High-Expansion Open-Cell Polylactide Foams Prepared by Microcellular Foaming Based on Stereocomplexation Mechanism with Outstanding Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Experimental Materials
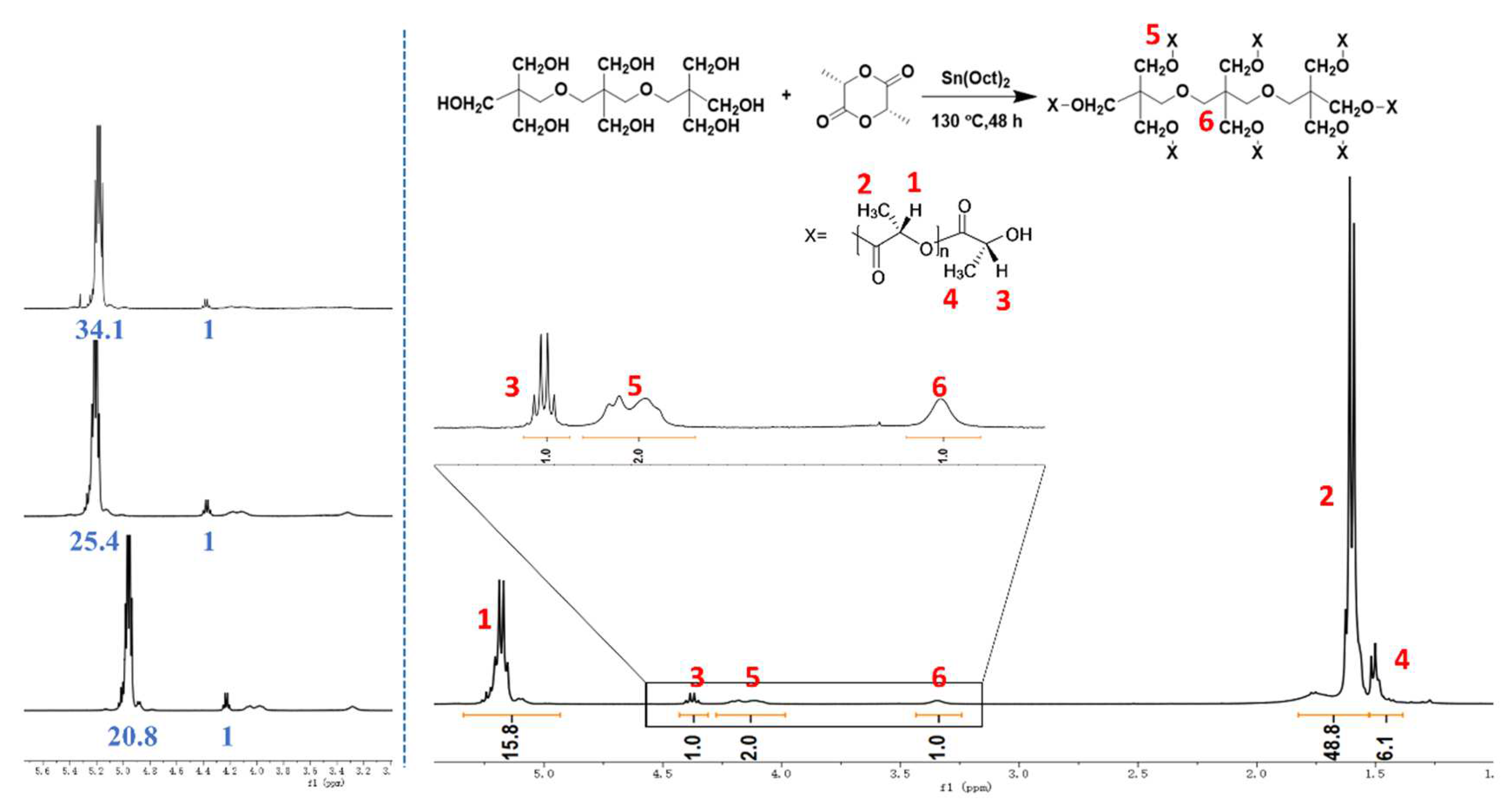
2.2. Synthesis of Star-Shaped PDLA with Eight Arms (8-s-PDLA)
2.3. Preparation of PLLA/8-s-PDLA
2.4. Supercritical Carbon Dioxide Microcellular Foaming
2.5. Characterization and Analysis
3. Results and Discussion
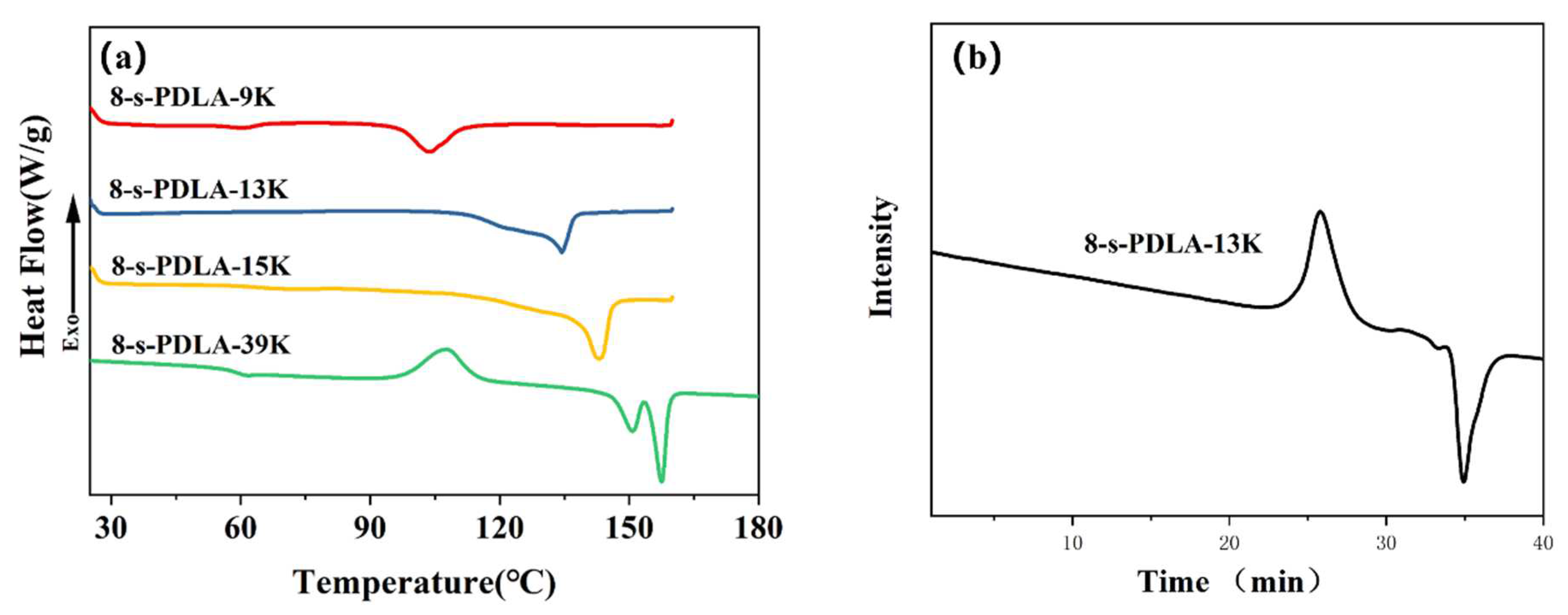
3.1. Characterization of Synthesized 8-s-PDLA
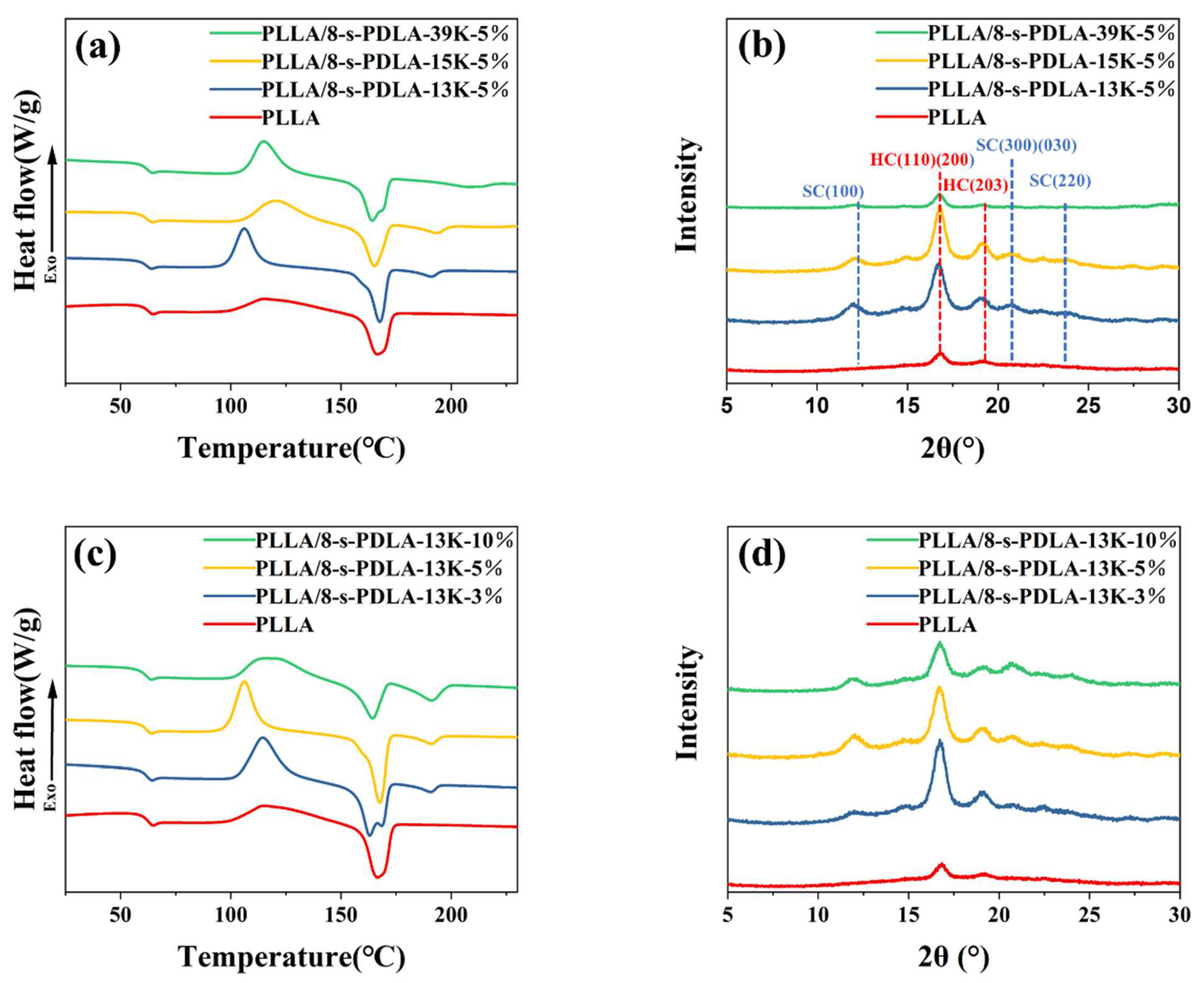
3.2. Foaming Behavior of PLLA/8-s- PDLA Blends during Microcellular Foaming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Lee, R.E.; Wang, G.; Chu, R.K.; Zhao, J.; Park, C.B. Use of stereocomplex crystallites for fully-biobased microcellular low-density poly(lactic acid) foams for green packaging. Chem. Eng. J. 2017, 327, 1151–1162. [Google Scholar] [CrossRef]
- Atiénzar-Navarro, R.; del Rey, R.; Alba, J.; Sánchez-Morcillo, V.J.; Picó, R. Sound Absorption Properties of Perforated Recycled Polyurethane Foams Reinforced with Woven Fabric. Polymers 2020, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Fang, W.; Zhong, M.; Jin, J.; Fan, P.; Yang, J.; Fei, Z.; Xu, L.; Chen, F. Extrusion Foaming of Lightweight Polystyrene Composite Foams with Controllable Cellular Structure for Sound Absorption Application. Polymers 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhao, G.; He, X. Non-acoustical parameters and sound absorption characteristics of porous polyurethane foams. Phys. Fluids 2019, 31, 037106. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, J.; Guo, H.; Guo, X.; Wang, X.; Shen, Y.; Li, Q. Fabrication of fibrillated and interconnected porous poly(ε-caprolactone) vascular tissue engineering scaffolds by microcellular foaming and polymer leaching. RSC Adv. 2020, 10, 10055–10066. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Montes, A.; Valor, D.; Delgado, L.; Pereyra, C.; de la Ossa, E.M. An Attempt to Optimize Supercritical CO2 Polyaniline-Polycaprolactone Foaming Processes to Produce Tissue Engineering Scaffolds. Polymers 2022, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Hailan, S.M.; Ponnamma, D.; Krupa, I. The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review. Polymers 2021, 13, 4142. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Du, J.; Yang, K.; Ren, T.; Wan, D.; Pu, H. Superhydrophobic and superoleophilic polystyrene/carbon nanotubes foam for oil/water separation. J. Environ. Chem. Eng. 2021, 9, 106038. [Google Scholar] [CrossRef]
- Fan, L.; Wang, R.; Zhang, Q.; Liu, S.; He, R.; Zhang, R.; Shen, M.; Xiang, X.; Zhou, Y. In situ self-foaming preparation of hydrophobic polyurethane foams for oil/water separation. New J. Chem. 2021, 45, 13902–13908. [Google Scholar] [CrossRef]
- Tabatabaei, A.; Park, C.B. In-situ visualization of PLA crystallization and crystal effects on foaming in extrusion. Eur. Polym. J. 2017, 96, 505–519. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams. Polymers 2020, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Naguib, H.E.; Park, C.B.; Wang, J. Increase of open-cell content by plasticizing soft regions with secondary blowing agent. Polym. Eng. Sci. 2005, 45, 1445–1451. [Google Scholar] [CrossRef]
- Kong, W.-L.; Bao, J.-B.; Wang, J.; Hu, G.-H.; Xu, Y.; Zhao, L. Preparation of open-cell polymer foams by CO2 assisted foaming of polymer blends. Polymer 2016, 90, 331–341. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heuzey, M.-C.; Carreau, P.J.; Taguet, A. Interfacial localization of CNCs in PLA/PBAT blends and its effect on rheological, thermal, and mechanical properties. Polymer 2021, 233, 124229. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.; Carreau, P.; Heuzey, M.-C.; Kamal, M. Mechanical and bead foaming behavior of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Liu, Y.; Ma, Z.; Jing, Z.; Fan, X. Microcellular foaming of polylactide and poly(butylene adipate-co-terphathalate) blends and their CaCO3reinforced nanocomposites using supercritical carbon dioxide. Polym. Adv. Technol. 2016, 27, 550–560. [Google Scholar] [CrossRef]
- Yu, P.; Mi, H.-Y.; Huang, A.; Geng, L.-H.; Chen, B.-Y.; Kuang, T.-R.; Mou, W.-J.; Peng, X.-F. Effect of Poly(butylenes succinate) on Poly(lactic acid) Foaming Behavior: Formation of Open Cell Structure. Ind. Eng. Chem. Res. 2015, 54, 6199–6207. [Google Scholar] [CrossRef]
- Chai, J.; Wang, G.; Zhao, J.; Zhang, A.; Shi, Z.; Wei, C.; Zhao, G. Microcellular PLA/PMMA foam fabricated by CO2 foaming with outstanding shape-memory performance. J. CO2 Util. 2021, 49, 101553. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, C.; Wang, G.; Li, S.; Zhang, A.; Dong, G.; Zhao, G. Miscible polymethyl methacrylate/polylactide blend with enhanced foaming behavior and foam mechanical properties. J. CO2 Util. 2022, 61, 102065. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, H.; Wang, Y.; Wu, L.; Li, G. Unique interfacial and confined porous morphology of PLA/PS blends in supercritical carbon dioxide. RSC Adv. 2014, 4, 45109–45117. [Google Scholar] [CrossRef]
- Xu, D.; Yu, K.; Qian, K.; Park, C.B. Foaming behavior of microcellular poly(lactic acid)/TPU composites in supercritical CO2. J. Thermoplast. Compos. Mater. 2018, 31, 61–78. [Google Scholar] [CrossRef]
- Ahmed, M.; Li, Y.; Yao, Z.; Cao, K.; Zeng, C. TPU/PLA blend foams: Enhanced foamability, structural stability, and implications for shape memory foams. J. Appl. Polym. Sci. 2019, 136, 47416. [Google Scholar] [CrossRef]
- Wang, W.; Liao, X.; Guo, F.; Wang, G.; Yan, Z.; Liu, F.; Li, G. Facile Fabrication of Lightweight Shape Memory Thermoplastic Polyurethane/Polylactide Foams by Supercritical Carbon Dioxide Foaming. Ind. Eng. Chem. Res. 2020, 59, 7611–7623. [Google Scholar] [CrossRef]
- Liu, M.-J.; Chen, S.-C.; Yang, K.-K.; Wang, Y.-Z. Biodegradable polylactide based materials with improved crystallinity, mechanical properties and rheological behaviour by introducing a long-chain branched copolymer. RSC Adv. 2015, 5, 42162–42173. [Google Scholar] [CrossRef]
- Gigmes, D.; Trimaille, T. Advances in amphiphilic polylactide/vinyl polymer based nano-assemblies for drug delivery. Adv. Colloid Interface Sci. 2021, 294, 102483. [Google Scholar] [CrossRef]
- Valderrama, M.A.M.; van Putten, R.-J.; Gruter, G.-J.M. PLGA Barrier Materials from CO2. The influence of Lactide Co-monomer on Glycolic Acid Polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G. Rheology and crystallization behavior of asymmetric PLLA/PDLA blends based on linear PLLA and PDLA with different structures. Polym. Adv. Technol. 2016, 27, 1108–1120. [Google Scholar] [CrossRef]
- Shi, X.; Qin, J.; Wang, L.; Ren, L.; Rong, F.; Li, D.; Wang, R.; Zhang, G. Introduction of stereocomplex crystallites of PLA for the solid and microcellular poly(lactide)/poly(butylene adipate-co-terephthalate) blends. RSC Adv. 2018, 8, 11850–11861. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, Q.; Gao, Y.; Zhang, G.; Huang, F.; Xiao, R.; Liu, W.; Wang, F.; Qin, J.; Bilotti, E.; et al. Microcellular epoxy/graphene nanocomposites with outstanding electromagnetic interference shielding and mechanical performance by overcoming nanofiller loading/dispersion dichotomy. Compos. Sci. Technol. 2021, 215, 109000. [Google Scholar] [CrossRef]
- Chen, Q.; Auras, R.; Corredig, M.; Kirkensgaard, J.J.K.; Mamakhel, A.; Uysal-Unalan, I. New opportunities for sustainable bioplastic development: Tailorable polymorphic and three-phase crystallization of stereocomplex polylactide by layered double hydroxide. Int. J. Biol. Macromol. 2022, 222, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, H.; Wang, K.; Xu, H.; He, Y.; Wang, X. Influence of scPLA microsphere on the crystallization behavior of PLLA/PDLA composites. Compos. Commun. 2020, 21, 100380. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Green fabrication method of layered and open-cell polylactide foams for oil-sorption via pre-crystallization and supercritical CO2-induced melting. J. Supercrit. Fluids 2020, 162, 104854. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Li, X.; Hu, Z.; Wang, B.; Li, M.; Dong, W. Preparation of high-expansion open-cell polylactic acid foam with superior oil-water separation performance. Int. J. Biol. Macromol. 2021, 193, 1059–1067. [Google Scholar] [CrossRef]
- Wei, X.; Meng, R.; Bai, Y.; Liu, W.; Zhou, H.; Wang, X.; Xu, B. Hydrophobic and oleophilic open-cell foams from in-situ microfibrillation blends of poly(lactic acid) and polytetrafluoroethylene: Selective oil-adsorption behaviors. Int. J. Biol. Macromol. 2023, 227, 273–284. [Google Scholar] [CrossRef]
- Hua, M.; Chen, D.; Xu, Z.; Fang, Y.; Song, Y. Fabrication of high-expansion, fully degradable polylactic acid-based foam with exponent oil/water separation. J. Appl. Polym. Sci. 2022, 139, e53234. [Google Scholar] [CrossRef]


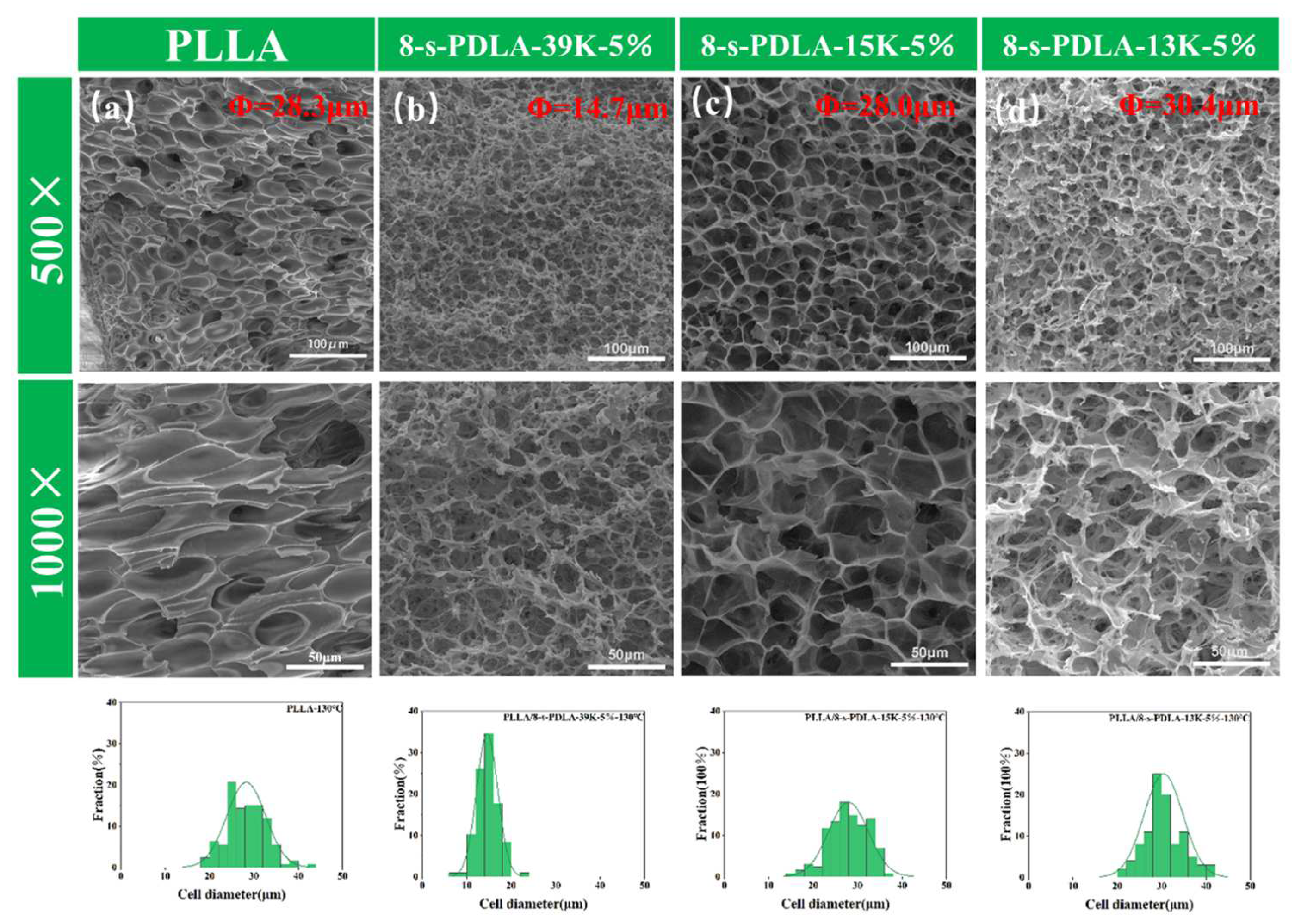

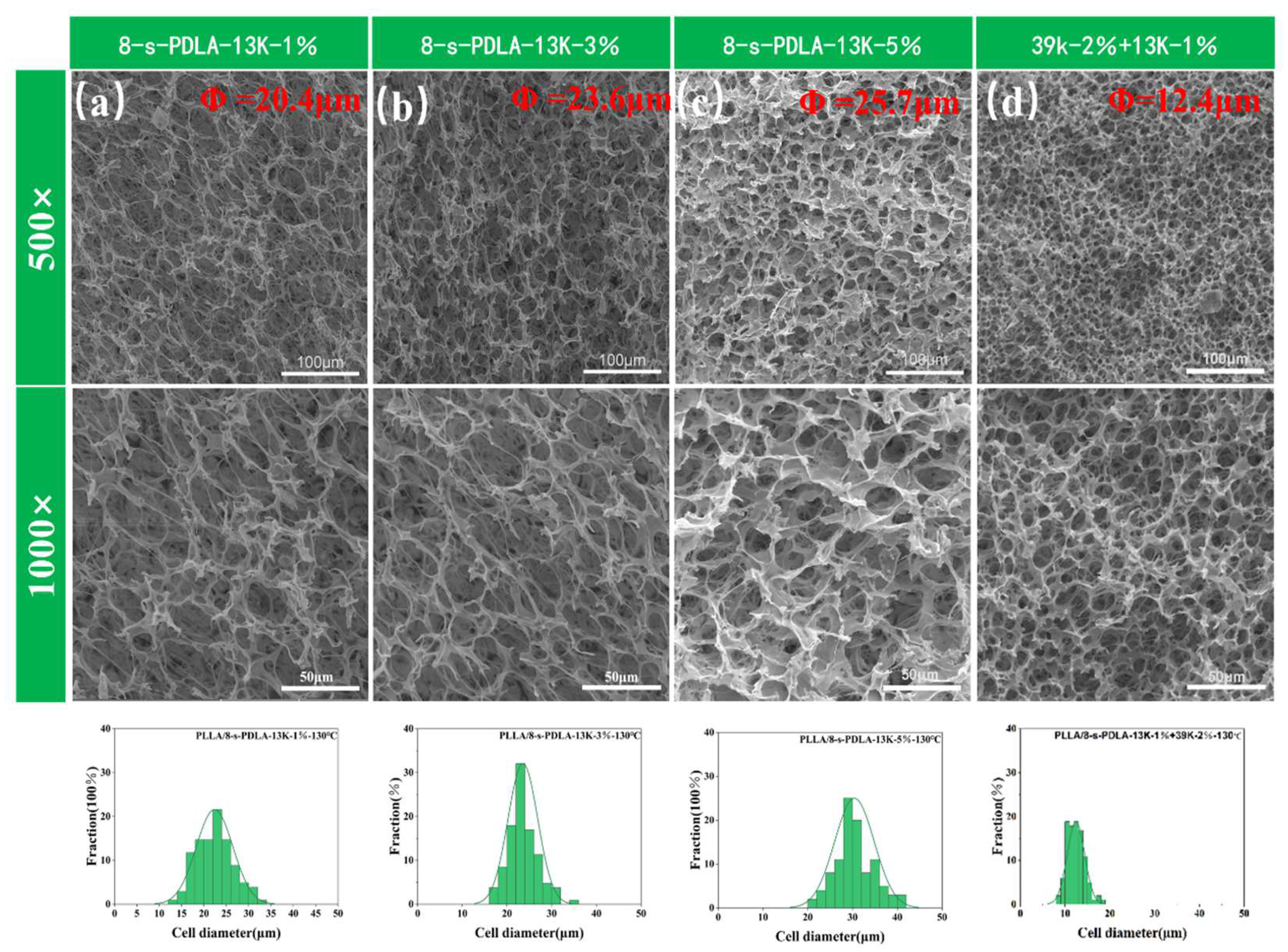


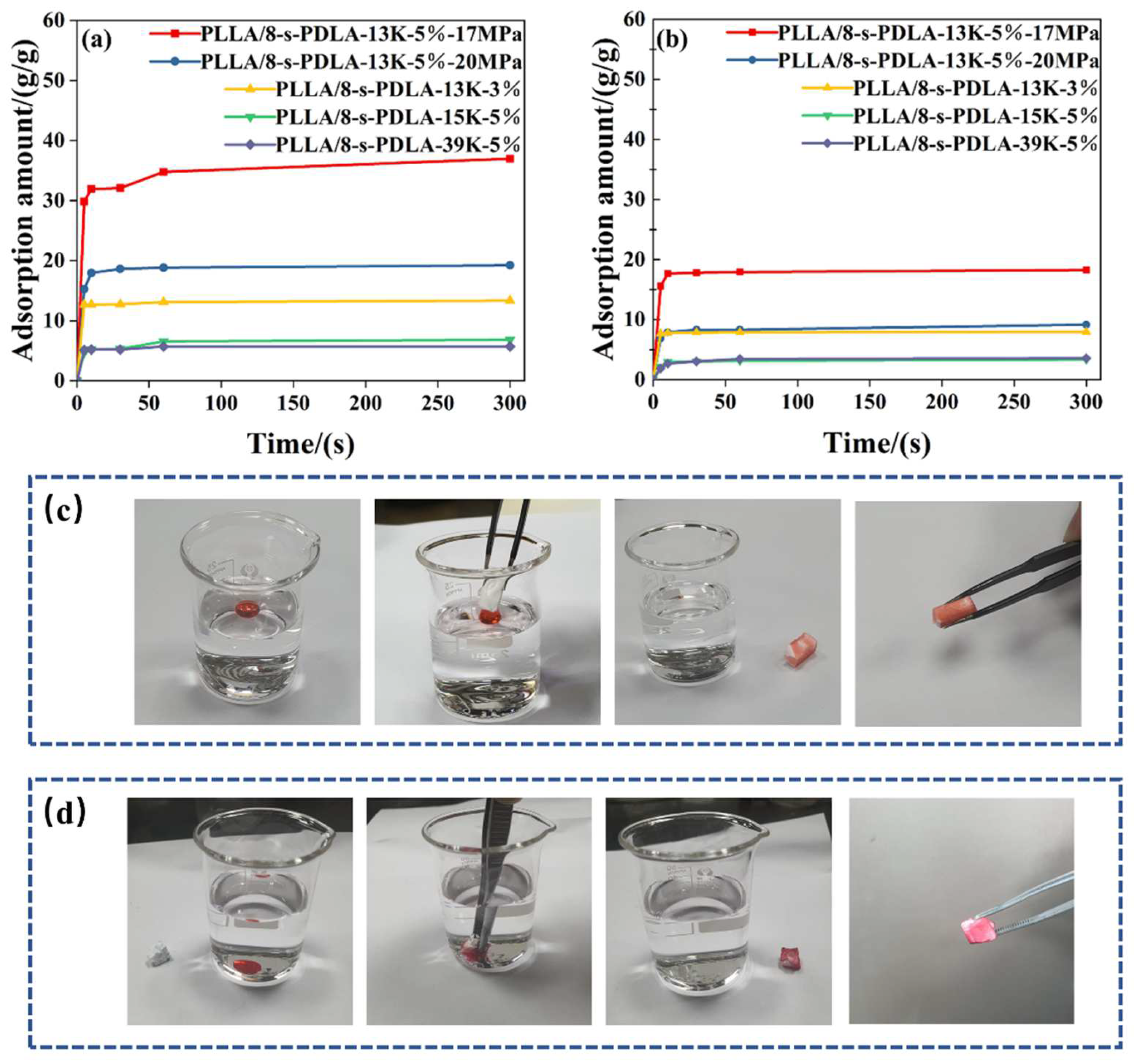
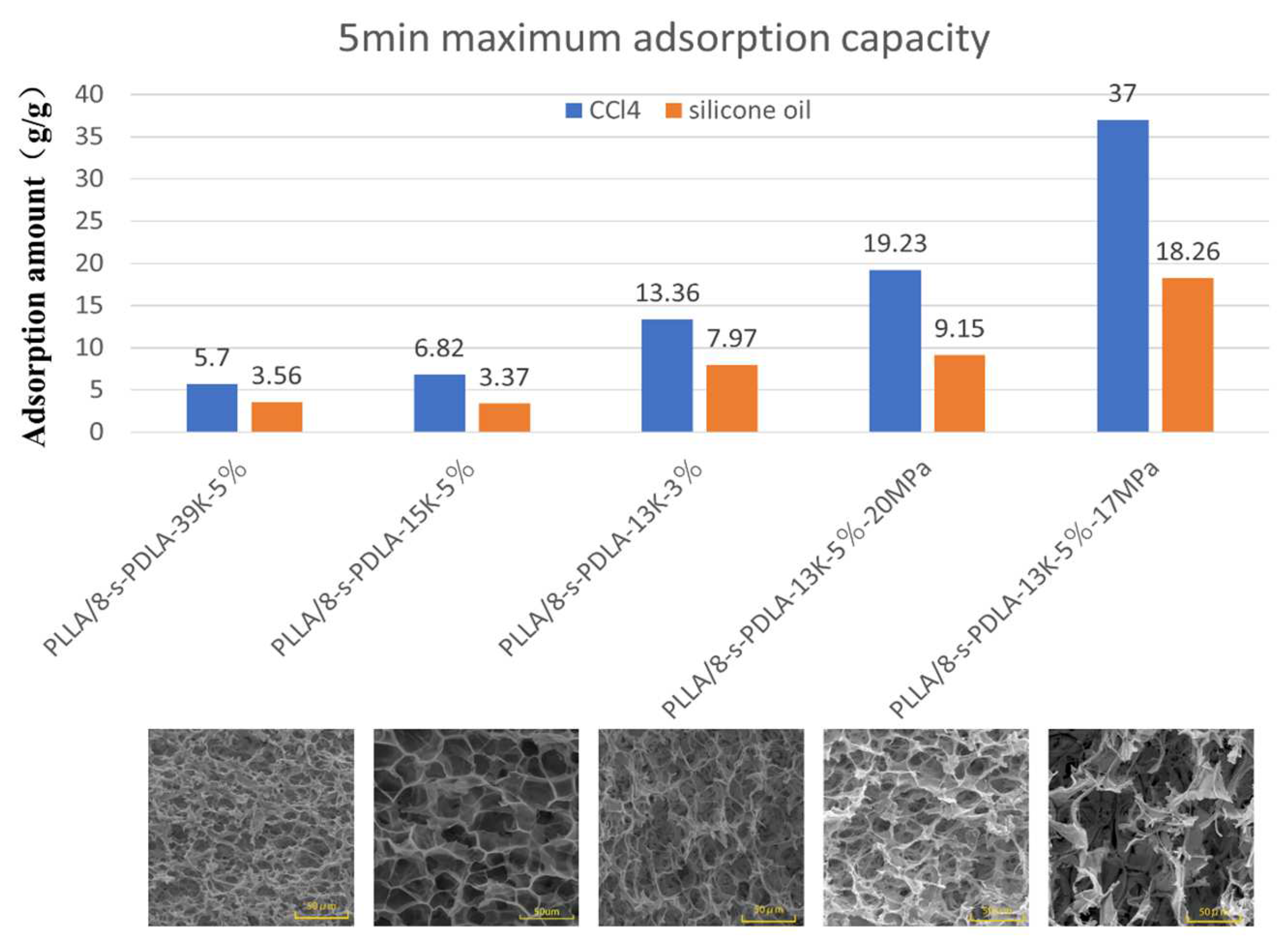
| Specimens | Cell Size(μm) | Density (g/cm3) | Expansion Ratio | Cell Density |
|---|---|---|---|---|
| PLLA | 28.26 | 0.42 | 3.03 | 1.93 × 108 |
| PLLA/8-s-PDLA-39K-5% | 14.60 | 0.17 | 7.59 | 3.50 × 109 |
| PLLA/8-s-PDLA-15K-5% | 27.95 | 0.15 | 8.63 | 5.68 × 108 |
| PLLA/8-s-PDLA-13K-5% | 20.52 | 0.09 | 14.45 | 2.40 × 109 |
| PLLA/8-s-PDLA-39K-3% | 23.55 | 0.10 | 13.50 | 1.49 × 109 |
| PLLA/8-s-PDLA-13K-5% 17MPa | 61.74 | 0.0532 | 24.06 | 1.47 × 108 |
| PLLA/8-s-PDLA-13K-1%+39K-2% | 12.43 | 0.18 | 7.03 | 5.26 × 109 |
| Authors | Materials | Expansion Multiplier | Maximum Adsorption Capacity(g/g) | Required Adsorption Time (min) | Paper |
|---|---|---|---|---|---|
| Those of this paper | PLLA/s-PDLA | 24.1 | 37.0 | <1 | |
| Li et al. | PLA/PBS | 43.6 | 21.9 | >15 | [19] |
| Li et al. | PLA | - | 4.1 | - | [34] |
| Wang et al. | PLA | 59.7 | 15.0 | - | [35] |
| Wei et al. | PLLA/PTFE | 10.2 | 6.1 | >20 | [36] |
| Hua et al. | PLLA/m-LA | 40.17 | 12.4 | >5 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, S.; Zhao, Z.; Miao, Z.; Zhang, G.; Shi, X. High-Expansion Open-Cell Polylactide Foams Prepared by Microcellular Foaming Based on Stereocomplexation Mechanism with Outstanding Oil–Water Separation. Polymers 2023, 15, 1984. https://doi.org/10.3390/polym15091984
Li D, Zhang S, Zhao Z, Miao Z, Zhang G, Shi X. High-Expansion Open-Cell Polylactide Foams Prepared by Microcellular Foaming Based on Stereocomplexation Mechanism with Outstanding Oil–Water Separation. Polymers. 2023; 15(9):1984. https://doi.org/10.3390/polym15091984
Chicago/Turabian StyleLi, Dongsheng, Shuai Zhang, Zezhong Zhao, Zhenyun Miao, Guangcheng Zhang, and Xuetao Shi. 2023. "High-Expansion Open-Cell Polylactide Foams Prepared by Microcellular Foaming Based on Stereocomplexation Mechanism with Outstanding Oil–Water Separation" Polymers 15, no. 9: 1984. https://doi.org/10.3390/polym15091984




