Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6
Abstract
1. Introduction
2. Experimental Section
3. Theoretical Background
3.1. Avrami Analysis
3.2. Nakamura Model
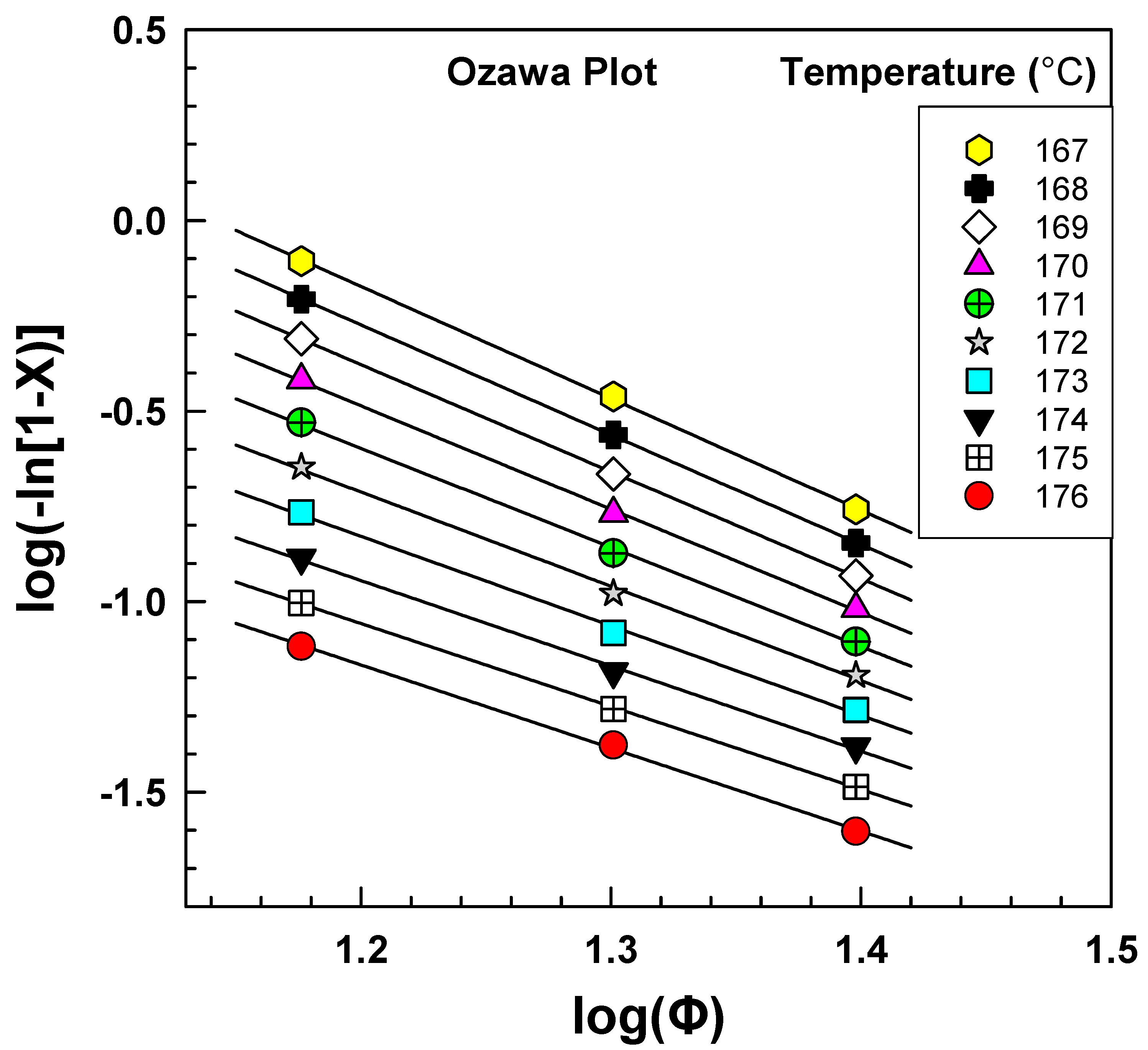
3.3. Ozawa Model
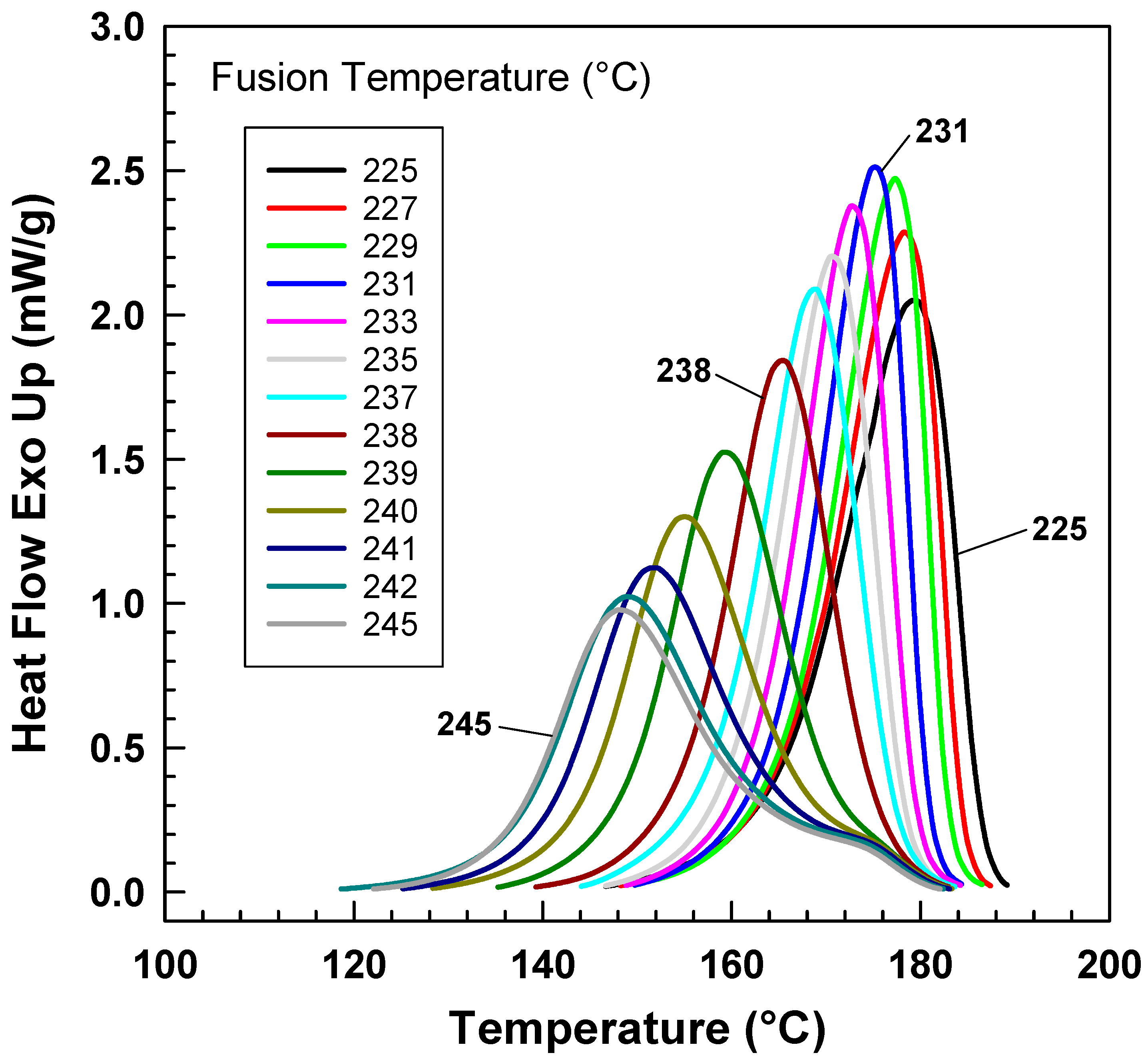
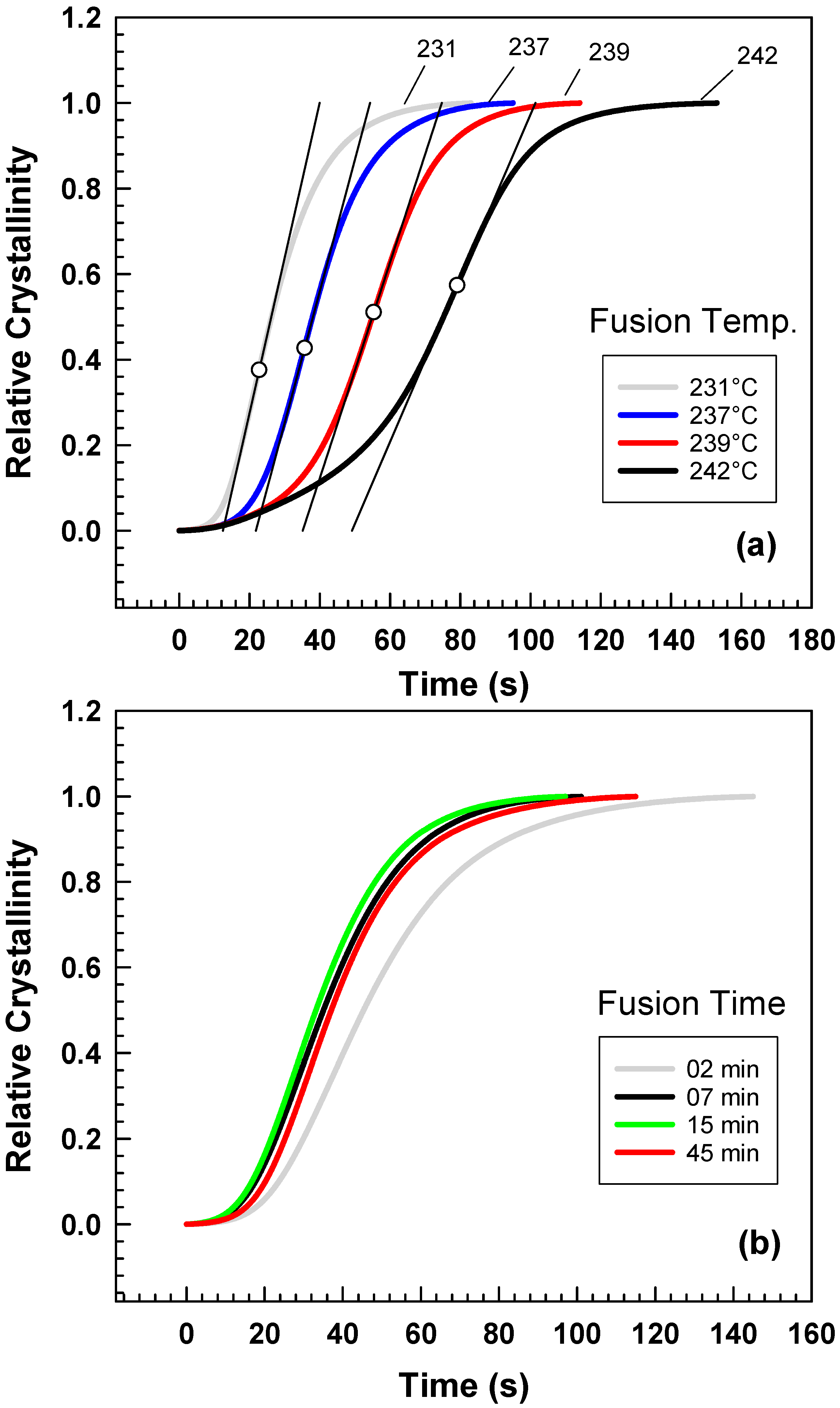
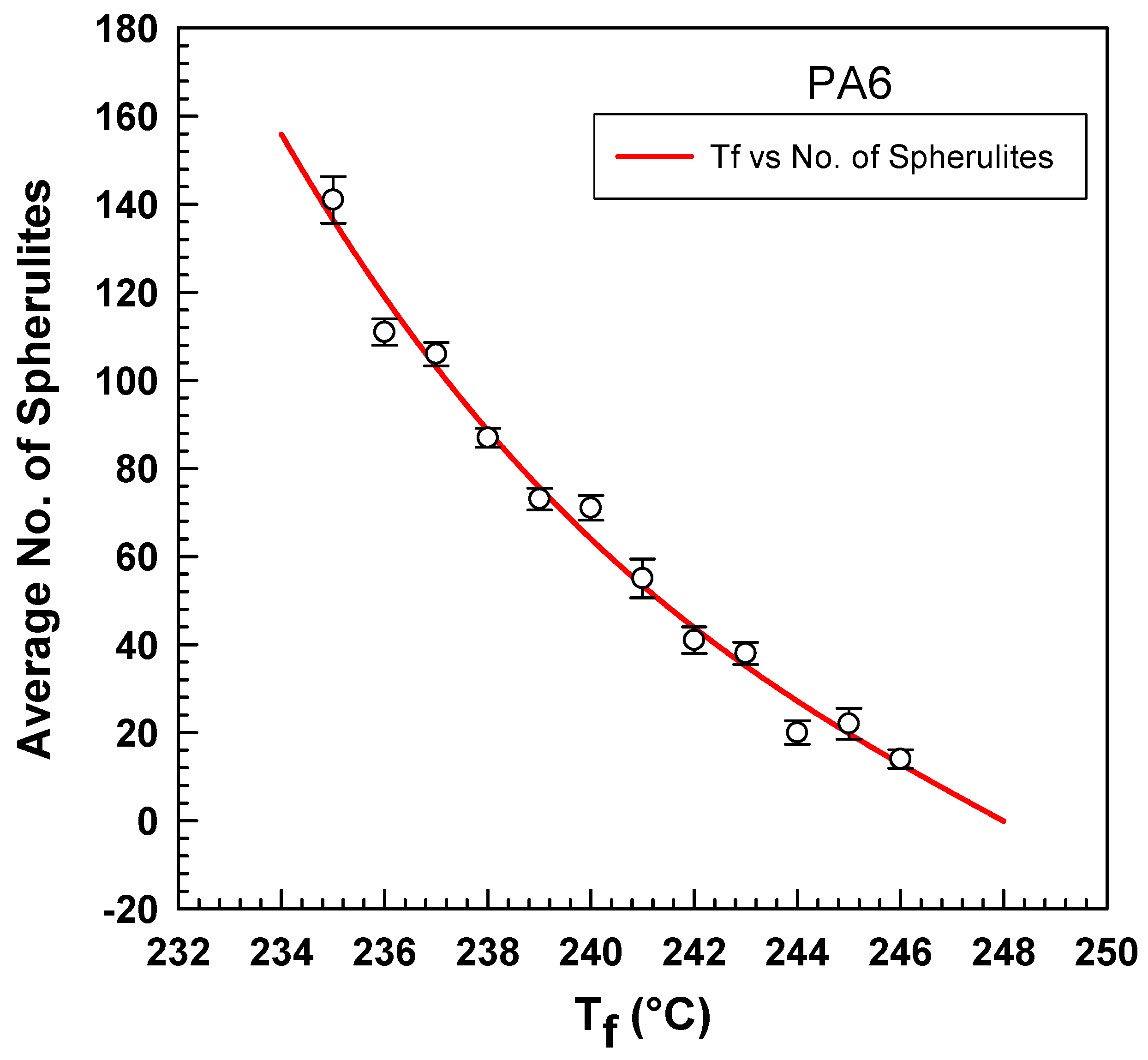
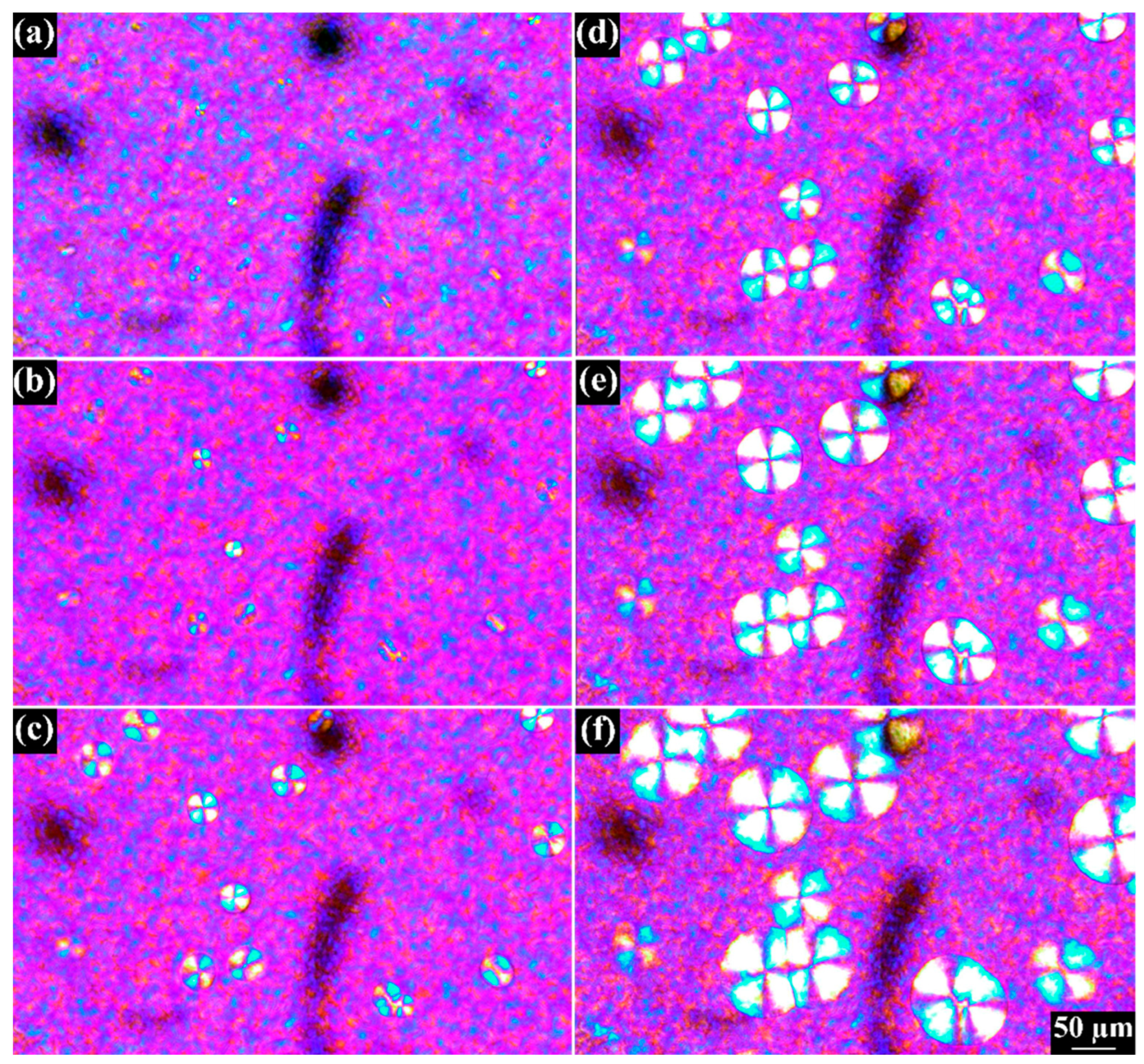
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kausar, A. Physical properties of hybrid polymer/clay composites. In Hybrid Polymer Composite Materials: Properties and Characterisation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–132. [Google Scholar] [CrossRef]
- Gaymans, R.J. Polyamides, Synthetic Methods in Step-Growth Polymers; Rogers, M.E., Long, T.E., Eds.; For the Ring-Opening Aminolysis-Condensation (ROAC) of Diamines and Dilactones, See Refs. [5e, g]; Wiley: New York, NY, USA, 2003; pp. 135–195. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Ziabicki, A. Memory effects in isothermal crystallization II. Isotactic polypropylene. Colloid Polym. Sci. 1995, 273, 317–323. [Google Scholar] [CrossRef]
- Supaphol, P.; Lin, J.-S. Crystalline memory effect in isothermal crystallization of syndiotactic polypropylenes: Effect of fusion temperature on crystallization and melting behavior. Polymer 2001, 42, 9617–9626. [Google Scholar] [CrossRef]
- Millot, C.; Fillot, L.-A.; Lame, O.; Sotta, P.; Seguela, R. Assessment of polyamide-6 crystallinity by DSC. J. Therm. Anal. Calorim. 2015, 122, 307–314. [Google Scholar] [CrossRef]
- Rudin, A. (Ed.) Chapter 1—Introductory Concepts and Definitions. In The Elements of Polymer Science and Engineering; Academic Press: San Diego, CA, USA, 1982; pp. 1–40. [Google Scholar]
- Payal, R.; Sommer, J.-U. Crystallization of Polymers under the Influence of an External Force Field. Polymers 2021, 13, 2078. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I. Crystallization of bulk polymers with chain folding: Theory of growth of lamellar spherulites. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65A, 297–336. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kelnar, I. A simple method of evaluating non-isothermal crystallization kinetics in multicomponent polymer systems. Polym. Test. 2015, 47, 79–86. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. The effect of morphology and chemical characteristics of cellulose reinforcements on the crystallinity of polylactic acid. J. Appl. Polym. Sci. 2006, 101, 300–310. [Google Scholar] [CrossRef]
- Ramanujam, B.T.S.; Annamalai, P.K. Conducting polymer-graphite binary and hybrid composites: Structure, properties, and applications. In Hybrid Polymer Composite Materials: Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Ziabicki, A.; Alfonso, G.C. Memory effects in isothermal crystallization. I. Theory. Colloid Polym. Sci. 1994, 272, 1027–1042. [Google Scholar] [CrossRef]
- Ishida, K.; Han, S.-I.; Im, S.-S.; Inoue, Y. Effects of Fusion Temperature and Metal Ion Variation on Crystallization of Lightly Ionized Poly(butylene succinate). Macromol. Chem. Phys. 2007, 208, 146–154. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Kuhn, W.P. Measurement of crystalline index in nylons by DSC: Complexities and recommendations. J. Polym. Sci. Part B-Polym. Phys. 1997, 35, 2219–2231. [Google Scholar] [CrossRef]
- Seguela, R. Overview and critical survey of polyamide6 structural habits: Misconceptions and controversies. J. Polym. Sci. 2020, 58, 2971–3003. [Google Scholar] [CrossRef]
- Mondal, A.; Sohel, A.; Arif, P.M.; Thomas, S.; SenGupta, A. Effect of ABS on non-isothermal crystallization kinetics of polyamide 6. J. Therm. Anal. Calorim. 2021, 146, 2489–2501. [Google Scholar] [CrossRef]
- Wu, B.; Gong, Y.; Yang, G. Non-isothermal crystallization of polyamide 6 matrix in all-polyamide composites: Crystallization kinetic, melting behavior, and crystal morphology. J. Mater. Sci. 2011, 46, 5184–5191. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Nakamura, K.; Katayama, K.; Amano, T. Some aspects of nonisothermal crystallization of polymers. II. Consideration of the isokinetic condition. J. Appl. Polym. Sci. 1973, 17, 1031–1041. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Jiang, S.; Zhou, K.; Bai, Z.; Wang, B.; Tai, Q.; Song, L.; Hu, Y. Thermal degradation and combustion behaviors of flame retarded glass fiber reinforced polyamide 6 composites based on cerium hypophosphite. Polym. Compos. 2016, 37, 3073–3082. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Silvestre, C. Non-isothermal crystallization of polymers. Prog. Polym. Sci. 1999, 24, 917–950. [Google Scholar] [CrossRef]
- Cebe, P.; Thomas, D.; Merfeld, J.; Partlow, B.P.; Kaplan, D.L.; Alamo, R.G.; Wurm, A.; Zhuravlev, E.; Schick, C. Heat of fusion of polymer crystals by fast scanning calorimetry. Polymer 2017, 126, 240–247. [Google Scholar] [CrossRef]
- Freire, L.; Combeaud, C.; Monge, G.; Billon, N.; Haudin, J. Transcrystallinity versus spherulitic crystallization in polyamide 66: An experimental study. Polym. Cryst. 2019, 2, e10028. [Google Scholar] [CrossRef]
- He, C.; Cao, X.; Huo, G.; Luo, S.; He, X. Non-Isothermal Crystallization Behaviour and Kinetics of LLDPE/REDMUD Blends. Polym. Polym. Compos. 2015, 23, 483–494. [Google Scholar] [CrossRef]
- Wang, H.-L.; Shi, T.-J.; Yang, S.-Z.; Hang, G.-P. Crystallization behavior of PA6/SiO2 organic–inorganic hybrid material. Mater. Res. Bull. 2006, 41, 298–306. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.; Wang, X.; Lu, L. Non-isothermal crystallization kinetics of nylon 6/attapulgite nanocomposites. Polym. Test. 2010, 29, 596–602. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Zhang, H. The morphology and non-isothermal crystallization characteristics of poly(trimethylene terephthalate)/BaSO4 nanocomposites prepared by in situ polycondensation. Polym. Test. 2009, 28, 402–411. [Google Scholar] [CrossRef]
- Poisson, C.; Colaers, M.; Van Puyvelde, P.; Goderis, B. Memory Effects in the Quiescent Crystallization of Polyamide 12: Self-Seeding, Post-Condensation, Disentangling, and Self-Nucleation beyond the Equilibrium Melting Temperature. Macromolecules 2023, 56, 2747–2760. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, F.; Chen, J.; Kang, J.; Yang, F.; Cao, Y.; Xiang, M. Regulating polycrystalline behavior of the β-nucleated isotactic polypropylene/graphene oxide composites by melt memory effect. Polym. Compos. 2019, 40 (Suppl. S1), E440–E448. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Pourali, M.; Peterson, A.M. A tale of two polyamides: Comparing the crystallization kinetics of a hot-melt adhesive and a PA 6/66 copolymer. Thermochim. Acta 2022, 710, 179176. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N.; Chrissafis, K. Crystallization and melting of propylene–ethylene random copolymers. Homogeneous nucleation and β-nucleating agents. Eur. Polym. J. 2013, 49, 1577–1590. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-based polyesters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Uthaipan, N.; Jarnthong, M.; Peng, Z.; Junhasavasdikul, B.; Nakason, C.; Thitithammawong, A. Effects of cooling rates on crystallization behavior and melting characteristics of isotactic polypropylene as neat and in the TPVs EPDM/PP and EOC/PP. Polym. Test. 2015, 44, 101–111. [Google Scholar] [CrossRef]
- Lee, Y.; Porter, R.S. Effects of thermal history on crystallization of poly(ether ether ketone) (PEEK). Macromolecules 1988, 21, 2770–2776. [Google Scholar] [CrossRef]
- Svoboda, P.; Trivedi, K.; Stoklasa, K.; Svobodova, D.; Ougizawa, T. Study of crystallization behaviour of electron beam irradiated polypropylene and high-density polyethylene. R. Soc. Open Sci. 2021, 8, 202250. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Schick, C. Crystal Nucleation of Polymers at High Supercooling of the Melt. Polym. Cryst. I Chain. Microstruct. Process. 2017, 276, 257–288. [Google Scholar] [CrossRef]
- Levy, A. Robust Numerical Resolution of Nakamura Crystallization Kinetics. Int. J. Theor. Appl. Math. 2017, 3, 143. [Google Scholar] [CrossRef]
- Seo, J.; Zhang, X.; Schaake, R.P.; Rhoades, A.M.; Colby, R.H. Dual Nakamura model for primary and secondary crystallization applied to nonisothermal crystallization of poly(ether ether ketone). Polym. Eng. Sci. 2021, 61, 2416–2426. [Google Scholar] [CrossRef]
- Lee, S.; Ree, M.; Park, C.; Jung, Y.; Jin, Y.; Bae, D. Synthesis and non-isothermal crystallization behaviors of poly(ethylene isophthalate-co-terephthalate)s. Polymer 1999, 40, 7137–7146. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zhao, Y.-K.; Wu, X.-F. Non-Isothermal Crystallization Kinetics of Graphene Oxide-Carbon Nanotubes Hybrids/Polyamide 6 Composites. J. Chem. Soc. Pak. 2019, 41, 394. [Google Scholar]
- Bassett, D.C. Polymer Spherulites: A Modern Assessment. J. Macromol. Sci. Part B 2007, 42, 227–256. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Wang, H.; Hu, G. Isothermal crystallization kinetics and melting behavior of in situ compatibilized polyamide 6/Polyethylene-octene blends. J. Polym. Res. 2010, 17, 429–437. [Google Scholar] [CrossRef]






| Property | Polyamide 6 F232-D |
|---|---|
| Melt temperature (10 °C/min) | 220 °C (ISO 11357) |
| Tensile modulus | 3300 MPa (ISO 527) |
| Charpy notched impact strength | 6 kJ/m2 at 23 °C (ISO 179) |
| Density | 1130 kg/m3 (ISO 1183) |
| Viscosity | 214 cm3/g (ISO 307) |
| Fusion Temp. (°C) | Avrami Parameters | |||
|---|---|---|---|---|
| R2 | Slope | n | k (s−1) | |
| 225 | 0.9992 | 0.02014 | 2.45 | 0.59 × 10−4 |
| 227 | 0.9993 | 0.02313 | 2.27 | 1.76 × 10−4 |
| 229 | 0.9993 | 0.02714 | 2.28 | 2.29 × 10−4 |
| 231 | 0.9990 | 0.02912 | 2.48 | 1.22 × 10−4 |
| Fusion Temp. (°C) | Nakamura K = k(1/n) (s−1) | t1/2−1(s−1) | Slope |
| 231 | 0.0337 | 0.0380 | 0.0363 |
| 233 | 0.0291 | 0.0326 | 0.0350 |
| 235 | 0.0279 | 0.0315 | 0.0333 |
| 237 | 0.0235 | 0.0263 | 0.0308 |
| 238 | 0.0208 | 0.0231 | 0.0289 |
| 239 | 0.0166 | 0.0182 | 0.0252 |
| 240 | 0.0140 | 0.0153 | 0.0225 |
| 241 | 0.0129 | 0.0140 | 0.0207 |
| 242 | 0.0122 | 0.0133 | 0.0191 |
| 245 | 0.0121 | 0.0132 | 0.0193 |
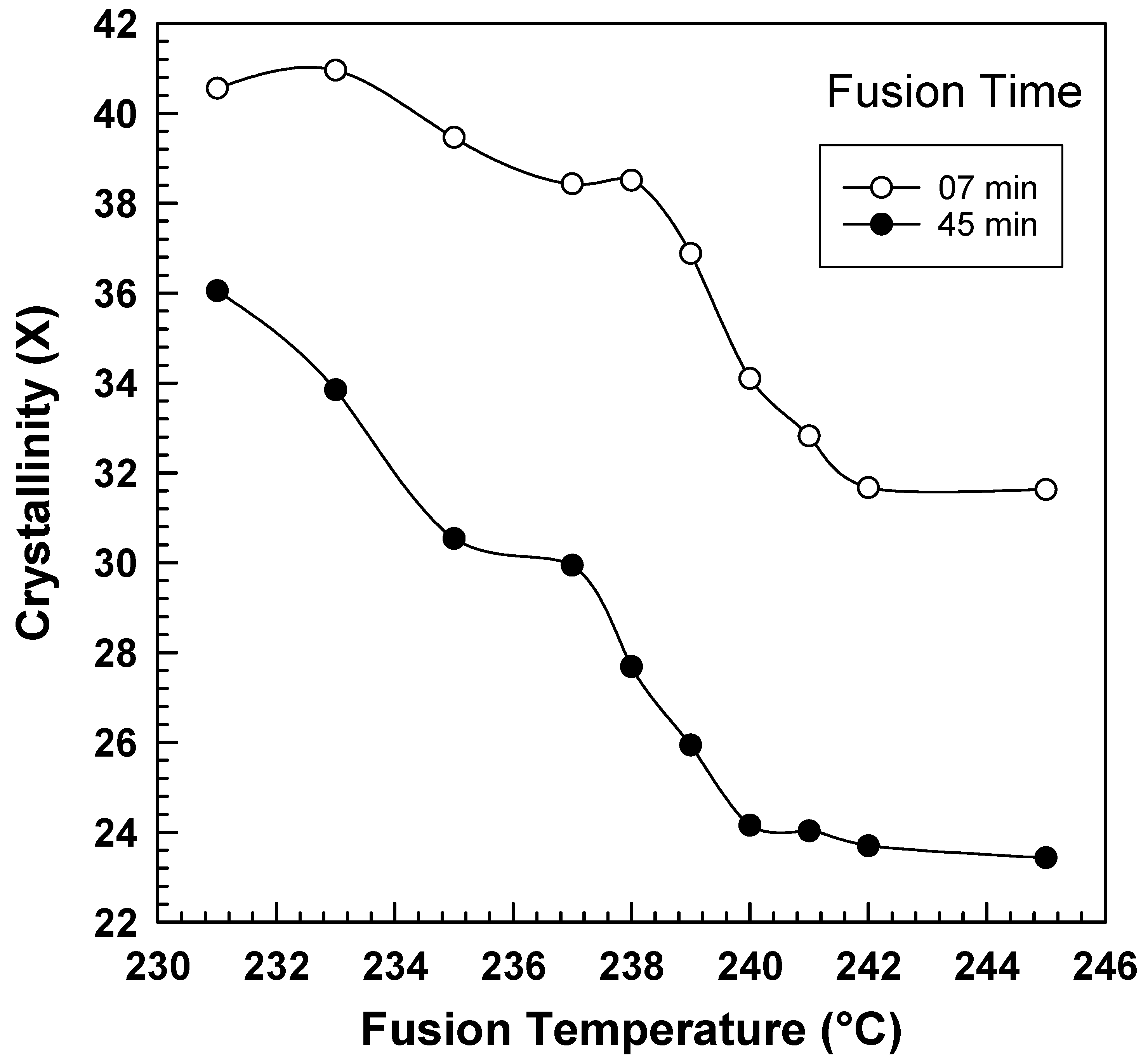
| Fusion Temperature (°C) | Crystallinity | |
|---|---|---|
| 7 min | 45 min | |
| 231 | 40.56 | 36.05 |
| 233 | 40.96 | 33.85 |
| 235 | 39.46 | 30.53 |
| 237 | 38.43 | 29.94 |
| 238 | 38.51 | 27.68 |
| 239 | 36.88 | 25.94 |
| 240 | 34.1 | 24.15 |
| 241 | 32.82 | 24.03 |
| 242 | 31.67 | 23.70 |
| 245 | 31.63 | 23.43 |
| Temperature (°C) | Slope | Intercept | RSQ |
|---|---|---|---|
| m | log[K(T)] | R2 | |
| 176 | 2.2 | 1.449 | 0.9988 |
| 175 | 2.2 | 1.552 | 0.9997 |
| 174 | 2.2 | 1.741 | 0.9979 |
| 173 | 2.3 | 1.986 | 0.9970 |
| 172 | 2.5 | 2.255 | 0.9974 |
| 171 | 2.6 | 2.521 | 0.9985 |
| 170 | 2.7 | 2.770 | 0.9994 |
| 169 | 2.8 | 2.993 | 0.9999 |
| 168 | 2.9 | 3.186 | 0.9999 |
| 167 | 2.9 | 3.349 | 0.9995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, A.; Svoboda, P. Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6. Polymers 2023, 15, 1952. https://doi.org/10.3390/polym15081952
Nasr A, Svoboda P. Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6. Polymers. 2023; 15(8):1952. https://doi.org/10.3390/polym15081952
Chicago/Turabian StyleNasr, Ahmed, and Petr Svoboda. 2023. "Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6" Polymers 15, no. 8: 1952. https://doi.org/10.3390/polym15081952
APA StyleNasr, A., & Svoboda, P. (2023). Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6. Polymers, 15(8), 1952. https://doi.org/10.3390/polym15081952






