Studies of the Tarragon Essential Oil Effects on the Characteristics of Doped Hydroxyapatite/Chitosan Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.2.1. Physico-Chemical Characterization
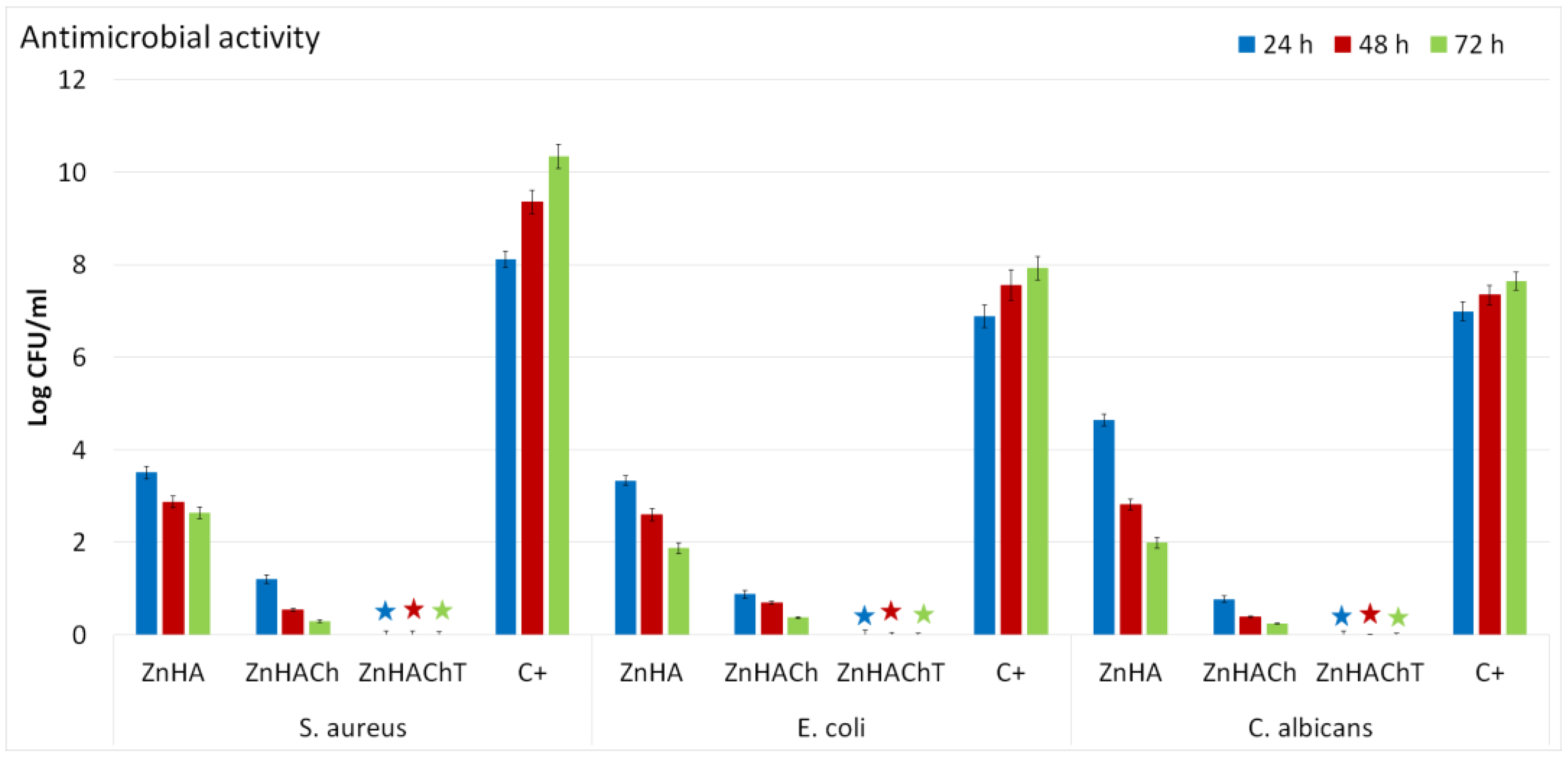
2.2.2. In Vitro Antimicrobial Assay
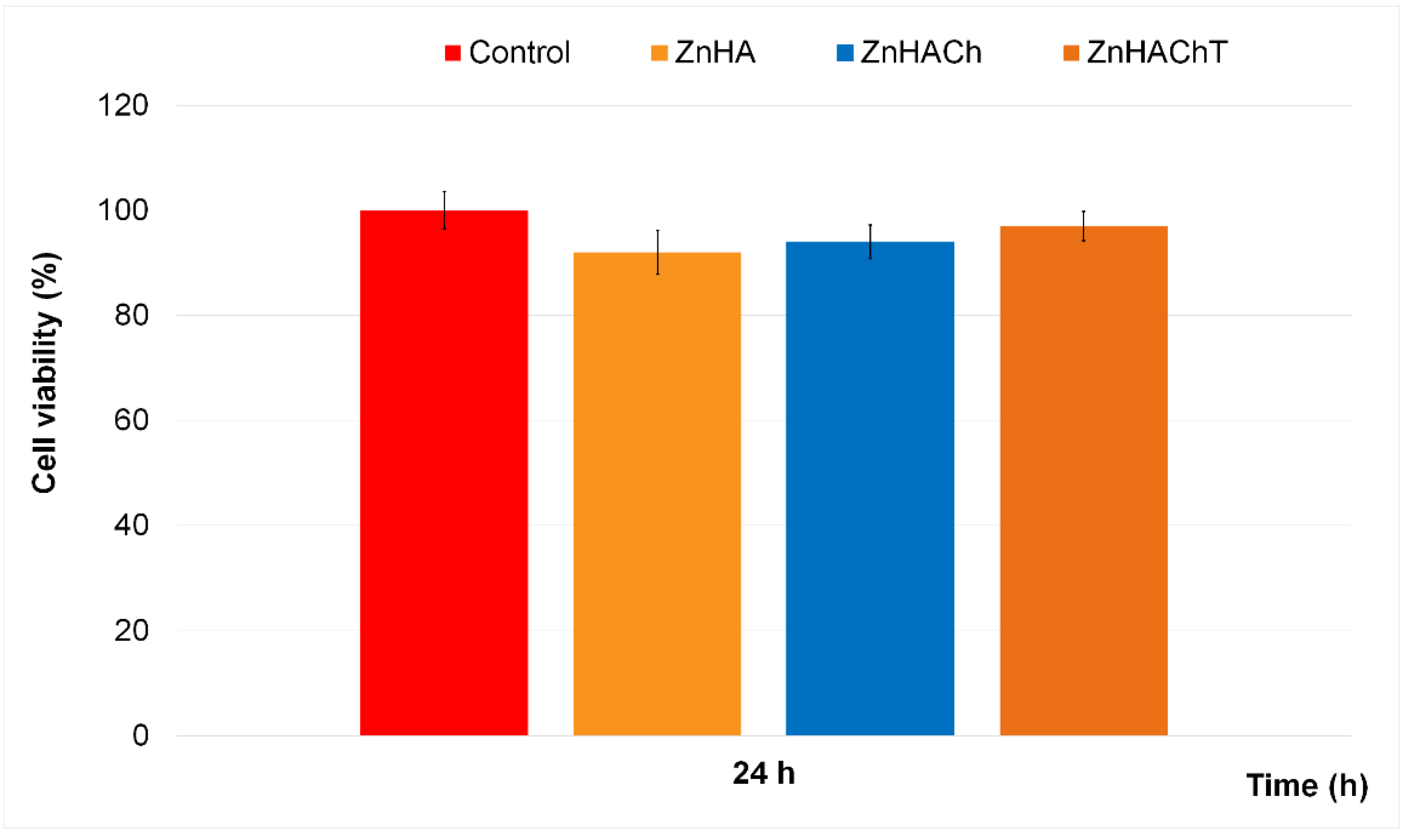
2.2.3. Cytotoxicity Assay
2.2.4. Statistical Analysis
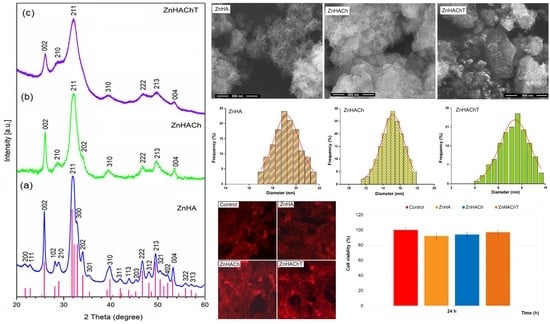
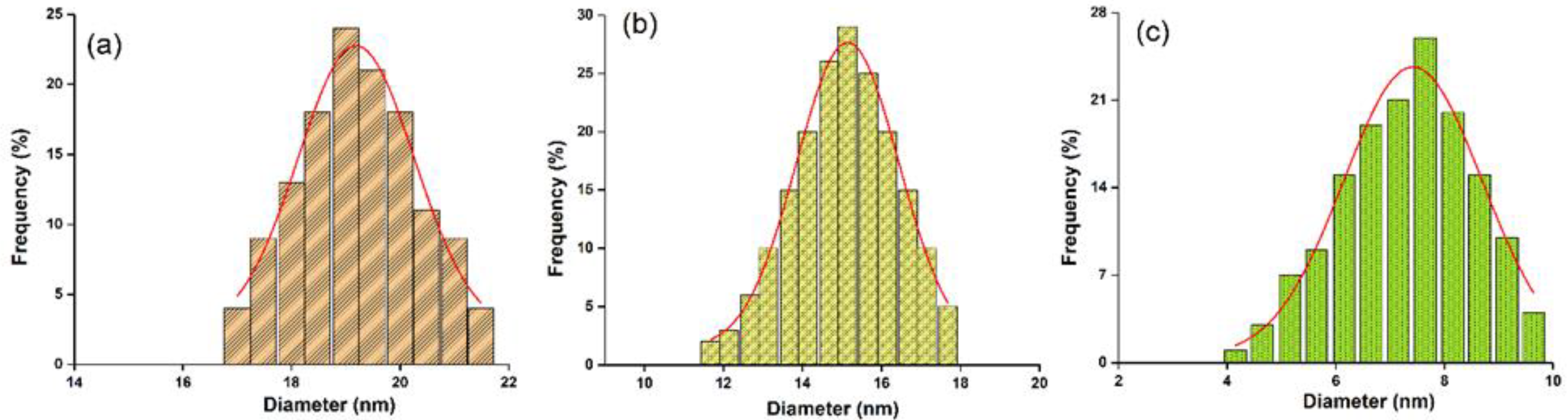
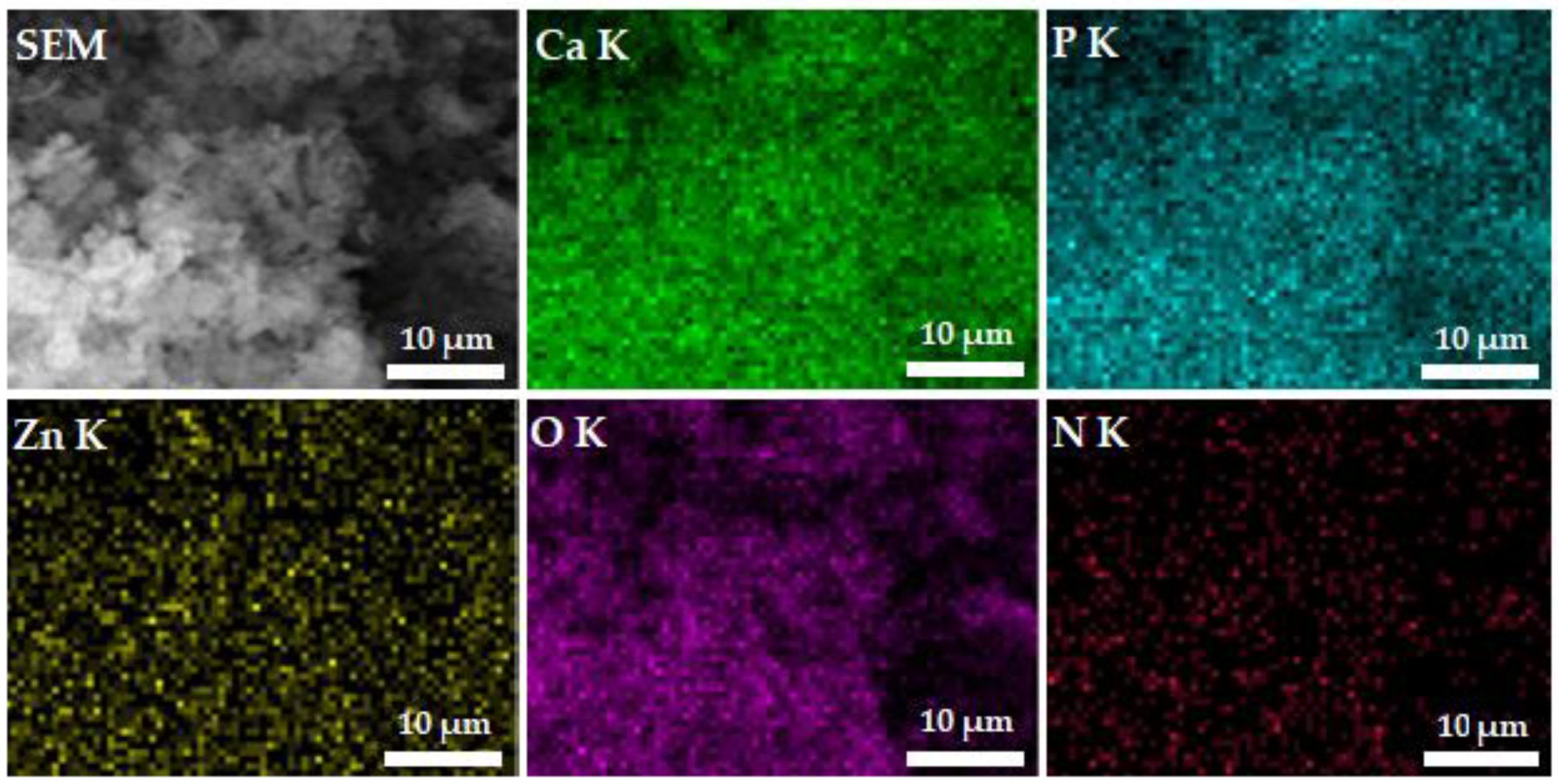
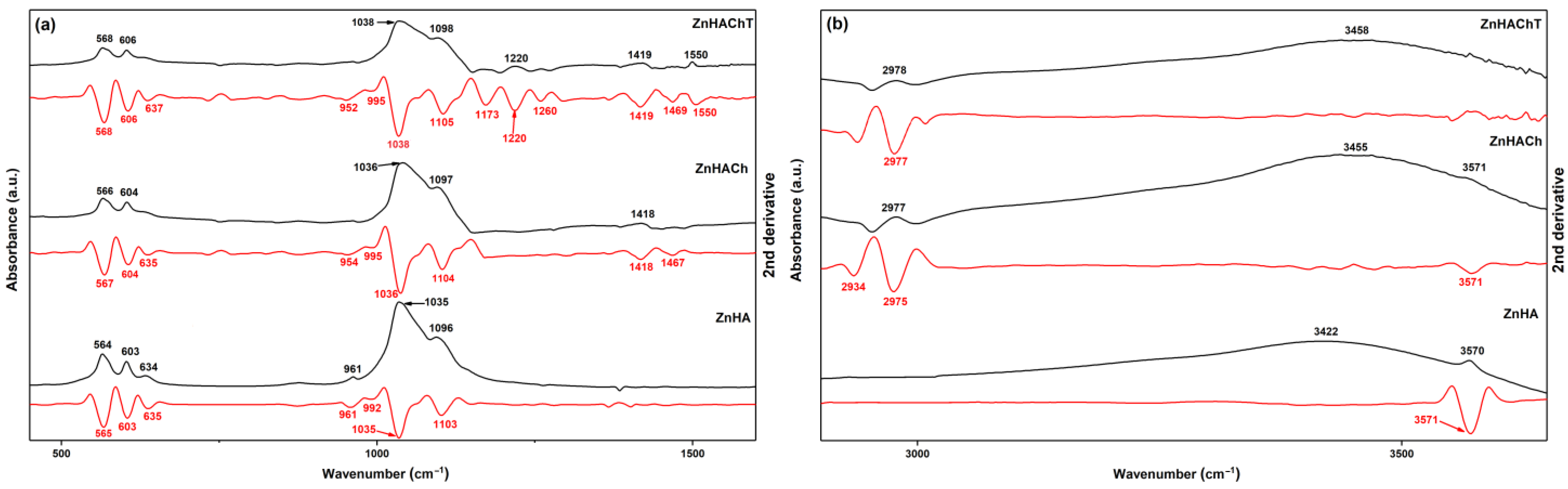
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T. The staggering death toll of drug-resistant bacteria. Nature, 2022; ahead of print. [Google Scholar] [CrossRef]
- Gupta, A.; Pratt, R.; Mishra, B. Physicochemical characterization of ferric pyrophosphate citrate. Biometals 2018, 31, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.; Kamaraj, Y.; Kumaresan, V.; Kannaiyan, M.; David, E.; Ranganathan, B.; Selvaraj, V.; Balupillai, A. Green synthesized zinc oxide nanoparticles induce apoptosis by suppressing PI3K/Akt/mTOR signaling pathway in osteosarcoma MG63 cells. Global Transl. Med. 2022, 1, 34. [Google Scholar] [CrossRef]
- Karahan, M.; Karahan, N.; Ozkan, F.; Yildirim, K. Characterization of Natural Reinforcements and their Composites. J. Compos. Biodegrad. Polym. 2021, 9, 17–34. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, H.; Cai, J. Preparation and characterization of nano-hydroxyapatite/chitosan composite with enhanced compressive strength by urease-catalyzed method. Mater. Lett. 2014, 116, 293–295. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, C.; Cao, G.; Wu, D.; Gao, C.; Lakshmanan, S. Cryogel biocomposite containing chitosan-gelatin/cerium-zinc doped hydroxyapatite for bone tissue engineering. Saudi J. Biol. Sci. 2020, 27, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Paterson, T.E.; Shi, R.; Tian, J.; Harrison, C.J.; De Sousa Mendes, M.; Hatton, P.V.; Li, Z.; Ortega, I. Electrospun Scaffolds Containing Silver-Doped Hydroxyapatite with Antimicrobial Properties for Applications in Orthopedic and Dental Bone Surgery. J. Funct. Biomater. 2020, 11, 58. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial Effect and Cytotoxic Evaluation of Mg-Doped Hydroxyapatite Functionalized with Au-Nano Rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Vinicius Beserra dos Santos, M.; Bastos Nogueira Rocha, L.; Gomes Vieira, E.; Leite Oliveira, A.; Oliveira Lobo, A.; de Carvalho, M.A.M.; Anteveli Osajima, J.; Cavalcanti Silva-Filho, E. Development of Composite Scaffolds Based on Cerium Doped-Hydroxyapatite and Natural Gums—Biological and Mechanical Properties. Materials 2019, 12, 2389. [Google Scholar] [CrossRef]
- Nenen, A.; Maureira, M.; Neira, M.; Orellana, S.L.; Covarrubias, C.; Moreno-Villoslada, I. Synthesis of antibacterial silver and zinc doped nano-hydroxyapatite with potential in bone tissue engineering applications. Ceram. Int. 2022, 48, 34750–34759. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Golus, J.; Kolmas, J.; Wojcik, M.; Kolodynska, D.; Przekora, A. Noncytotoxic zinc-doped nanohydroxyapatite-based bone scaffolds with strong bactericidal, bacteriostatic, and antibiofilm activity. Biomat. Adv. 2022, 139, 213011. [Google Scholar] [CrossRef]
- Rasyida, A.; Wicaksono, S.T.; Pradita, N.N.; Ardhyananta, H.; Purnomo, A. Effect of chitosan addition to characteristic and antimicrobial activity of zinc doped hydroxyapatite. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012063. [Google Scholar] [CrossRef]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Hazarika, U.; Kovács, Z.; Bodor, Z.; Gosztola, B. Phytochemicals and organoleptic properties of French tarragon (Artemisia dracunculus L.) influenced by different preservation methods. LWT 2022, 169, 114006. [Google Scholar] [CrossRef]
- Liu, T.; Lin, P.; Bao, T. Essential oil composition and antimicrobial activity of Artemisia dracunculus L. var. qinghaiensis Y. R. Ling (Asteraceae) from Qinghai-Tibet Plateau. Ind. Crops Prod. 2018, 125, 1–4. [Google Scholar] [CrossRef]
- Fildan, A.P.; Pet, I.; Stoin, D.; Bujanca, G. Artemisia dracunculus essential oil chemical composition and antioxidant properties. Rev. Chim. 2019, 70, 59–62. [Google Scholar] [CrossRef]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zedan, H.; Hosseini, S.M.; Mohammadi, A. The effect of tarragon (Artemisia dracunculus) essential oil and high molecular weight chitosan on sensory properties and shelf life of yogurt. LWT 2021, 147, 111613. [Google Scholar] [CrossRef]
- Predoi, S.A.; Ciobanu, S.C.; Chifiriuc, M.C.; Motelica-Heino, M.; Predoi, D.; Iconaru, S.L. Hydroxyapatite Nanopowders for Effective Removal of Strontium Ions from Aqueous Solutions. Materials 2023, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, L.; An, J. Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Cololabis saira. Crystals 2017, 7, 103. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.-L.; Predoi, M.-V.; Buton, N. Development of Novel Tetracycline and Ciprofloxacin Loaded Silver Doped Hydroxyapatite Suspensions for Biomedical Applications. Antibiotics 2023, 12, 74. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Gundle, R.; Beresford, N.J. Isolation and culture of bone forming cells (osteoblasts) from human bone. Hum. Cell Cult. Protoc. 1996, 2, 233–263. [Google Scholar]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic Layers with Antifungal Properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; Wiley: Brisbane, Australia, 1996. [Google Scholar]
- Brundavanam, R.K.; Jiang, Z.T.; Chapman, P.; Le, X.T.; Mondinos, N.; Fawcett, D.; Poinern, G.E.J. Effect of dilute gelatine on the ultrasonic thermally assisted synthesis of nano hydroxyapatite. Ultrason. Sonochem. 2011, 18, 697–703. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Chu, M.C.; Balakrishnan, A.; Lee, Y.J.; Kim, T.N.; Cho, S.J. Synthesis of nano hydroxyapatite powder that simulate teeth particle morphology and composition. Curr. Appl. Phys. 2009, 9, 1459–1462. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Tiji, S.; Lakrat, M.; Rokni, Y.; Mejdoubi, E.M.; Hano, C.; Addi, M.; Asehraou, A.; Mimouni, M. Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite. Antibiotics 2022, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Danylchenko, S.M.; Kalinkevich, O.V.; Pogorelov, M.V. Chitosan–hydroxyapatite composite biomaterials made by a one step co-precipitation method: Preparation, characterization and in vivo tests. J. Biol. Phys. Chem. 2009, 9, 119–126. [Google Scholar] [CrossRef]
- Osanloo, M.; Sedaghat, M.M.; Sereshti, H.; Rahmani, M.; Saeedi Landi, F.; Amani, A. Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J. Nanostruct. 2019, 9, 723–735. [Google Scholar]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Dertli, E. A green nano-biosynthesis of selenium nanoparticles with Tarragon extract: Structural, thermal, and antimicrobial characterization. LWT 2021, 141, 110969. [Google Scholar] [CrossRef]
- Osanloo, M.; Firooziyan, S.; Abdollahi, A.; Hatami, S.; Nematollahi, A.; Elahi, N.; Zarenezhad, E. Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; larvicidal effect and antibacterial activity. BMC Res. Notes 2022, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral. Sci. 1991, 15, 1–201. [Google Scholar]
- Prabakaran, K.; Rajeswari, S. Spectroscopic investigations on the synthesis of nano-hydroxyapatite from calcined eggshell by hydrothermal method using cationic surfactant as template. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 1127–1134. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, X.; Younis, M.R.; Blum, N.T.; Lei, S.; Zhang, D.; Luo, Y.; Huang, P.; Lin, J. Non-invasive monitoring of in vivo bone regeneration based on alkaline phosphatase-responsive scaffolds. Chem. Eng. J. 2021, 408, 127959. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazinska, M.; Kryszak, B.; Grzymajło, M.; Pigłowski, J.; Wiglusz, R.J.; Okamoto, M. The influence of hydroxyapatite content on properties of poly(L-lactide)/hydroxyapatite porous scaffolds obtained using thermal induced phase separation technique. Eur. Polym. J. 2019, 113, 313–320. [Google Scholar] [CrossRef]
- Yu, J.; Xu, L.; Li, K.; Xie, N.; Xi, Y.; Wang, Y.; Zheng, X.; Chen, X.; Wang, M.; Ye, X. Zinc-modified calcium silicate coatings promote osteogenic differentiation through TGF-β/Smad pathway and osseointegration in osteopenic rabbits. Sci. Rep. 2017, 7, 3440. [Google Scholar] [CrossRef]
- Gazinska, M.; Krokos, A.; Kobielarz, M.; Włodarczyk, M.; Skibinska, P.; Stepak, B.; Antończak, A.; Morawiak, M.; Płocinski, P.; Rudnicka, K. Influence of hydroxyapatite surface functionalization on thermal and biological properties of poly(L-lactide)-and poly(l-lactide-co-glycolide)-based composites. Int. J. Mol. Sci. 2020, 21, 6711. [Google Scholar] [CrossRef] [PubMed]
- Korbut, A.; Włodarczyk, M.; Rudnicka, K.; Szwed, A.; Płociński, P.; Biernat, M.; Tymowicz-Grzyb, P.; Michalska, M.; Karska, N.; Rodziewicz-Motowidło, S.; et al. Three Component Composite Scaffolds Based on PCL, Hydroxyapatite, and L-Lysine Obtained in TIPS-SL: Bioactive Material for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 13589. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Qing, Y.A.; Li, R.; Tang, X.; Guo, D.; Qin, Y. Enhancing ZnO-NP antibacterial and osteogenesis properties in orthopedic applications: A review. Int. J. Nanomed. 2020, 15, 6247–6262. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. T Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotech. 2020, 8, 61. [Google Scholar] [CrossRef]
- Miyakoshi, N. Effects of parathyroid hormone on cancellous bone mass and structure in osteoporosis. Curr. Pharm. Des. 2004, 10, 2615–2627. [Google Scholar] [CrossRef]
- Paterson, A.H. Evaluating bone mass and bone quality in patients with breast cancer. Clin. Breast Cancer. 2005, 5, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N. Natural medicinal compounds in bone tissue engineering. Trends Biotechnol. 2020, 38, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug. Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.L.; Haleem, A. Prosthetic joint infections: An update. Curr. Infect. Dis. Rep. 2018, 20, 15. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, J.; Ren, Q.; Liu, Y.; Zhou, P.; Li, H. Development of Novel Thermal Sprayed Hydroxyapatite-Rare Earth (HA-Re) Coatings for Potential Antimicrobial Applications in Orthopedics. J. Therm. Spray. Tech. 2021, 30, 886–897. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Rouahi, M.; Gallet, O.; Champion, E.; Dentzer, J.; Hardouin, P.; Anselme, K. Influence of hydroxyapatite microstructure on human bone cell response. J. Biomed. Mater. 2006, 78, 222–235. [Google Scholar] [CrossRef]
- Ponche, A.; Bigerelle, M.; Anselme, K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: Physico-chemical effects. Proc. Inst. Mech. Eng. H 2010, 224, 1471–1486. [Google Scholar] [CrossRef]
- Mann, V.; Grimm, D.; Corydon, T.J.; Krüger, M.; Wehland, M.; Riwaldt, S.; Sahana, J.; Kopp, S.; Bauer, J.; Reseland, J.E.; et al. Changes in Human Foetal Osteoblasts Exposed to the Random Positioning Machine and Bone Construct Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1357. [Google Scholar] [CrossRef]
- Gustafson, T.; Wolpert, L. Cellular mechanisms in the morphogenesis of the sea urchin larva. The formation of arms. Exp. Cell Res. 1961, 22, 509–520. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, Ž.; Veljović, D.; Jokić, B.; Dimitrijević, S.; Bogdanović, G.; Kojić, V.; Petrović, R.; Janaćković, D. Biocompatibility and antimicrobial activity of zinc(II)-doped hydroxyapatite, synthesized by a hydrothermal method. J. Serb. Chem. Soc. 2012, 77, 1787–1798. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Huang, T.; Yan, G.; Guan, M. Zinc Homeostasis in Bone: Zinc Transporters and Bone Diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef]
- Lin, W.; Li, D. Zinc and zinc transporters: Novel regulators of ventricular myocardial development. Pediatr. Cardiol. 2018, 39, 1042–1051. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Maimaiti, B.; Zhang, N.; Yan, L.; Luo, J.; Xie, C.; Wang, Y.; Ma, C.; Ye, T. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar] [CrossRef]
- Cassone, A.; Cauda, R. Candida and candidiasis in HIV-infected patients: Where com-mensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS 2012, 26, 1457–1472. [Google Scholar] [CrossRef]
- Dall, T.M.; Gallo, P.D.; Chakrabarti, R.; West, T.; Semilla, A.P.; Storm, M.V. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff. 2013, 32, 2013–2020. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid. Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An open multicenter comparative randomized clinical study on chitosan. Wound Repair Regen. 2015, 23, 518–524. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with antimicrobial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Shaaban, H.A. Essential oil as antimicrobial agents: Efficacy, stability, and safety issues for food application. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; de Oliveira, M.S., Silva, S., Da Costa, W.A., Eds.; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Betts, T.J. Chemical characterisation of the different types of volatile oil constituents by various solute retention ratios with the use of conventional and novel commercial gas chromatographic stationary phases. J. Chromatogr. A 2001, 936, 33–46. [Google Scholar] [CrossRef]
- Mousavi, M.; Maroufpoor, N.; Valizadegan, O. Fumigant toxicity of tarragon (Artemisia dracunculus L.) and dill (Anethum graveolens L.) essential oils on different life stages of Trialeurodes vaporariorum (Westwood). Acta Phytopathol. Entomol. Hung. 2018, 53, 29–42. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Fiestel, B.; Glotov, N. Artemisia dracunculus L. (tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef]
- Rajabian, A.; Khayyat, M.H.; Emami, S.A.; Tayarani-Najaran, Z.; Oskooie, R.R.; Asili, J. Phytochemical evaluation and antioxidant activity of essential oil, and aqueous and organic extracts of Artemisia dracunculus. J. Nat. Pharm. Prod. 2017, 12, 323–325. [Google Scholar] [CrossRef]
- Kumar, V.; Marković, T.; Emerald, M.; Dey, A. Herbs: Composition and Dietary Importance. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 3, pp. 332–337. [Google Scholar]
- Petrosyan, M.; Sahakyan, N.; Trchounian, A. Chemical composition and antimicrobial potential of essential oil of Artemisia dracunculus L., cultivated at high altitude armenian landscape. Proc. Yerevan State Univ. Chem. Biol. 2018, 52, 116–121. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Razavi, R.S.; Maham, M.; Moradi, M.; Hajimohammadi, B.; Naghili, H.; Hashemi, M.; Mehdizadeh, T. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran. J. Microbiol. 2012, 4, 30–34. [Google Scholar]
- Chaleshtori, R.S.; Rokni, N.; Razavilar, V.; Kopaei, M.R. The Evaluation of the Antibacterial and Antioxidant Activity of Tarragon (Artemisia dracunculus L.) Essential Oil and Its Chemical Composition. Jundishapur J. Microbiol. 2013, 6, e7877. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Iconaru, S.L.; Ciobanu, C.S.; Raita, M.S.; Ghegoiu, L.; Trusca, R.; Badea, M.L.; Cimpeanu, C. Studies of the Tarragon Essential Oil Effects on the Characteristics of Doped Hydroxyapatite/Chitosan Biocomposites. Polymers 2023, 15, 1908. https://doi.org/10.3390/polym15081908
Predoi D, Iconaru SL, Ciobanu CS, Raita MS, Ghegoiu L, Trusca R, Badea ML, Cimpeanu C. Studies of the Tarragon Essential Oil Effects on the Characteristics of Doped Hydroxyapatite/Chitosan Biocomposites. Polymers. 2023; 15(8):1908. https://doi.org/10.3390/polym15081908
Chicago/Turabian StylePredoi, Daniela, Simona Liliana Iconaru, Carmen Steluta Ciobanu, Mariana Stefania Raita, Liliana Ghegoiu, Roxana Trusca, Monica Luminita Badea, and Carmen Cimpeanu. 2023. "Studies of the Tarragon Essential Oil Effects on the Characteristics of Doped Hydroxyapatite/Chitosan Biocomposites" Polymers 15, no. 8: 1908. https://doi.org/10.3390/polym15081908