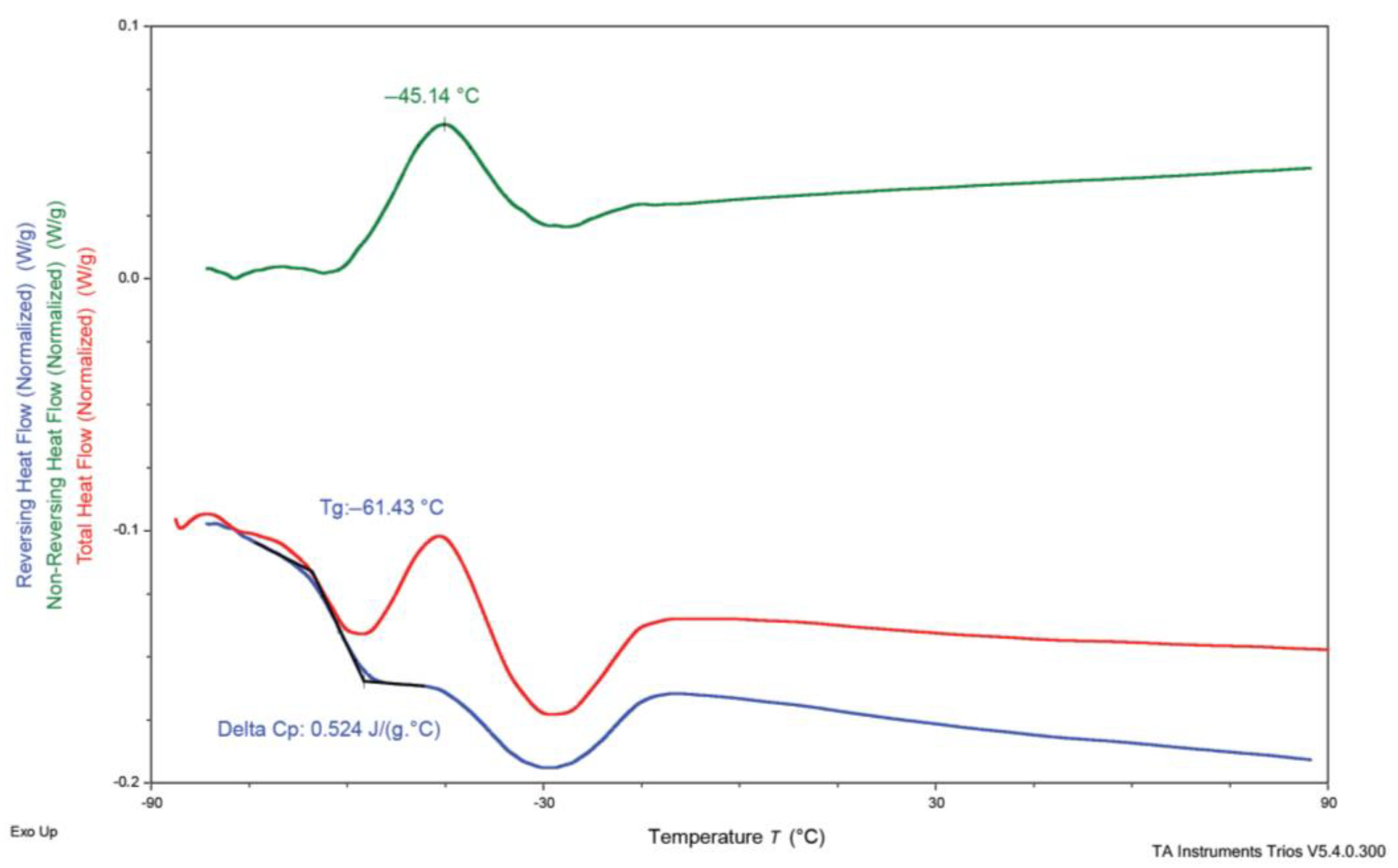
3.3. Differential Scanning Calorimetry (DSC)
3.4. Rheology
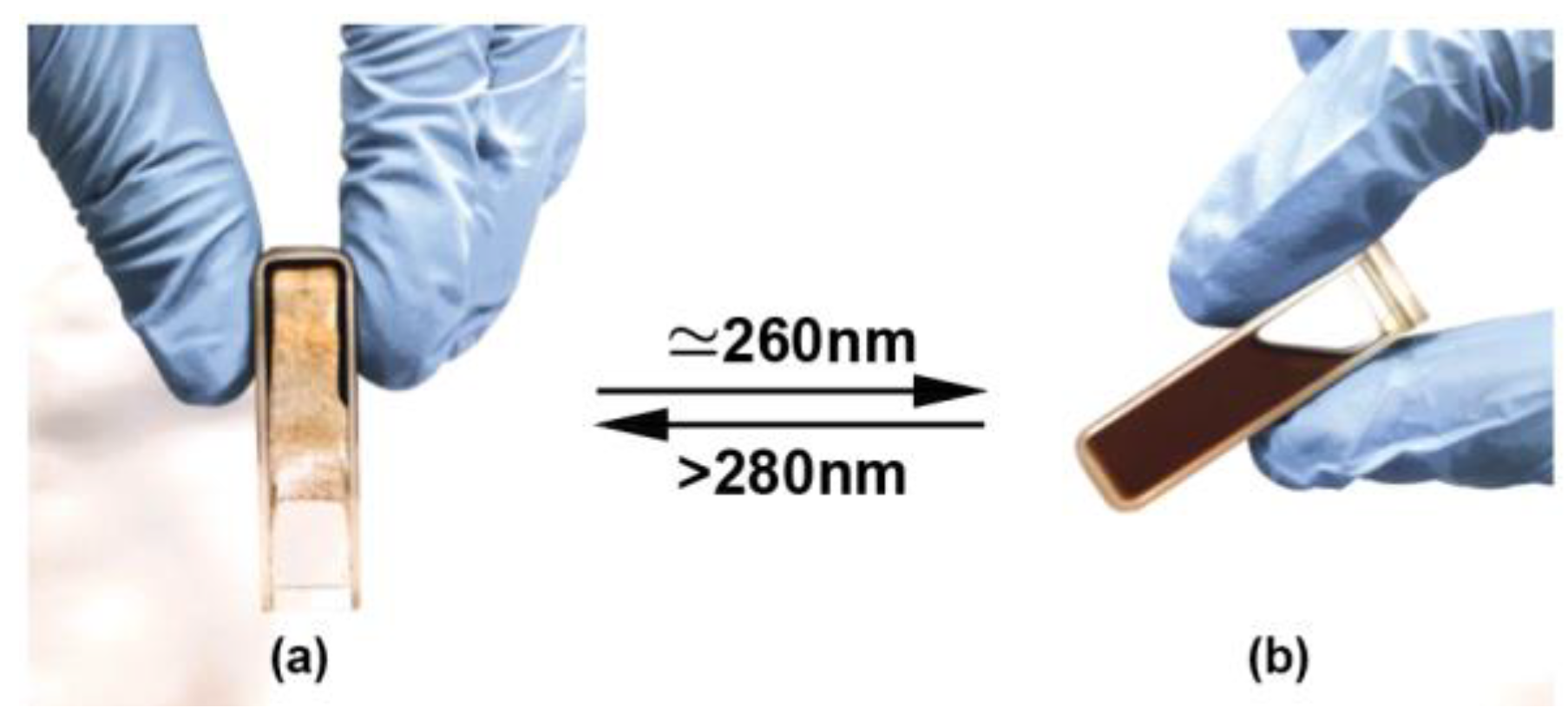
3.5. Photo-Reversible Crosslinking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mooney, D.J. Designing hydrogels for tissue engineering. Nat. Mater. 2012, 11, 23–36. [Google Scholar]
- Ha, H.-J.; Kim, J.-H.; Kim, J.-H.; Lee, Y.-S. UV-Curable Semi-Interpenetrating Polymer Network-Integrated, Highly Bendable Plastic Crystal Composite Electrolytes for Shape-Conformable All-Solid-State Lithium Ion Batteries. Energy Environ. Sci. 2012, 5, 7122–7129. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Liu, Z.; Ren, J.; Li, X. Light-Triggered Topological Programmability in a Dynamic Covalent Polymer Network. Sci. Adv. 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Jellali, R.; Bertrand, V.; Alexandre, M.; Rosière, N.; Grauwels, M.; De Pauw-Gillet, M.-C.; Jérôme, C. Photoreversibility and Bio-compatibility of Polydimethylsiloxane-Coumarin as Adjustable Intraocular Lens Material. Macromol. Biosci. 2017, 17, 1600049. [Google Scholar] [CrossRef]
- Lopez, S.; Rodríguez-Lopez, J.; García, M.T.; Rodríguez, J.F.; Pérez-Ortiz, J.M.; Ramos, M.J.; Gracia, I. Self-assembled coumarin- and 5-fluorouracil-PEG micelles as multifunctional drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 74, 103582. [Google Scholar] [CrossRef]
- Van Lijsebetten, F.; Debsharma, T.; Winne, J.M.; Du Prez, F.E. A Highly Dynamic Covalent Polymer Network without Creep: Mission Impossible? Angew. Chem. Int. Ed. 2021, 61, 544–549. [Google Scholar]
- Nagata, M.; Yamamoto, Y. Photoreversible Poly(ethylene glycol)s with Pendant Coumarin Group and Their Hydrogels. Biomacromolecules 2012, 13, 4213–4221. [Google Scholar]
- Mitov, M.; Kopeček, J.K.; Thomas, E.L. Photoresponsive polymers for biomedical applications. Prog. Polym. Sci. 2011, 36, 1835–1866. [Google Scholar]
- Qin, X.-H.; Ovsianikov, A.; Stampfl, J.; Liska, R. Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMaterials 2014, 15, 49–70. [Google Scholar] [CrossRef] [Green Version]
- Chujo, Y.; Sada, K.; Saegusa, T. Polyoxazoline Having a Coumarin Moiety as a Pendant Group: Synthesis and Photogelation. Macromolecules. 1989, 23, 2693–2697. [Google Scholar] [CrossRef]
- Chujo, Y.; Sada, K.; Saegusa, T. Reversible gelation of polyoxazoline by means of Diels-Alder reaction. Macromolecules 1990, 23, 2636–2641. [Google Scholar] [CrossRef]
- Mir, M.; Najabat Ali, M.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Wu, Q.; Han, X.; Zhang, W.; Wei, W.; Chen, L.; Li, L.; Huang, W. Photosensitive hydrogels: From structure, mechanisms, design to bioapplications. Sci. China Life Sci. 2020, 63, 1813–1828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, J.H.; An, D.H.; Park, S.J.; Lee, J.H. Development of photoresponsive hydrogels for controlled drug delivery. J. Control. Release 2009, 140, 196–205. [Google Scholar]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels in biomedical applications. Adv. Drug Deliv. Rev.. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Mooney, D.J. Photoresponsive Hydrogels for Tissue Engineering. In Tissue Engineering; Lanza, R., Langer, R., Vacanti, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 1, pp. 183–195. [Google Scholar]
- Li, Y.; Li, Y.; Wang, H. Recent Progress in Photoresponsive Hydrogels for Biomedical Applications. Adv. Healthc. Mater. 2014, 3, 1135–1154. [Google Scholar]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural Polymers-Based Light-Induced Hydrogels: Promising Biomaterials for Biomedical Applications. Chem. Cell Res. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Zarei, M.; El Fray, M. Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers 2021, 13, 3177. [Google Scholar] [CrossRef]
- Niedźwiedź, M.J.; Demirci, G.; Kantor-Malujdy, N.; Sobolewski, P.; El Fray, M. Fatty-Acid-Derived Ester-Urethane Macromonomers Synthesized Using Bismuth and Zinc Catalysts. Eur. Polym. J. 2022, 170, 111168. [Google Scholar] [CrossRef]
- Skrobot, J.; Ignaczak, W.; El Fray, M. Hydrolytic and Enzymatic Degradation of Flexible Polymer Networks Comprising Fatty Acid Derivatives. Polymers 2015, 7, 215–220. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [Green Version]
- Parcheta, M.; Swisłocka, R.; Orzechowska, S.; Akimowicz, M.; Choinska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Ponnaiya, B.; Buonanno, M.; Welch, D.; Shuryak, I.; Randers-Pehrson, G.; Brenner, D.J. Far-UVC light prevents MRSA infection of superficial wounds in vivo. PLoS ONE 2018, 13, e0192053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zein El Abedin, S.; Endres, F. Raman and FTIR Spectroscopic Studies of 1-Ethyl-3-methylimidazolium Trifluoromethylsulfonate, its Mixtures with Water and the Solvation of Zinc Ions. Molecules 2015, 20, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Pourhossein, M.; Heravizadeh, O.R.; Omidi, F.; Khadem, M.; Shahtaheri, S.J. Ultrasound-Assisted Emulsified Microextraction Based on Deep Eutectic Solvent for Trace Residue Analysis of Metribuzin in Urine Samples. Molecules 2021, 26, 3957. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chen, Z. Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study. Nanomaterials 2017, 7, 398. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Ahmad, I.; Zheng, Y. Fourier Transform Infrared Spectroscopy of “Bisphenol A”. Spectrosc. Lett. 2020, 53, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Rashad, M.M.; Nooman, M.U.; Ali, M.M.; Al-kashef, A.S.; Mahmoud, A.E. Production, Characterization and Anticancer Activity of Candida bombicola Sophorolipids by Means of Solid State Fermentation of Sunflower Oil Cake and Soybean Oil. Molecules 2011, 16, 4179–4195. [Google Scholar] [CrossRef] [Green Version]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing Amorphous Silica, Short-Range-Ordered Silicates and Silicic Acid Species by FTIR. Minerals 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, P.; Zong, Y.; Xu, Z.; Chen, S. Analysis of FT-IR Spectra of Dicyclopentadienyl (Bis-Substituted Cyclopentadienyl) Dithiocyano of Titanium, Zirconium and Hafnium. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.J.; Leonard, P.; Tormena, C.F. 1H NMR Spectra. Part 28†: Proton chemical shifts and couplings in three-membered rings. A ring current model for cyclopropane and a novel dihedral angle dependence for 3JHH couplings involving the epoxy proton. Magn. Reson. Chem. 2009, 47, 263–268. [Google Scholar]
- Dai, J.; Kim, J.-C. Photo responsive monoolein cubic phase containing coumarin-Tween 20 conjugates. Drug Dev. Ind. Pharm. 2013, 39, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Kapur, G.S.; Berger, S. Unambiguous Resolution of α-Methyl and α-Methylene Protons in 1H NMR Spectra of Heavy Petroleum Fractions. Energy Fuels 2005, 19, 135–137. [Google Scholar] [CrossRef]
- Mori, K.; Murai, O.; Hashimoto, S.; Nakamura, Y. Highly Regio- and Stereoselective Photocycloaddition between Coumarin and Thymine by Molecular Recognition. Tetrahedron Lett. 1996, 37, 4079–4082. [Google Scholar] [CrossRef]


| Sample | Dynamic Viscosity (mPa⋅s) at 25 °C |
|---|---|
| Before crosslinking | 43,660 |
| After de-crosslinking | 45,670 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elchiev, I.; Demirci, G.; El Fray, M. Bio-Based Photoreversible Networks Containing Coumarin Groups for Future Medical Applications. Polymers 2023, 15, 1885. https://doi.org/10.3390/polym15081885
Elchiev I, Demirci G, El Fray M. Bio-Based Photoreversible Networks Containing Coumarin Groups for Future Medical Applications. Polymers. 2023; 15(8):1885. https://doi.org/10.3390/polym15081885
Chicago/Turabian StyleElchiev, Iskenderbek, Gokhan Demirci, and Miroslawa El Fray. 2023. "Bio-Based Photoreversible Networks Containing Coumarin Groups for Future Medical Applications" Polymers 15, no. 8: 1885. https://doi.org/10.3390/polym15081885
APA StyleElchiev, I., Demirci, G., & El Fray, M. (2023). Bio-Based Photoreversible Networks Containing Coumarin Groups for Future Medical Applications. Polymers, 15(8), 1885. https://doi.org/10.3390/polym15081885







