How Do Phosphorus Compounds with Different Valence States Affect the Flame Retardancy of PET?
Abstract
:1. Introduction
2. Experimental
2.1. Materials
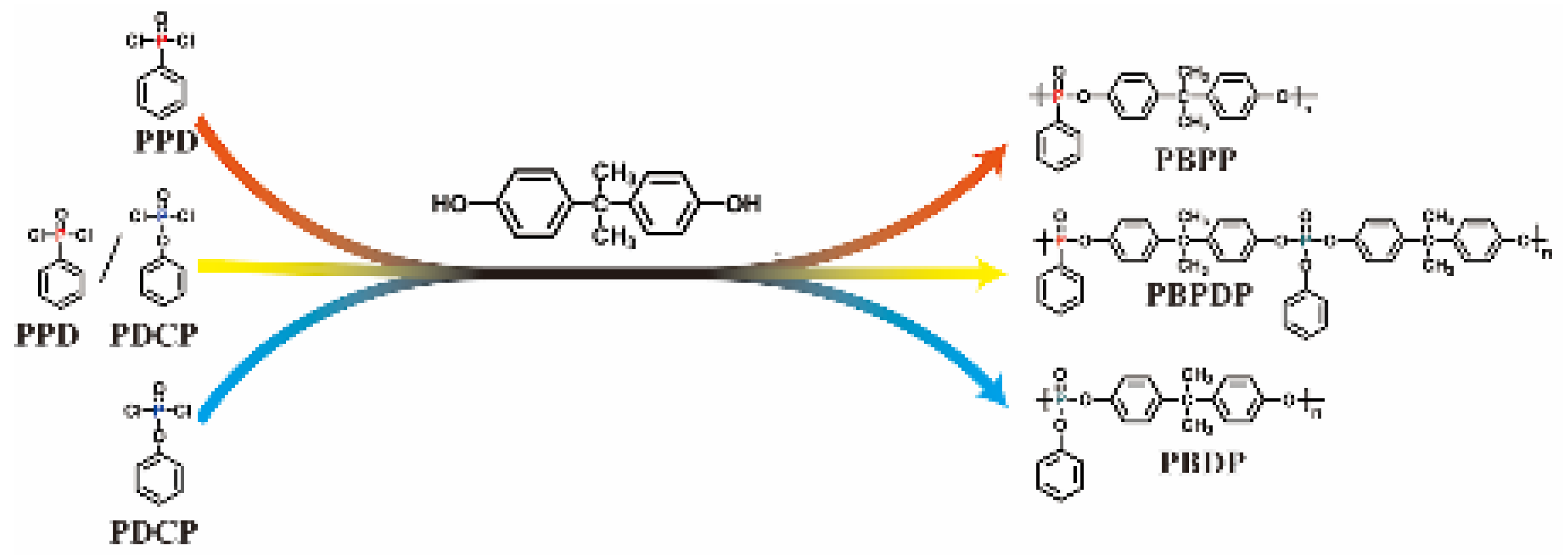
2.2. Synthesis of Polyphosphates
2.3. Preparation of PET Composites
2.4. Characterization
3. Results and Discussion
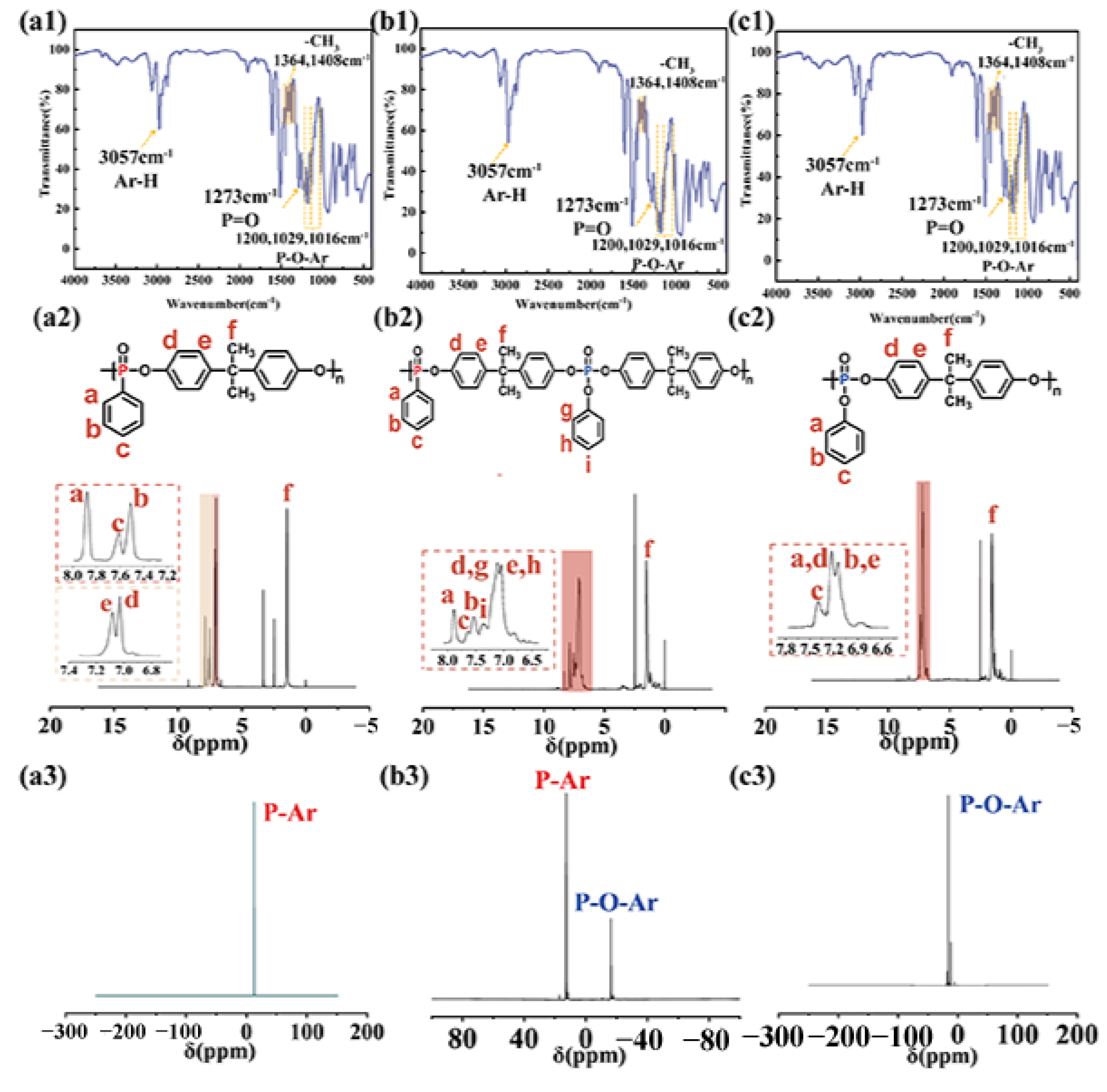
3.1. Chemical Structure and Pyrolysis Behavior of Flame Retardants
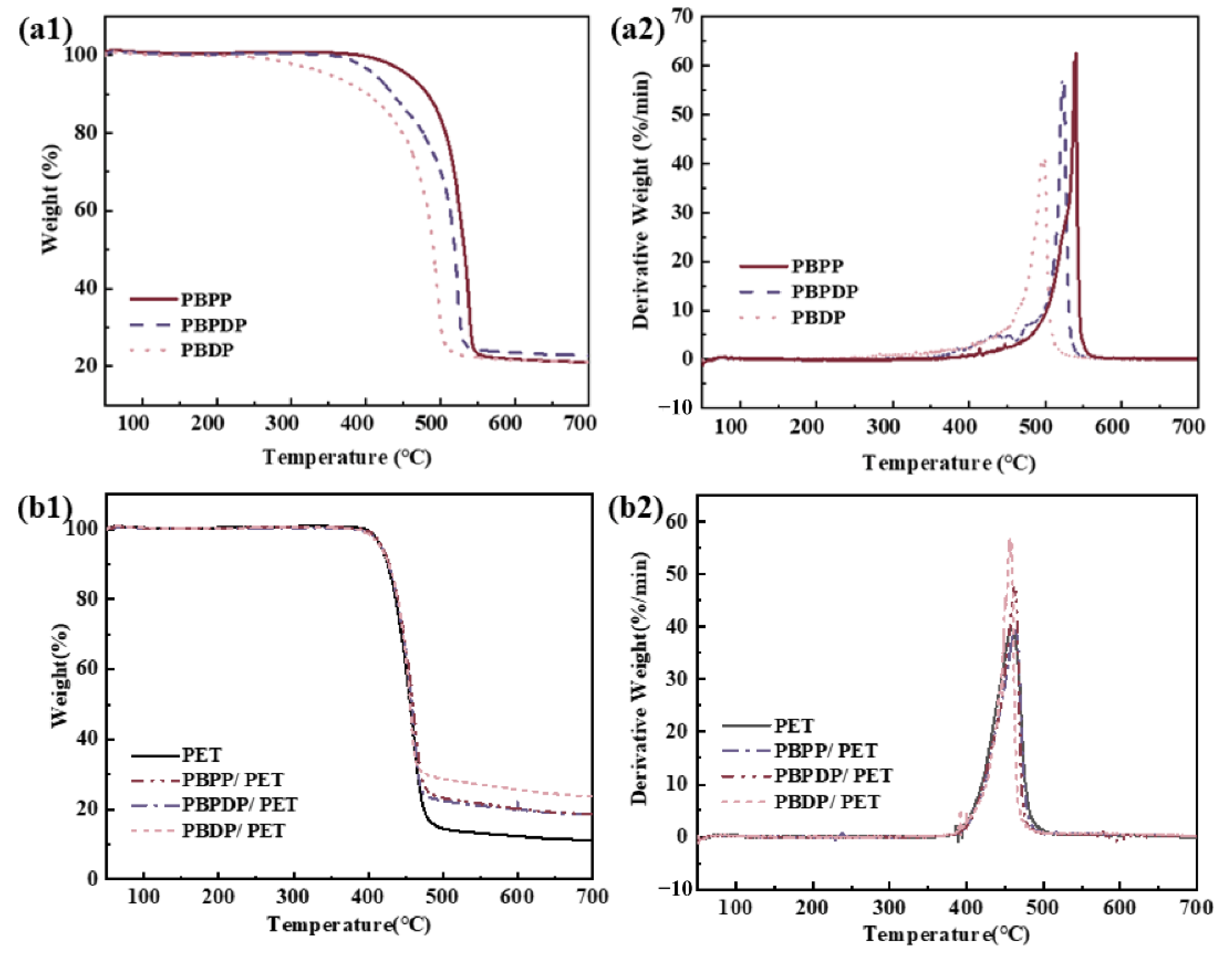
3.2. Thermal Stability Analysis
- W: The content of the additions in the composites.
- R: The residue result after TGA test.
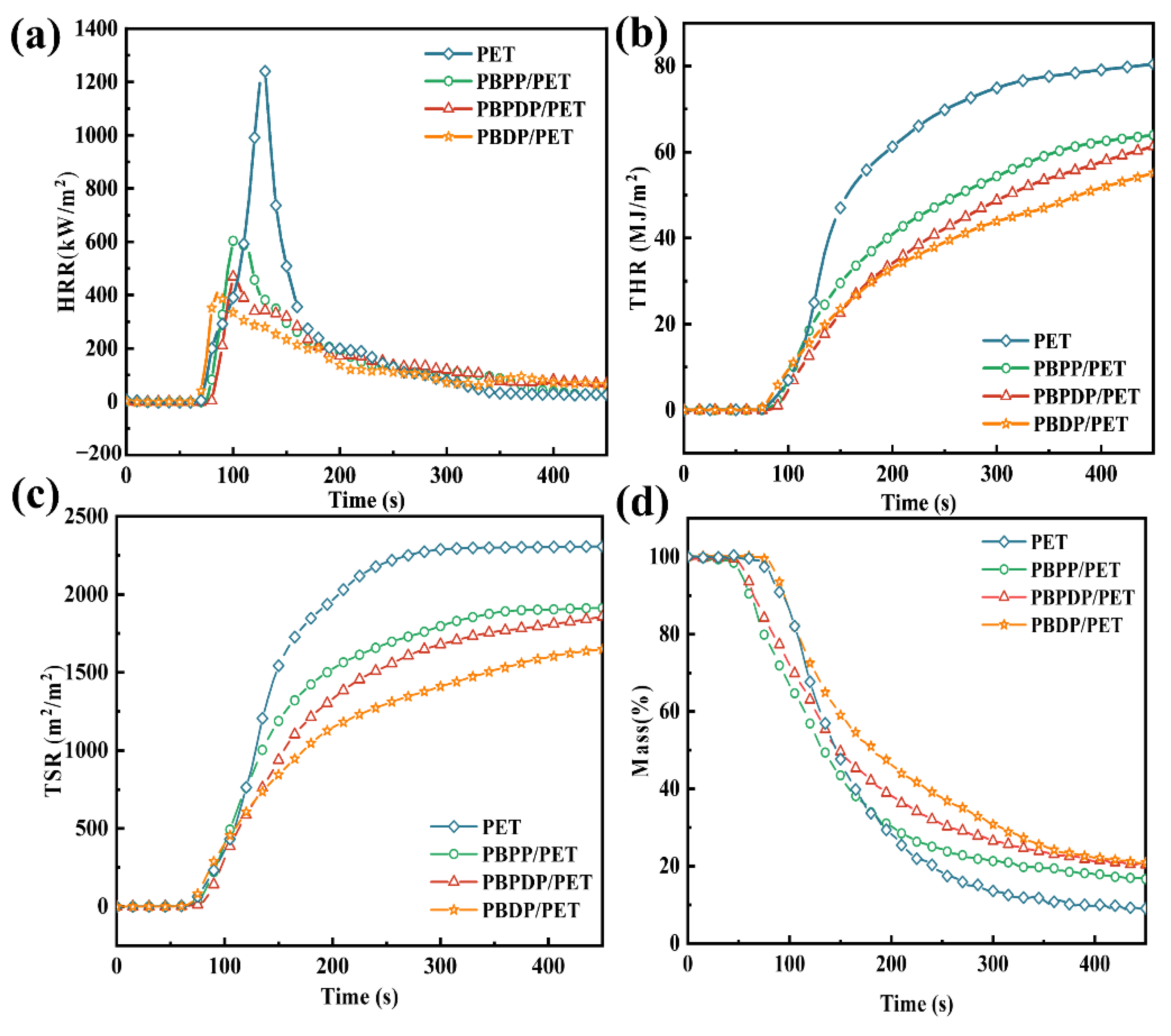
3.3. Force Combustion
3.4. Physical and Chemical Analysis of Char Residue
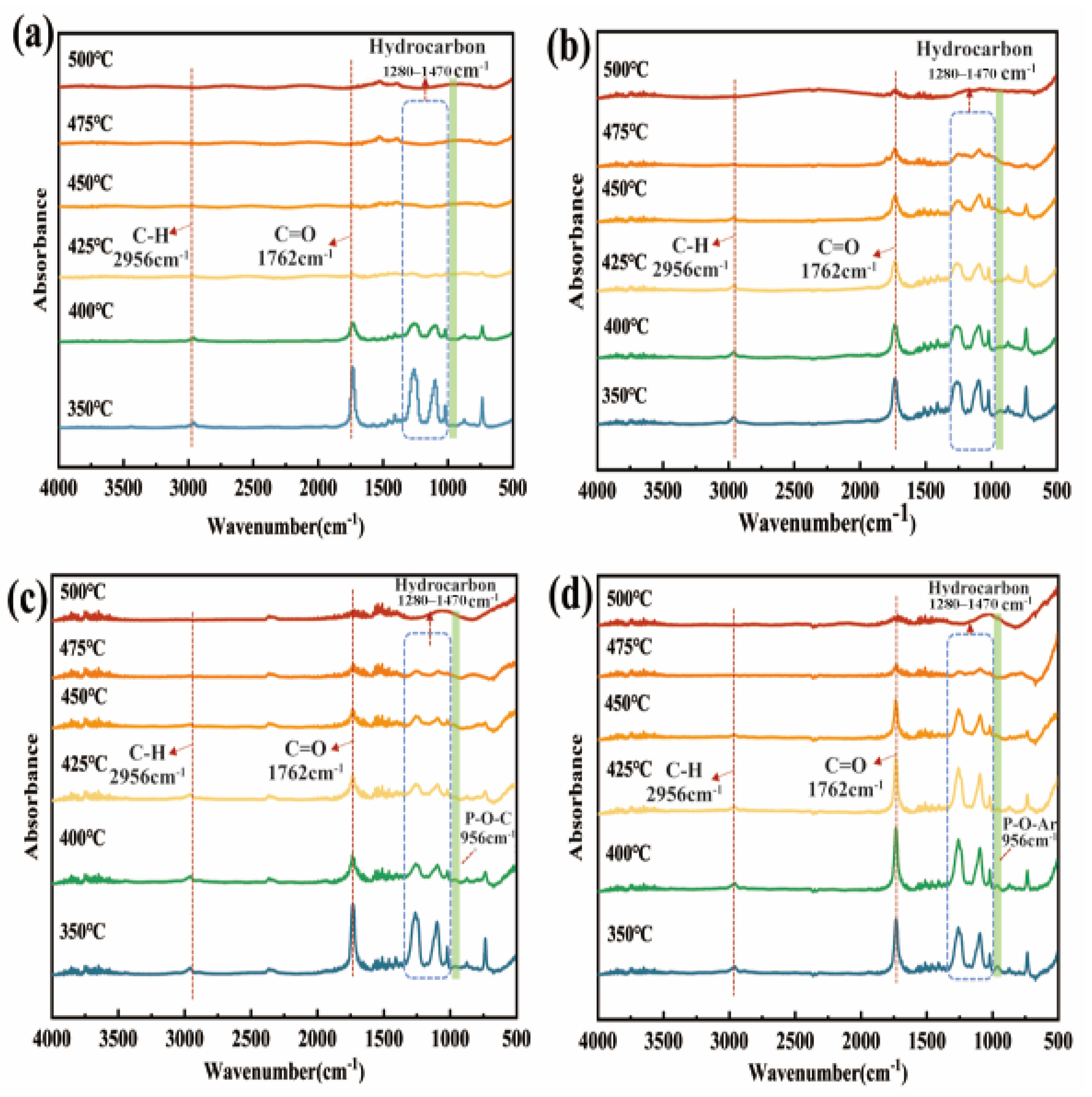
3.5. Gas-Phase Products Analysis of Flame Retardants and PET Composites
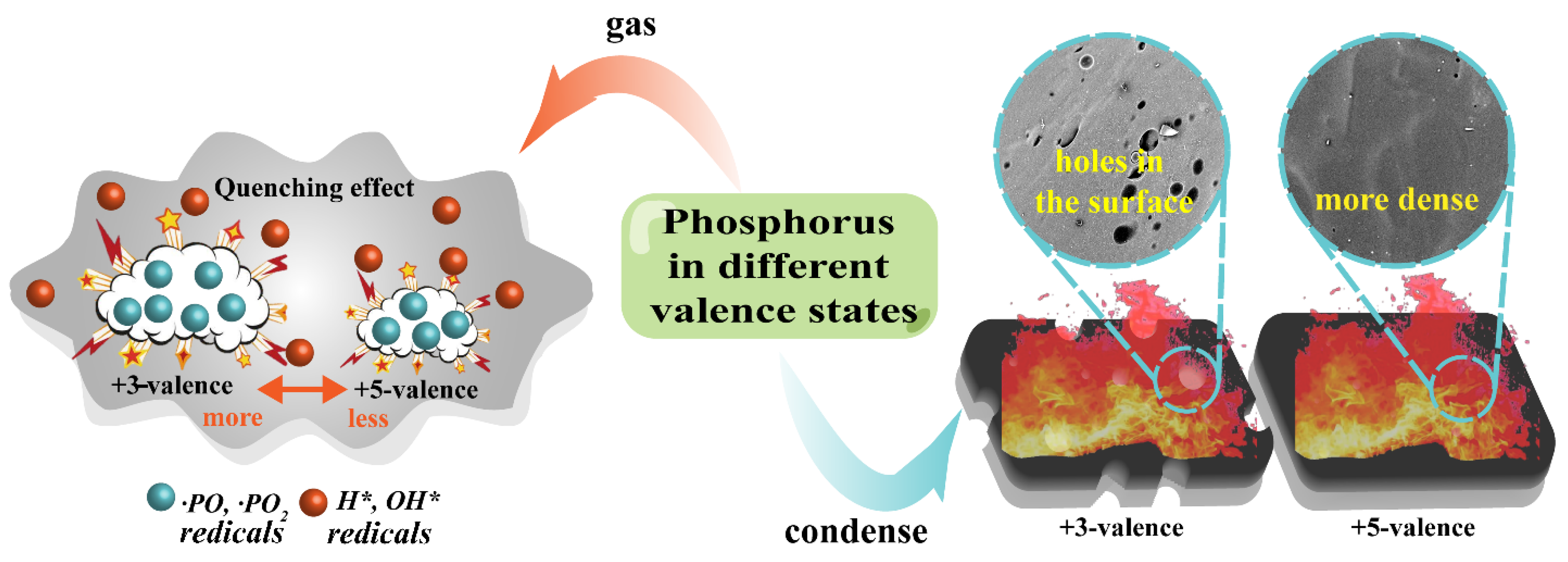
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ao, X.; Du, Y.; Yu, D.; Wang, W.; Yang, W.Z.; Sun, B.; Zhu, M.F. Synthesis, characterization of a DOPO-based polymeric flame retardant and its application in polyethylene terephthalate. Prog. Nat. Sci. 2020, 30, 200–207. [Google Scholar] [CrossRef]
- Aytan, E.; Aytan, T.A.; Kahraman, M.V. Phosphorus Ester Containing Mesoporous Silica as Novel High-Effective Flame Retardant in Polyurethane and Polyester Coatings. Chemistryselect 2021, 6, 6541–6547. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Knittel, D.; Gutmann, J.S.; Opwis, K. Permanent Flame Retardant Finishing of Textiles by Allyl-Functionalized Polyphosphazenes. ACS Appl. Mater. Interfaces 2015, 7, 9349–9363. [Google Scholar] [CrossRef]
- Ban, D.M.; Wang, Y.Z.; Yang, B.; Zhao, G.M. A novel non-dripping oligomeric flame retardant for polyethylene terephthalate. Eur. Polym. J. 2004, 40, 1909–1913. [Google Scholar] [CrossRef]
- Chen, L.; Bian, X.C.; Yang, R.; Wang, Y.Z. PET in situ composites improved both flame retardancy and mechanical properties by phosphorus-containing thermotropic liquid crystalline copolyester with aromatic ether moiety. Compos. Sci. Technol. 2012, 72, 649–655. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. Aryl Polyphosphonates: Useful Halogen-Free Flame Retardants for Polymers. Materials 2010, 3, 4746–4760. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Shakeri, A.; Abbasi, A. Synthesize and Characterization of Flame Retardant Copolyesters PET/Phosphorus Compound. J. Vinyl Addit. Technol. 2019, 25, 262–270. [Google Scholar] [CrossRef]
- Li, C.; Yu-Zhong, W. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar]
- Jian, R.K.; Lin, X.B.; Liu, Z.Q.; Zhang, W.; Zhang, J.; Zhang, L.; Li, Z.; Wang, D.Y. Rationally designed zinc borate@ZIF-8 core-shell nanorods for curing epoxy resins along with low flammability and high mechanical property. Compos. Part B Eng. 2020, 200, 108349. [Google Scholar] [CrossRef]
- Kilinc, M.; Cakal, G.O.; Bayram, G.; Eroglu, I.; Ozkar, S. Flame retardancy and mechanical properties of pet-based composites containing phosphorus and boron-based additives. J. Appl. Polym. Sci. 2015, 132, 42016. [Google Scholar] [CrossRef]
- Fang, Y.C.; Zhou, X.; Xing, Z.Q.; Wu, Y.R. An effective flame retardant for poly(ethylene terephthalate) synthesized by phosphaphenanthrene and cyclotriphosphazene. J. Appl. Polym. Sci. 2017, 134, 45246. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, S.H.; Shan, H.; Qian, L.J.; Wang, J.Y.; Xin, F. Effect of two boron compounds on smoke-suppression and flame-retardant properties for rigid polyurethane foams. Polym. Int. 2022, 71, 1210–1219. [Google Scholar] [CrossRef]
- Xu, B.; Shao, L.S.; Wang, J.Y.; Liu, Y.T.; Qian, L.J. Enhancement of the intumescent flame retardant efficiency in polypropylene by synergistic charring effect of a hypophosphite/cyclotetrasiloxane bi-group compound. Polym. Degrad. Stab. 2020, 181, 109281. [Google Scholar] [CrossRef]
- Shao, L.; Xu, B.; Ma, W.; Wang, J.; Liu, Y.; Qian, L. Flame retardant application of a hypophosphite/cyclotetrasiloxane bigroup compound on polycarbonate. J. Appl. Polym. Sci. 2020, 137, 48699. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doring, M.; Perez, R.; Sandler, J.K.W.; Altstadt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Ozukanar, O.; Cakmakci, E.; Daglar, O.; Durmaz, H.; Kumbaraci, V. A double-click strategy for the synthesis of P and N-containing hydrolytically stable reactive flame retardant for photocurable networks. J. Appl. Polym. Sci. 2022, 139, e52837. [Google Scholar] [CrossRef]
- Bo, X.; Zhao, S.H.; Shan, H.; Wei, S.M.; Zhang, Q.L. A Sulfur-Containing Polyphosphonate Flame Retardant for Polyethylene Terephthalate. Combust. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Qiu, S.L.; Guo, W.W.; Chu, F.K.; Zhou, X.; Chen, W.J.; Wang, J.W.; Zhang, K.; Cheng, L.; Hu, Y. Construction of hierarchical Ti3C2TX@PHbP-PHC architecture with enhanced free-radical quenching capability: Effective reinforcement and fire safety performance in bismaleimide resin. Chem. Eng. J. 2022, 427, 131634. [Google Scholar] [CrossRef]
- Huang, H.Z.; Wang, Y.Z.; Jow, J.; Su, K. Transesterification-controlled compatibility and microfibrillation in PC–ABS composites reinforced by phosphorus-containing thermotropic liquid crystalline polyester. Polymer 2009, 50, 3037–3046. [Google Scholar]
- Rao, W.H.; Xu, H.X.; Xu, Y.J.; Qi, M.; Liao, W.; Xu, S.M.; Wang, Y.Z. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Cai, W.; Li, Z.X.; Mu, X.W.; He, L.X.; Zhou, X.; Guo, W.W.; Song, L.; Hu, Y. Barrier function of graphene for suppressing the smoke toxicity of polymer/black phosphorous nanocomposites with mechanism change. J. Hazard. Mater. 2021, 404, 124106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhao, J.P. Facile preparation of slag or fly ash geopolymer composite coatings with flame resistance. Constr. Build. Mater. 2019, 203, 655–661. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Qian, L.; Chen, Y.; Qiu, Y. Impact on flame retardancy and degradation behavior of intumescent flame-retardant EP composites by a hyperbranched triazine-based charring agent. Polym. Adv. Technol. 2020, 31, 3316–3327. [Google Scholar] [CrossRef]
- Xu, B.; Wu, X.; Ma, W.; Qian, L.; Xin, F.; Qiu, Y. Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J. Anal. Appl. Pyrolysis 2018, 134, 231–242. [Google Scholar] [CrossRef]
- Mariappan, T.; You, Z.; Hao, J.; Wilkie, C.A. Influence of oxidation state of phosphorus on the thermal and flammability of polyurea and epoxy resin. Eur. Polym. J. 2013, 49, 3171–3180. [Google Scholar] [CrossRef]
- Çakmakçiı, E.; Kahraman, M.V. Boron/phosphorus-containing flame-retardant photocurable coatings. RSC Smart Mater. 2015, 2015, 150–187. [Google Scholar]
- Wang, J.Y.; Xu, B.; Wang, X.D.; Liu, Y.T. A phosphorous-based bi-functional flame retardant for rigid polyurethane foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Wei, S.; Zhao, S.; Qian, L.; Chen, Y.; Shan, H.; Zhang, Q. A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset. Int. J. Mol. Sci. 2022, 23, 11256. [Google Scholar] [CrossRef]
- Denis, M.; Coste, G.; Sonnier, R.; Caillol, S.; Negrell, C. Influence of Phosphorus Structures and Their Oxidation States on Flame-Retardant Properties of Polyhydroxyurethanes. Molecules 2023, 28, 611. [Google Scholar] [CrossRef]



| Sample | Flame Retardants | PET Composites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Td,5% (°C) | Tpeak (°C) | Rpeak (%/min) | Residuals at 700 °C (wt.%) | Td,5% (°C) | Tpeak (°C) | Rpeak (%/min) | Residuals at 700 °C (wt.%) | Theoretical Residuals (wt.%) | |
| Neat PET | - | - | - | - | 417 | 459 | 39.6 | 11.3 | - |
| PBPP | 455 | 539 | 62.5 | 21.2 | 418 | 462 | 40.0 | 18.6 | 12.1 |
| PBPDP | 412 | 521 | 57.7 | 22.1 | 417 | 461 | 47.4 | 18.6 | 12.2 |
| PBDP | 351 | 495 | 40.6 | 21.2 | 416 | 456 | 56.4 | 23.7 | 12.1 |
| Samples | PHRR (kW/m2) | THR (MJ/m2) | av-EHC (MJ/kg) | TSR (m2/m2) | av-COY (kg/kg) | av-CO2Y (kg/kg) | Residue (wt.%) | FGI (kW/(m2·s)) |
|---|---|---|---|---|---|---|---|---|
| PET | 1240.0 | 80.4 | 19.6 | 2300 | 0.08 | 2.124 | 13.2 | 9.5 |
| PBPP/PET | 603.6 | 63.9 | 18.0 | 1896.9 | 0.10 | 1.85 | 20.9 | 6.0 |
| PBPDP/PET | 469.0 | 61.3 | 16.8 | 1845.0 | 0.08 | 1.71 | 22.8 | 4.7 |
| PBDP/PET | 408.4 | 55.1 | 16.2 | 1644.7 | 0.08 | 1.69 | 24.5 | 4.8 |
| PBPDP/PET-cal | 538.5 | 61.0 | 17.4 | 1812.8 | 0.09 | 1.80 | 22.1 | 5.6 |
| Samples | Flame-Inhibition Effect (%) | Charring Effect (%) | Barrier and Protective Effect (%) |
|---|---|---|---|
| PET | - | - | - |
| PBPP/PET | 8.16 | 8.87 | 38.75 |
| PBPDP/PET | 14.29 | 11.06 | 50.40 |
| PBDP/PET | 17.35 | 13.02 | 51.94 |
| Samples | P Ratio in Residues (%) | Char Yield (%) | Initial P Ratio in Samples (%) | Reserved P Ratio in Total P (%) | Released P Ratio in Total P (%) |
|---|---|---|---|---|---|
| PBPP/PET | 1.37 | 20.9 | 0.73 | 39.30% | 60.70% |
| PBPDP/PET | 1.97 | 22.8 | 0.74 | 60.40% | 39.60% |
| PBDP/PET | 3.18 | 24.5 | 0.84 | 92.70% | 7.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Xu, B.; Shan, H.; Zhang, Q.; Wang, X. How Do Phosphorus Compounds with Different Valence States Affect the Flame Retardancy of PET? Polymers 2023, 15, 1917. https://doi.org/10.3390/polym15081917
Zhao S, Xu B, Shan H, Zhang Q, Wang X. How Do Phosphorus Compounds with Different Valence States Affect the Flame Retardancy of PET? Polymers. 2023; 15(8):1917. https://doi.org/10.3390/polym15081917
Chicago/Turabian StyleZhao, Siheng, Bo Xu, Hao Shan, Qinglei Zhang, and Xiangdong Wang. 2023. "How Do Phosphorus Compounds with Different Valence States Affect the Flame Retardancy of PET?" Polymers 15, no. 8: 1917. https://doi.org/10.3390/polym15081917
APA StyleZhao, S., Xu, B., Shan, H., Zhang, Q., & Wang, X. (2023). How Do Phosphorus Compounds with Different Valence States Affect the Flame Retardancy of PET? Polymers, 15(8), 1917. https://doi.org/10.3390/polym15081917







