Cationic Nanogels Enable Gold Nanoparticle Immobilization and Regulated Catalytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterizations
2.2.1. UV-Vis Spectroscopy
2.2.2. Transmission Electron Microscope (TEM)
2.2.3. Light Scattering
2.2.4. H Nuclear Magnetic Resonance (1H NMR)
2.3. Preparation of PMETAC Nanogels
2.4. Synthesis of Au@PMETAC Nanogels
2.5. Catalytic Performance of Au@PMETAC Nanogel
3. Results
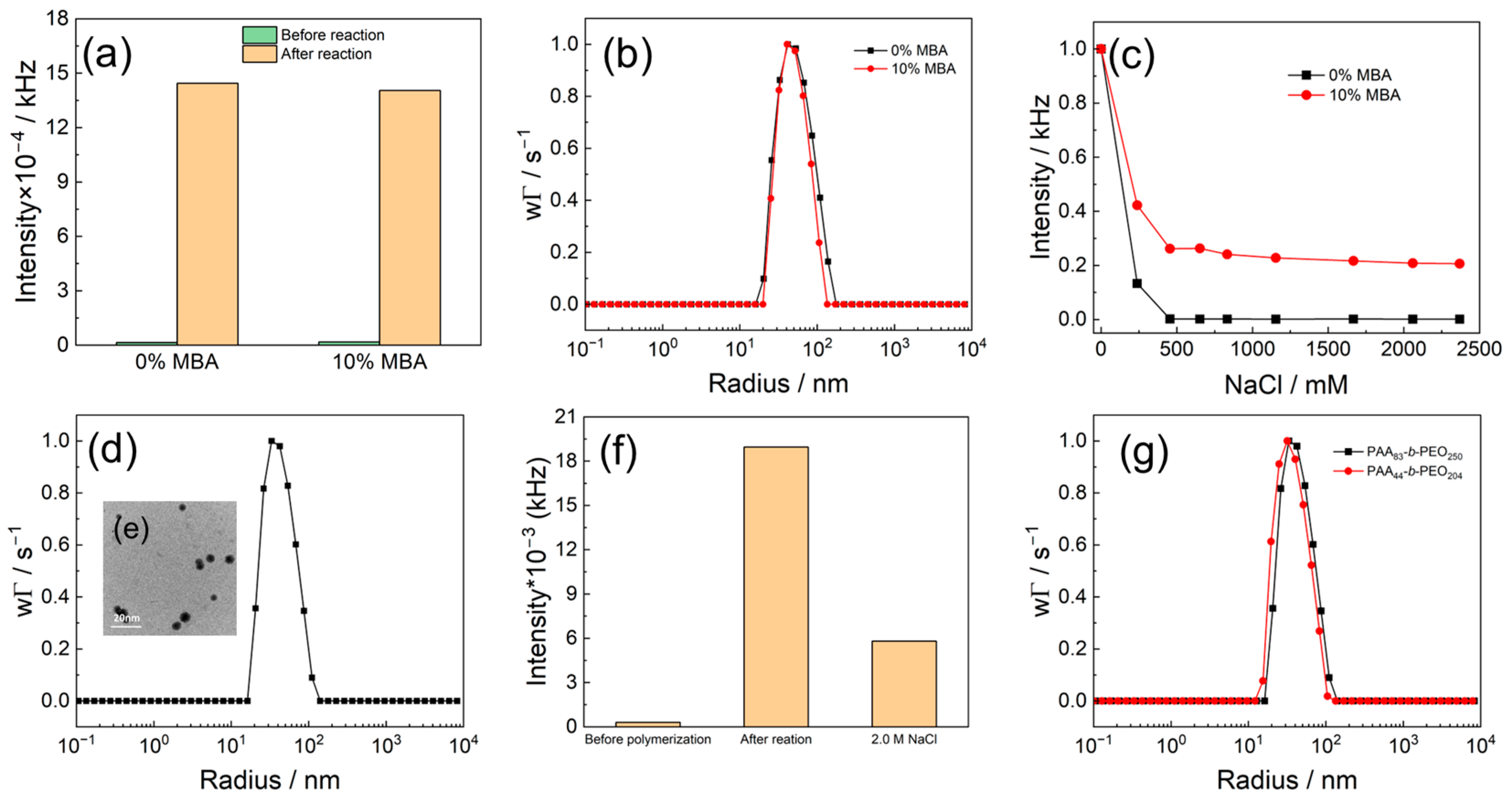
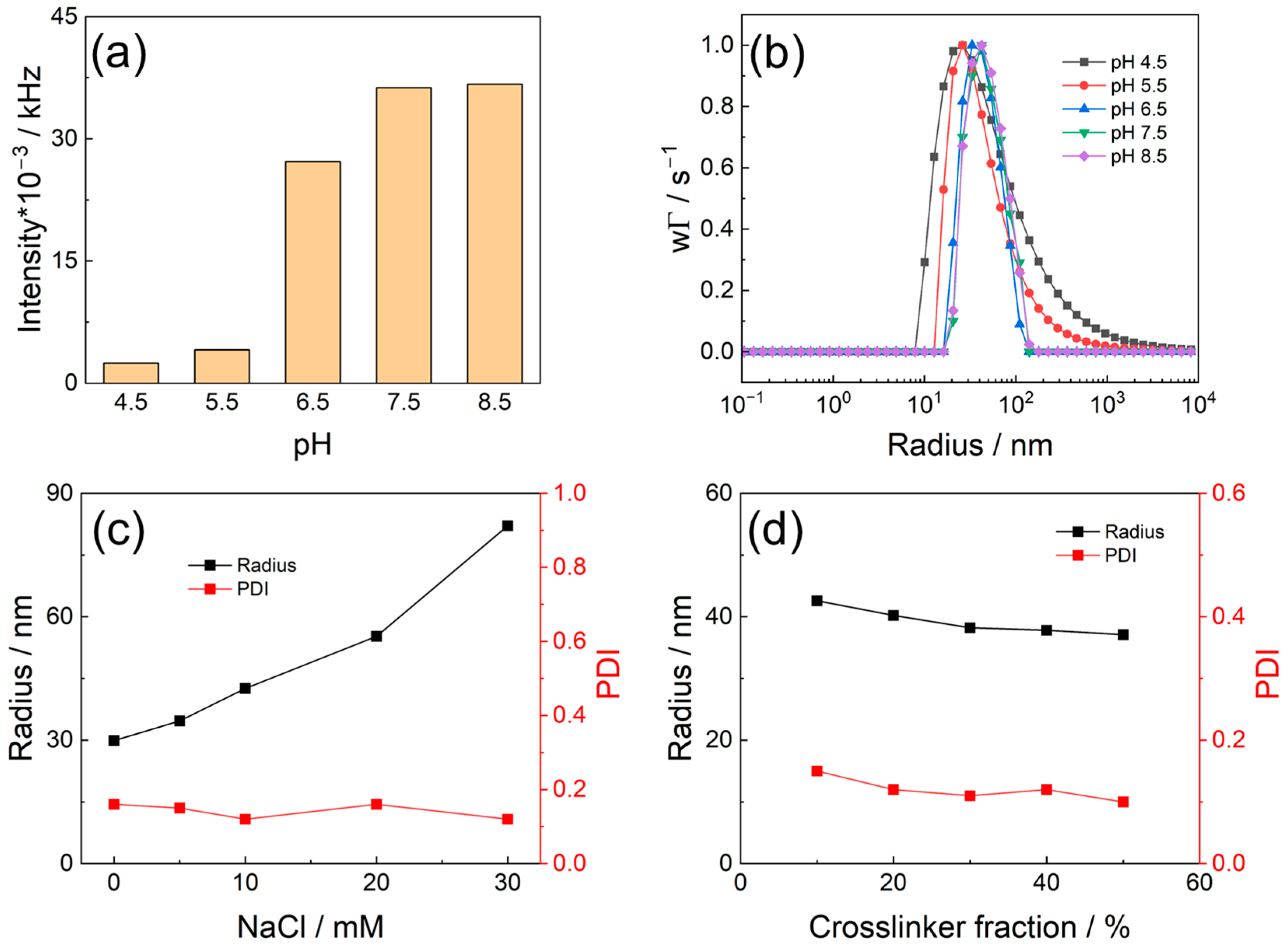
3.1. Preparation of PMETAC Nanogels
3.2. Synthesis of Au@PMETAC Nanogels
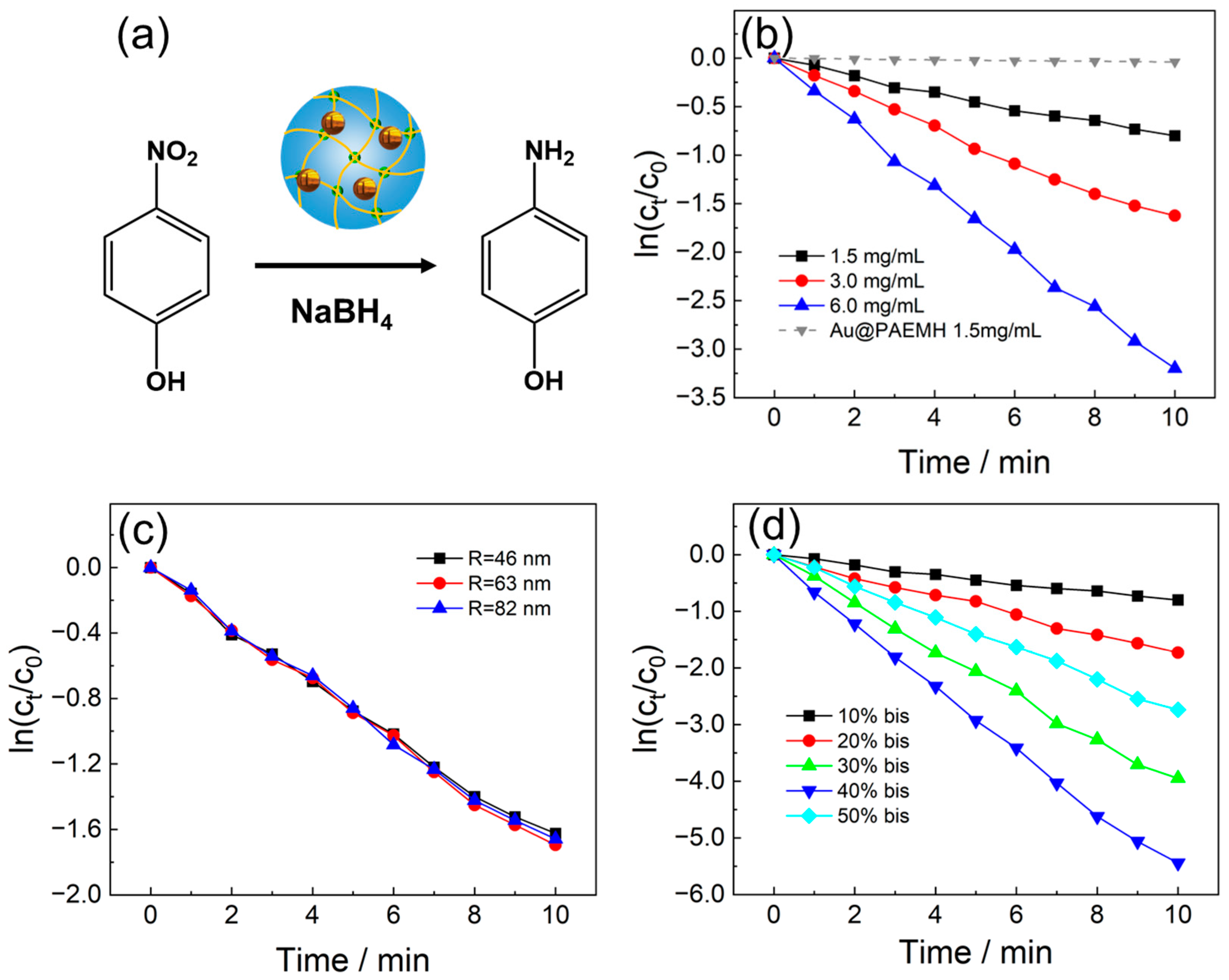
3.3. Catalytic Performance of Au@PMETAC Nanogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lara, P.; Martínez-Prieto, L.M. Metal Nanoparticle Catalysis. Catalysts 2021, 11, 1210. [Google Scholar] [CrossRef]
- Feng, S.; Song, X.; Liu, Y.; Lin, X.; Yan, L.; Liu, S.; Dong, W.; Yang, X.; Jiang, Z.; Ding, Y. In situ formation of mononuclear complexes by reaction-induced atomic dispersion of supported noble metal nanoparticles. Nat. Commun. 2019, 10, 5281. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef]
- Jia, Z.; Amar, M.B.; Yang, D.; Brinza, O.; Kanaev, A.; Duten, X.; Vega-González, A. Plasma catalysis application of gold nano-particles for acetaldehyde decomposition. Chem. Eng. J. 2018, 347, 913. [Google Scholar] [CrossRef]
- Zhu, J.; He, K.; Dai, Z.; Gong, L.; Zhou, T.; Liang, H.; Liu, J. Self-Assembly of Luminescent Gold Nanoparticles with Sensitive pH-Stimulated Structure Transformation and Emission Response toward Lysosome Escape and Intracellular Imaging. Anal. Chem. 2019, 91, 8237. [Google Scholar] [CrossRef]
- Mazloomi-Rezvani, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Pirayesh, A. Effect of surface modification with various thiol compounds on colloidal stability of gold nanoparticles. Appl. Organomet. Chem. 2017, 32, e4079. [Google Scholar] [CrossRef]
- Hove, J.B.T.; Wang, J.; van Oosterom, M.N.; van Leeuwen, F.W.B.; Velders, A.H. Size-Sorting and Pattern Formation of Nanoparticle-Loaded Micellar Superstructures in Biconcave Thin Films. ACS Nano 2017, 11, 11225–11231. [Google Scholar] [CrossRef]
- Zhao, F.; Li, L.; Tian, Y.-C.; Liu, J.-J.; Wang, J.-J.; Zhou, Z.-M.; Lv, C.-X.; Guo, X.-H. Preparation of Au/Ag multilayers via layer-by-layer self-assembly in spherical polyelectrolyte brushes and their catalytic activity. Chin. J. Polym. Sci. 2015, 33, 1421–1430. [Google Scholar] [CrossRef]
- Mota-Santiago, P.; Kremer, F.; Rizza, G.; Dufour, C.; Khomenkov, V.; Notthoff, C.; Hadley, A.; Kluth, P. Ion shaping of single-layer Au nanoparticles in amorphous silicon dioxide, in silicon nitride, and at their interface. Phys. Rev. Mater. 2020, 4, 096002. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, Y.; Yang, H.; Kang, X.-J.; Wang, C.; Wen, L.-L.; Lu, Z.-D. Ultrasmall Au Nanoparticles Embedded in 2D Mixed-Ligand Metal-Organic Framework Nanosheets Exhibiting Highly Efficient and Size-Selective Catalysis. Adv. Funct. Mater. 2018, 28, 1802021. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, D.; Wang, J.; Chen, C.; Yang, X.; Li, C. Multilayer Magnetic Composite Particles with Functional Polymer Brushes as Stabilizers for Gold Nanocolloids and Their Recyclable Catalysis. J. Phys. Chem. C 2013, 117, 6363–6372. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, S.; Chen, G.; Tu, K.; Jiang, H.; Wang, L.Q. Novel chitosan-based pH-sensitive and disintegrable polyelectrolyte nanogels. Colloid Surf. B-Biointerfaces 2014, 122, 194. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, Y.; Cai, Y.; Zhou, J.; Ding, P.; Stuart, M.A.C.; Wang, J. Controlled synthesis of PEGylated polyelectrolyte nanogels as efficient protein carriers. J. Colloid Interface Sci. 2022, 620, 322. [Google Scholar] [CrossRef]
- Ni, J.; Wan, Y.; Cai, Y.; Ding, P.; Stuart, M.A.C.; Wang, J. Synthesis of Anionic Nanogels for Selective and Efficient Enzyme Encapsulation. Langmuir 2022, 38, 3234. [Google Scholar] [CrossRef]
- Spencer, D.S.; Shodeinde, A.B.; Beckman, D.W.; Luu, B.C.; Hodges, H.R.; Peppas, N.A. Cytocompatibility, membrane disruption, and siRNA delivery using environmentally responsive cationic nanogels. J. Control. Release 2021, 332, 608–619. [Google Scholar] [CrossRef]
- Knipe, J.M.; Strong, L.E.; Peppas, N.A. Enzyme- and pH-Responsive Microencapsulated Nanogels for Oral Delivery of siRNA to Induce TNF-alpha Knockdown in the Intestine. Biomacromolecules 2016, 17, 788. [Google Scholar] [CrossRef]
- Ding, P.; Liu, W.; Guo, X.; Stuart, M.A.C.; Wang, J. Optimal synthesis of polyelectrolyte nanogels by electrostatic assembly directed polymerization for dye loading and release. Soft Matter 2021, 17, 887–892. [Google Scholar] [CrossRef]
- Cai, Y.; Ding, P.; Ni, J.; Zhou, L.; Ahmad, A.; Guo, X.; Stuart, M.A.C.; Wang, J. Regulated Polyelectrolyte Nanogels for Enzyme Encapsulation and Activation. Biomacromolecules 2021, 22, 4748–4757. [Google Scholar] [CrossRef]
- Ding, P.; Huang, J.; Wei, C.; Liu, W.; Zhou, W.; Wang, J.; Wang, M.; Guo, X.; Stuart, M.A.C.; Wang, J. Efficient and Generic Prep-aration of Diverse Polyelectrolyte Nanogels by Electrostatic Assembly Directed Polymerization. CCS Chem. 2020, 2, 1016. [Google Scholar] [CrossRef]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Provencher, S.W. Contin: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229. [Google Scholar] [CrossRef]
- Van Der Kooij, H.M.; Spruijt, E.; Voets, I.K.; Fokkink, R.; Stuart, M.A.C.; Van Der Gucht, J. On the Stability and Morphology of Complex Coacervate Core Micelles: From Spherical to Wormlike Micelles. Langmuir 2012, 28, 14180–14191. [Google Scholar] [CrossRef]
- Voets, I.K.; de Keizer, A.; Stuart, M.A.C. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147–148, 300. [Google Scholar] [CrossRef]
- Spruijt, E.; Westphal, A.H.; Borst, J.W.; Stuart, M.A.C.; van der Gucht, J. Binodal Compositions of Polyelectrolyte Complexes. Macromolecules 2010, 43, 6476. [Google Scholar] [CrossRef]
- Liu, Y.; Winter, H.H.; Perry, S.L. Linear viscoelasticity of complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 46–60. [Google Scholar] [CrossRef]
- Liu, Y.; Momani, B.; Winter, H.H.; Perry, S.L. Rheological characterization of liquid-to-solid transitions in bulk polyelectrolyte complexes. Soft Matter 2017, 13, 7332–7340. [Google Scholar] [CrossRef]
- Li, Z.; Wan, J.; Zhang, Y.; Dang, C.; Pan, F.; Fu, J. Influences of petroleum hydrocarbon pyrene on the formation, stability and antibacterial activity of natural Au nanoparticles. Sci. Total Environ. 2021, 795, 148813. [Google Scholar] [CrossRef]
- Tu, T.; Zhou, W.; Wang, M.; Guo, X.; Li, L.; Stuart, M.A.C.; Wang, J. One-Pot Synthesis of Small and Uniform Gold Nanoparticles in Water by Flash Nanoprecipitation. Ind. Eng. Chem. Res. 2020, 59, 11080–11086. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Ballauff, M.; Drechsler, M. Thermosensitive Core−Shell Particles as Carrier Systems for Metallic Nanoparticles. J. Phys. Chem. B 2006, 110, 3930–3937. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, X.; Cohen Stuart, M.A.; Wang, J.; Ding, P. Cationic Nanogels Enable Gold Nanoparticle Immobilization and Regulated Catalytic Activity. Polymers 2023, 15, 1935. https://doi.org/10.3390/polym15081935
Wang X, Guo X, Cohen Stuart MA, Wang J, Ding P. Cationic Nanogels Enable Gold Nanoparticle Immobilization and Regulated Catalytic Activity. Polymers. 2023; 15(8):1935. https://doi.org/10.3390/polym15081935
Chicago/Turabian StyleWang, Xin, Xuhong Guo, Martien A. Cohen Stuart, Junyou Wang, and Peng Ding. 2023. "Cationic Nanogels Enable Gold Nanoparticle Immobilization and Regulated Catalytic Activity" Polymers 15, no. 8: 1935. https://doi.org/10.3390/polym15081935
APA StyleWang, X., Guo, X., Cohen Stuart, M. A., Wang, J., & Ding, P. (2023). Cationic Nanogels Enable Gold Nanoparticle Immobilization and Regulated Catalytic Activity. Polymers, 15(8), 1935. https://doi.org/10.3390/polym15081935






