Development of Hybrid DSPC:DOPC:P(OEGMA950-DIPAEMA) Nanostructures: The Random Architecture of Polymeric Guest as a Key Design Parameter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
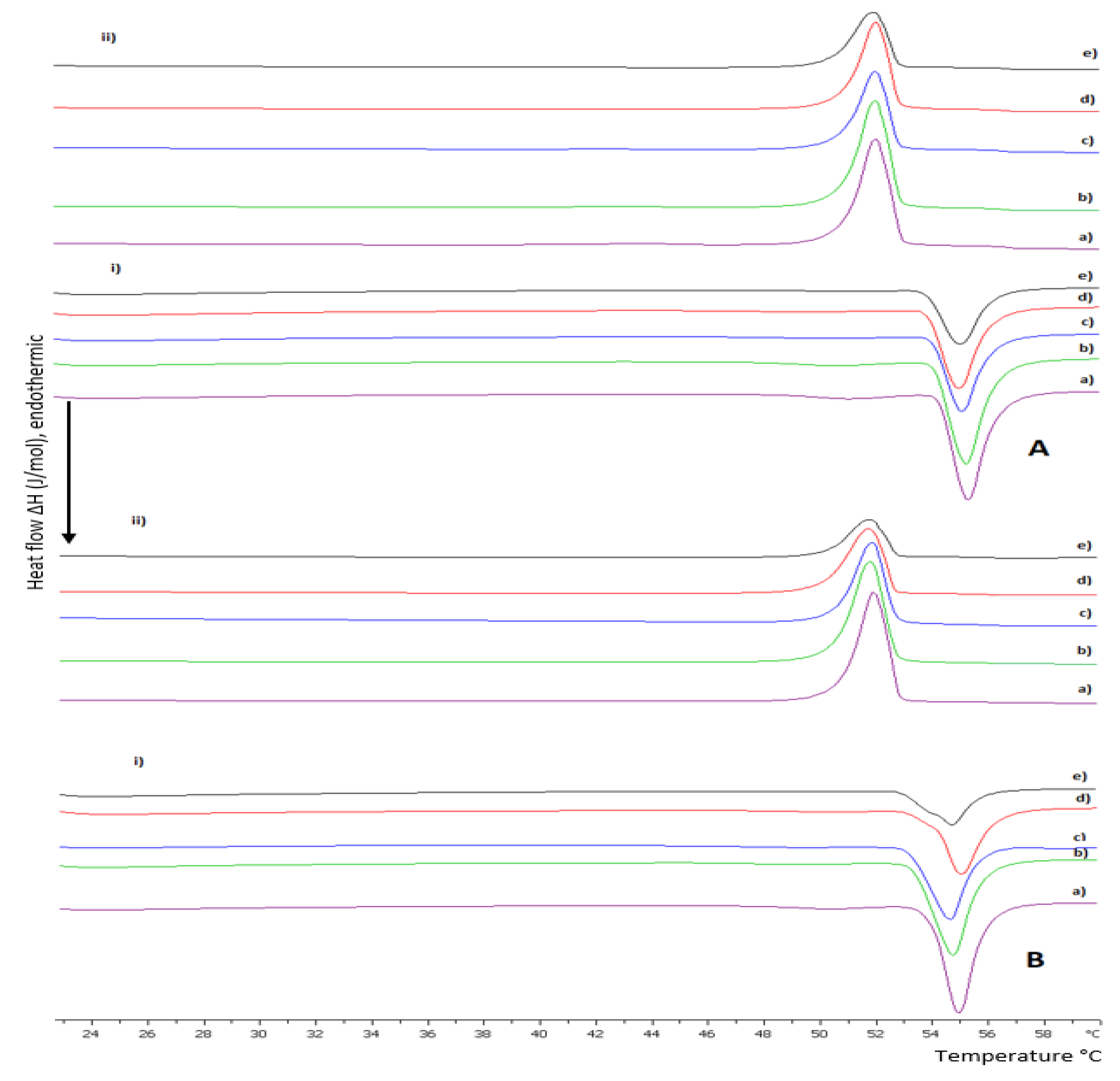
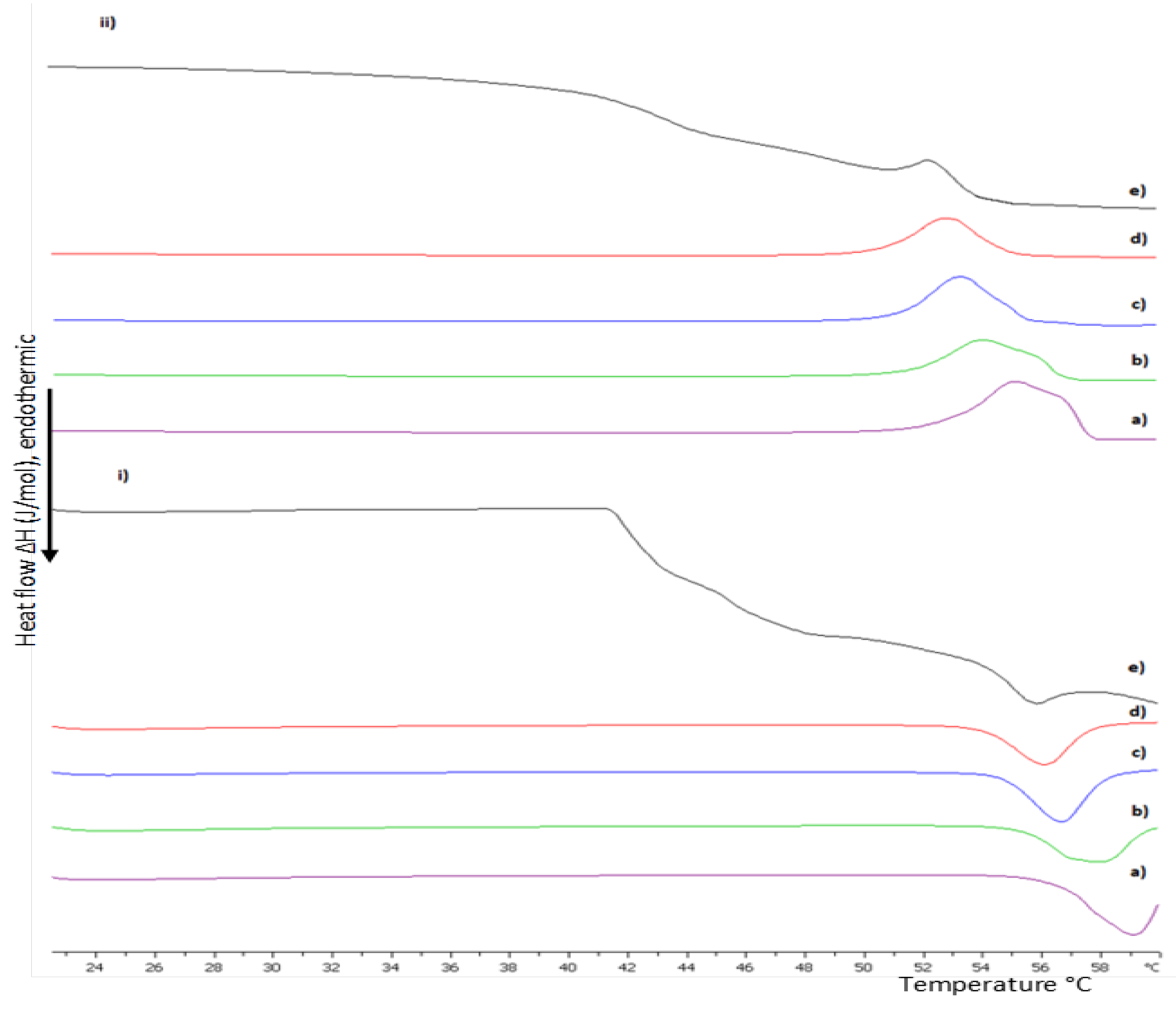
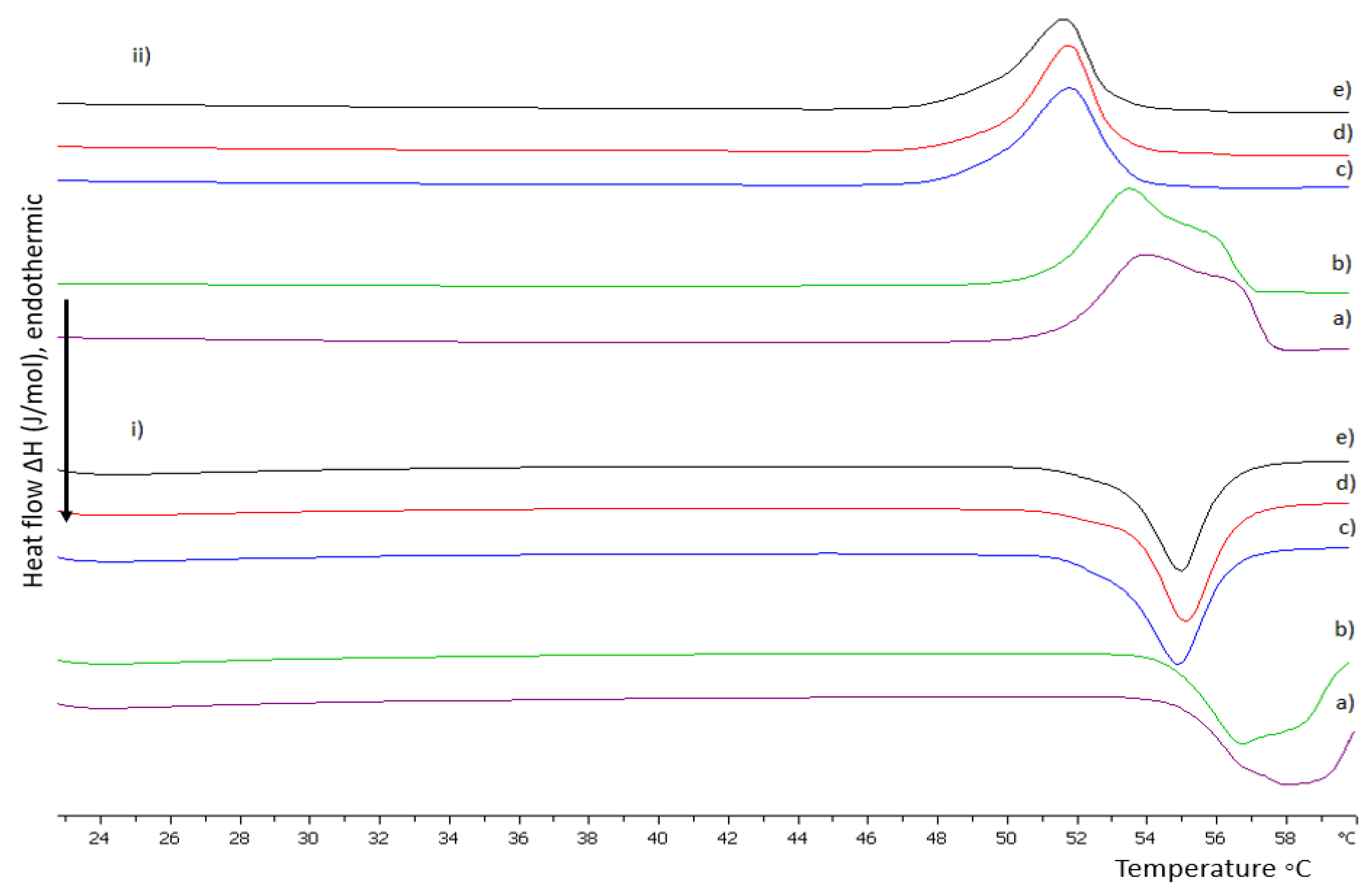
2.2.1. Differential Scanning Calorimetry
2.2.2. Preparation of Lipid/Copolymer Hybrid Systems
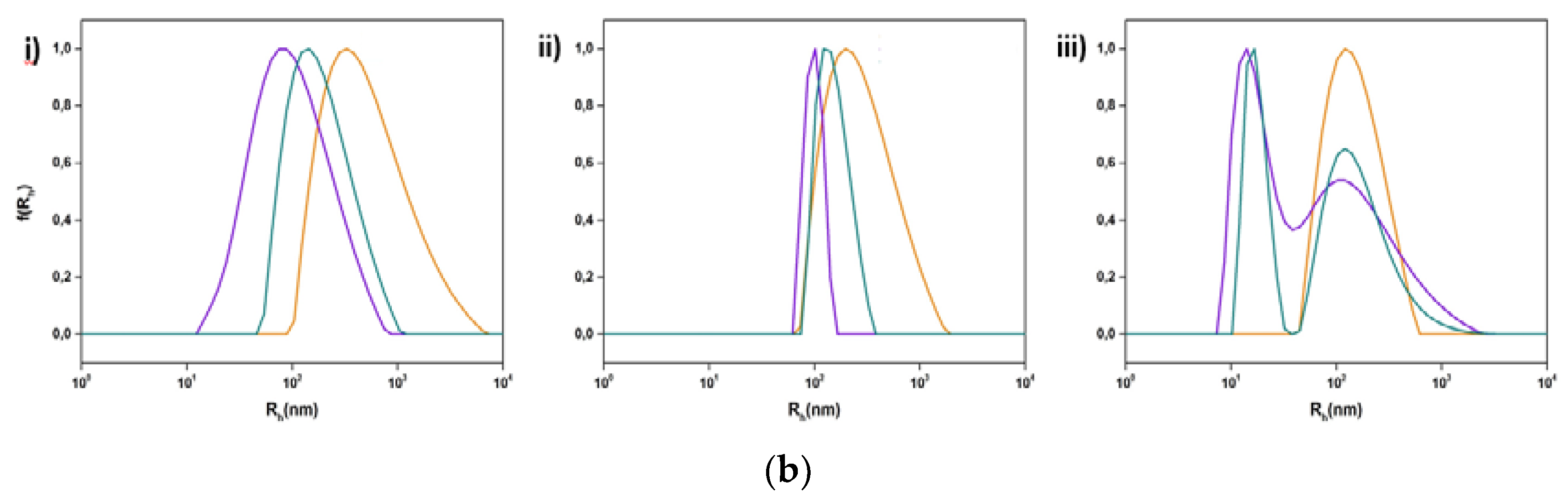
2.2.3. Light Scattering Methods
2.2.4. Fluorescence Spectroscopy
2.2.5. In Vitro Toxicity
3. Results and Discussion
3.1. Preformulation Studies of Lipid/Polymer Hybrid Bilayers
3.1.1. The Random Copolymer/Phospholipid Interactions in DSPC:P(OEGMA950-co-DIPAEMA) Hybrid Bilayers
3.1.2. The Influence of pH on DSPC:P(OEGMA950-co-DIPAEMA) Hybrid Bilayers
3.1.3. The Influence of Unsaturated DOPC in Hybrid Bilayers
3.2. Physicochemical Evaluation of Lipid/Polymer Hybrid Nanosystems
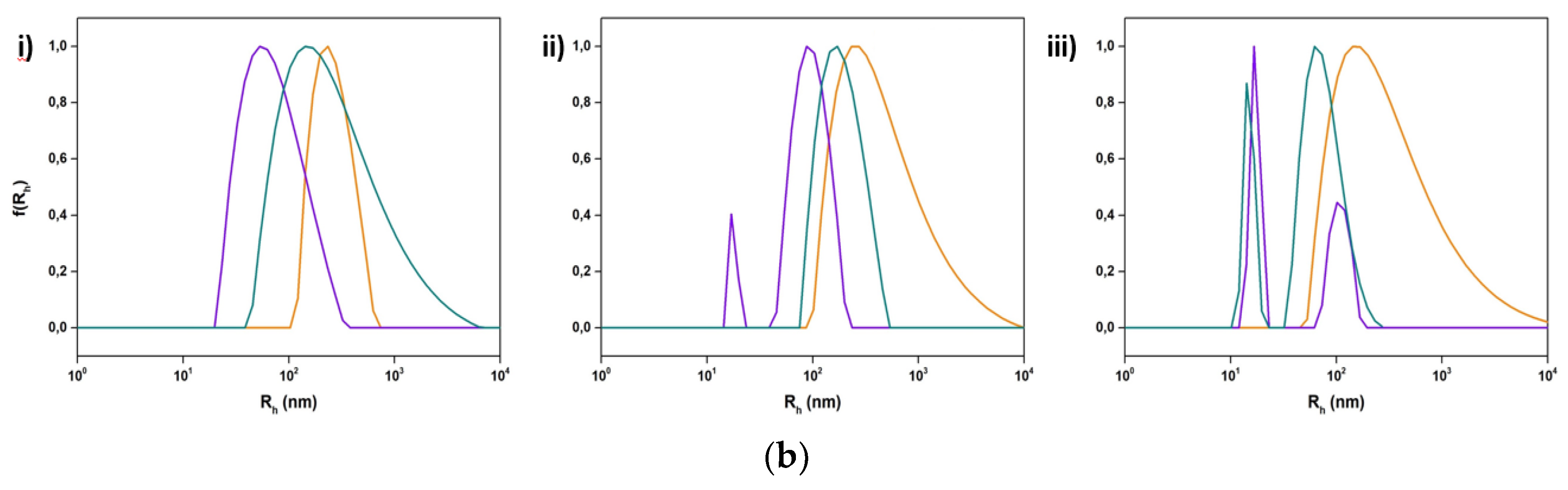
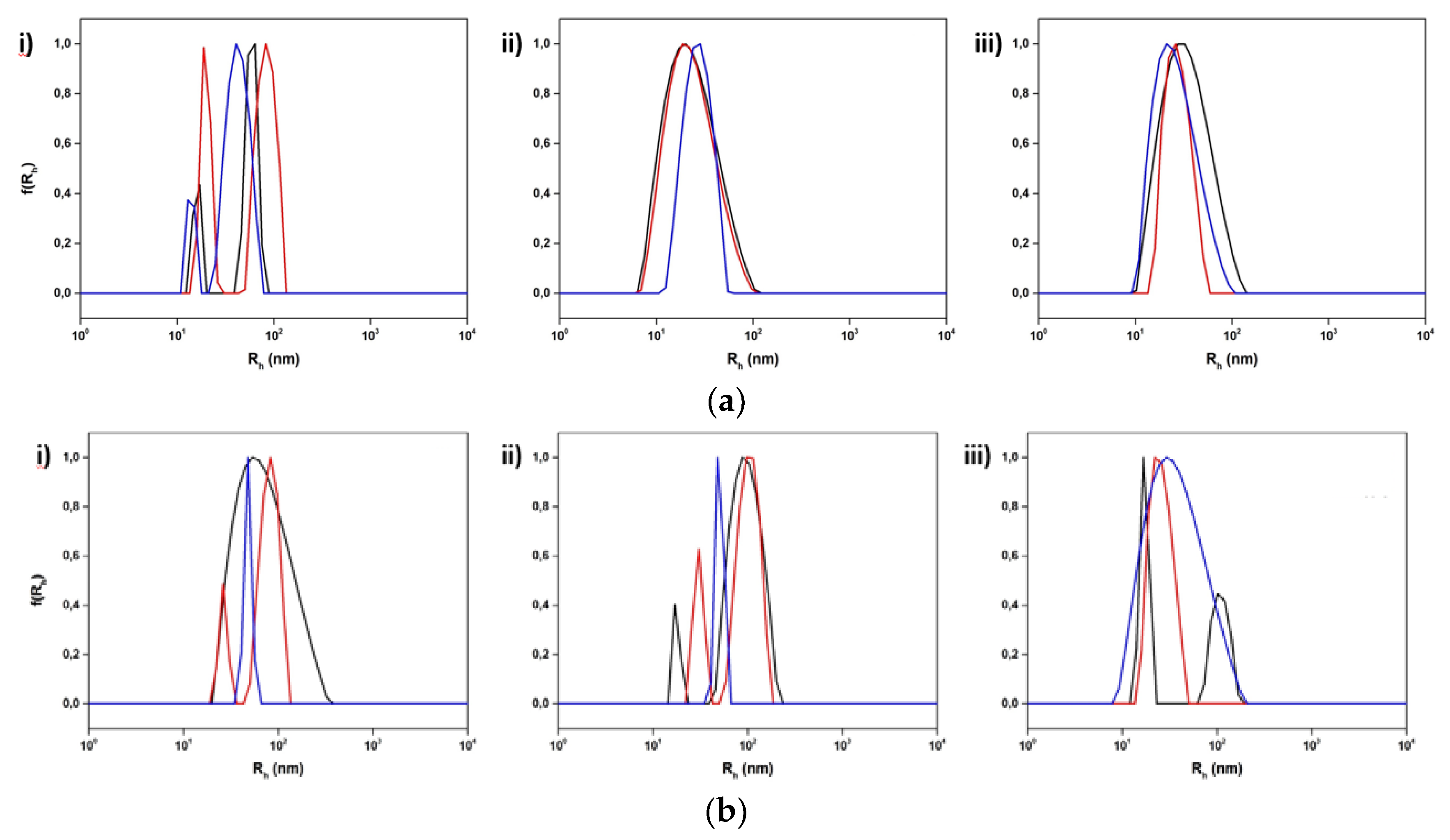
3.2.1. The Influence of Chemical Composition and Lipid to Copolymer Ratio
3.2.2. The Impact of Stimuli-Responsive Copolymers on the Physicochemical Properties of Hybrid Nanostructures
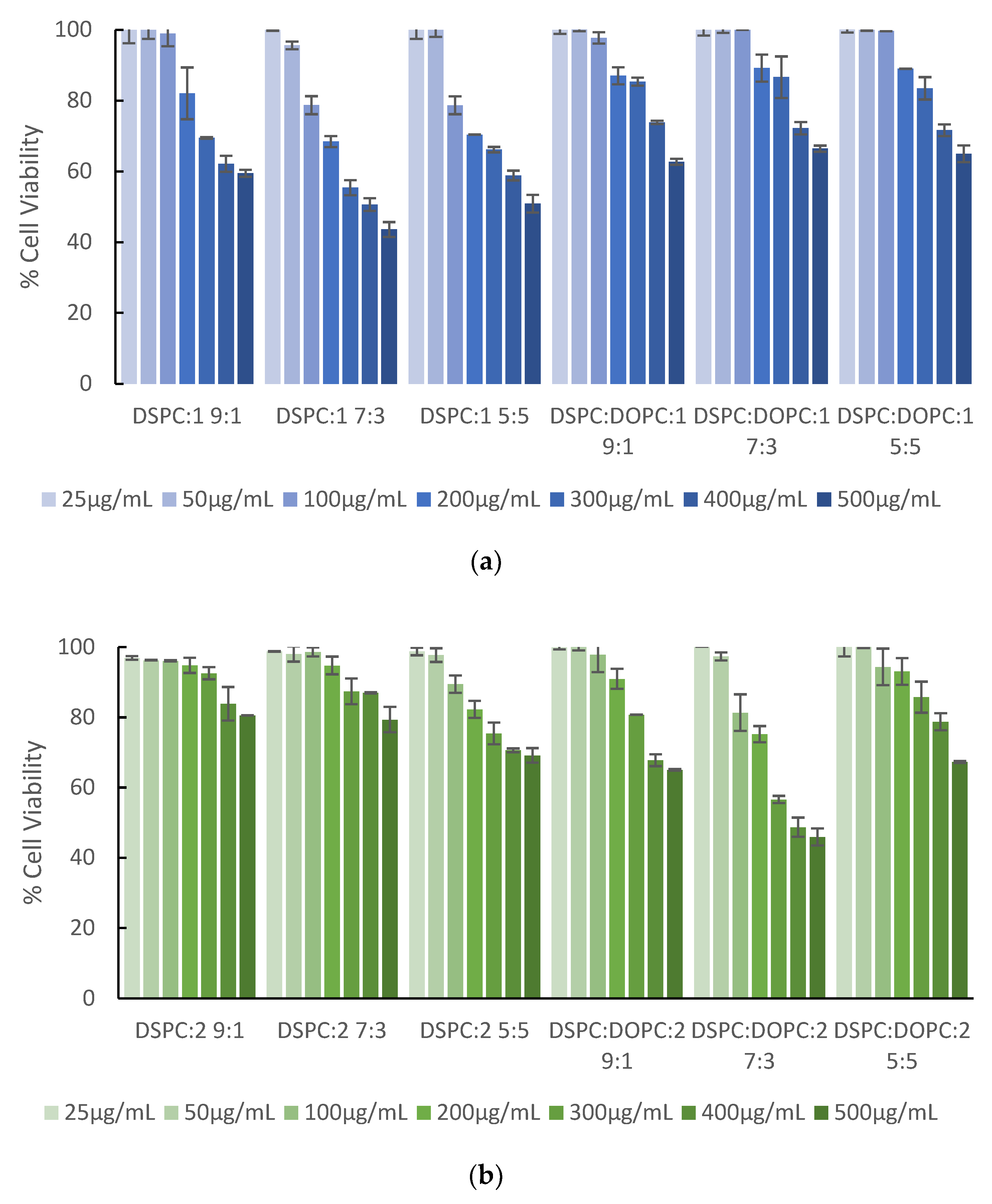
3.3. In Vitro Toxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid–polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J.-H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef]
- He, C.; Lu, J.; Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control. Release 2015, 219, 224–236. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Pispas, S.; Demetzos, C. Differential scanning calorimetry (DSC): An invaluable tool for the thermal evaluation of advanced chimeric liposomal drug delivery nanosystems. In Series in BioEngineering; Springer: Singapore, 2019; pp. 297–337. [Google Scholar]
- Zhang, H.; Liu, Z.; Shen, J. Cyclodextrins modified/coated metal-organic frameworks. Materials 2020, 13, 1273. [Google Scholar] [CrossRef]
- Zhang, L.I.; Zhang, L. Lipid–polymer hybrid nanoparticles: Synthesis, characterization and applications. Nano Life 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant phospholipid/block copolymer hybrid vesicles: Mixing behavior and domain formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bardoliwala, D.; Patel, V.; Misra, A.; Sawant, K. Systematic development and characterization of inhalable dry powder containing Polymeric Lipid Hybrid Nanocarriers co-loaded with ABCB1 shRNA and docetaxel using QbD approach. J. Drug. Deliv. Sci. Technol. 2021, 66, 102903. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert. Opin. Drug. Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bai, J.; Yang, W. Stimuli-responsive nanocarriers for therapeutic applications in cancer. Cancer Biol. Med. 2021, 18, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive polymers and Thermo-responsive liposomal drug delivery systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug. Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Peng, C.-L.; Yang, L.-Y.; Luo, T.-Y.; Lai, P.-S.; Yang, S.-J.; Lin, W.-J.; Shieh, M.-J. Development of pH sensitive 2-(diisopropylamino)ethyl methacrylate based nanoparticles for photodynamic therapy. Nanotechnology 2010, 21, 155103. [Google Scholar] [CrossRef]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Liu, M.; Leroux, J.-C.; Gauthier, M.A. Conformation–function relationships for the comb-shaped polymer pOEGMA. Prog. Polym. Sci. 2015, 48, 111–121. [Google Scholar] [CrossRef]
- Pires-Oliveira, R.; Tang, J.; Percebom, A.M.; Petzhold, C.L.; Tam, K.C.; Loh, W. Effect of molecular architecture and composition on the aggregation pathways of POEGMA random copolymers in water. Langmuir 2020, 36, 15018–15029. [Google Scholar] [CrossRef] [PubMed]
- Guntnur, R.T.; Muzzio, N.; Morales, M.; Romero, G. Phase transition characterization of poly(oligo(ethylene glycol)methyl ether methacrylate) brushes using the quartz crystal microbalance with dissipation. Soft Matter. 2021, 17, 2530–2538. [Google Scholar] [CrossRef]
- Pearson, R.T.; Warren, N.J.; Lewis, A.L.; Armes, S.P.; Battaglia, G. Effect of pH and temperature on PMPC–PDPA copolymer self-assembly. Macromolecules 2013, 46, 1400–1407. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. Multi-responsive poly(oligo(ethylene glycol)methyl methacrylate)-co-poly(2-(diisopropylamino)ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym. Chem. 2021, 12, 6582–6593. [Google Scholar] [CrossRef]
- Mohammadi, M.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Hybrid Vesicular Drug Delivery Systems for Cancer Therapeutics. Adv. Funct. Mater. 2018, 28, 1802136. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.; Mak, N.; McElhaney, R.N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry 1987, 26, 6118–6126. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef]
- Li, L.; Raghupathi, K.; Song, C.; Prasad, P.; Thayumanavan, S. Self-assembly of random copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef]
- Kimura, Y.; Terashima, T.; Sawamoto, M. Self-assembly of amphiphilic random copolyacrylamides into uniform and necklace micelles in water. Macromol. Chem. Phys. 2017, 218, 1700230. [Google Scholar] [CrossRef]
- Menger, F.M.; Wood, M.G.; Zhou, Q.Z.; Hopkins, H.P.; Fumero, J. Thermotropic properties of synthetic chain-substituted phosphatidylcholines: Effect of substituent size, polarity, number, and location on molecular packing in bilayers. J. Am. Chem. Soc. 1988, 110, 6804–6810. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Tribet, C.; Vial, F. Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter. 2007, 4, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Chountoulesi, M.; Kyrili, A.; Meristoudi, A.; Pispas, S.; Demetzos, C. Calorimetric study on pH-responsive block copolymer grafted lipid bilayers: Rational design and development of liposomes. J. Liposome Res. 2016, 26, 211–220. [Google Scholar] [CrossRef]
- Pippa, N.; Forys, A.; Katifelis, H.; Chrysostomou, V.; Trzebicka, B.; Gazouli, M.; Demetzos, C.; Pispas, S. Design and development of DSPC:DAP:PDMAEMA-b-PLMA nanostructures: From the adumbration of their morphological characteristics to in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127768. [Google Scholar] [CrossRef]
- Lee, A.G. Lipid phase transitions and phase diagrams I. Lipid phase transitions. Biochim. Biophys. Act. 1977, 472, 237–281. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Latowski, D.; Strzałka, K. Thermotropic phase behaviour of α-dipalmitoylphosphatidylcholine multibilayers is influenced to various extents by carotenoids containing different structural features—Evidence from differential scanning calorimetry. Biochim. Biophys. Acta Biomembr. 2003, 1609, 193–202. [Google Scholar] [CrossRef]
- Klajnert, B.; Epand, R.M. PAMAM dendrimers and model membranes: Differential scanning calorimetry studies. Int. J. Pharm. [Internet] 2005, 305, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Perinelli, D.R.; Pispas, S.; Bonacucina, G.; Demetzos, C.; Forys, A.; Trzebicka, B. Studying the colloidal behavior of chimeric liposomes by cryo-TEM, micro-differential scanning calorimetry and high-resolution ultrasound spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 539–547. [Google Scholar] [CrossRef]
- Shinitzky, M.; Barenholz, Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta. 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Naziris, N.; Pippa, N.; Stellas, D.; Chrysostomou, V.; Pispas, S.; Demetzos, C.; Libera, M.; Trzebicka, B. Development and evaluation of stimuli-responsive chimeric nanostructures. AAPS PharmSciTech. 2018, 19, 2971–2989. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllopoulou, E.; Pippa, N.; Demetzos, C. Protein-liposome interactions: The impact of surface charge and fluidisation effect on protein binding. J. Liposome Res. 2022, 33, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chemin, M.; Brun, P.-M.; Lecommandoux, S.; Sandre, O.; Le Meins, J.-F. Hybrid polymer/lipid vesicles: Fine control of the lipid and polymer distribution in the binary membrane. Soft Matter. 2012, 8, 2867. [Google Scholar] [CrossRef]
- Winzen, S.; Bernhardt, M.; Schaeffel, D.; Koch, A.; Kappl, M.; Koynov, K.; Landfester, K.; Kroeger, A. Submicron hybrid vesicles consisting of polymer–lipid and polymer–cholesterol blends. Soft Matter. 2013, 9, 5883. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Lipid Bilayer; Garland Science: London, UK, 2002. [Google Scholar]
- Frampton, M.B.; Yakoub, D.; Katsaras, J.; Zelisko, P.M.; Marquardt, D. A calorimetric, volumetric and combined SANS and SAXS study of hybrid siloxane phosphocholine bilayers. Chem. Phys. Lipids. 2021, 241, 105149. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Ruso, J.M.; Hidalgo-Alvarez, R.; Sarmiento, F. Size and stability of liposomes: A possible role of hydration and osmotic forces. Eur. Phys. J. E Soft Matter. 2006, 20, 401–408. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Soema, P.C.; Willems, G.-J.; Jiskoot, W.; Amorij, J.-P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Selianitis, D.; Perinelli, D.R.; Bonacucina, G.; Pippa, N.; Gazouli, M.; Pispas, S. Non-ionic surfactant effects on innate pluronic 188 behavior: Interactions, and physicochemical and biocompatibility studies. Int. J. Mol. Sci. 2022, 23, 13814. [Google Scholar] [CrossRef]
- Pippa, N.; Merkouraki, M.; Pispas, S.; Demetzos, C. DPPC:MPOx chimeric advanced Drug Delivery nano Systems (chi-aDDnSs): Physicochemical and structural characterization, stability and drug release studies. Int. J. Pharm. 2013, 450, 1–10. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. In Light Scattering from Polymers; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–124. [Google Scholar]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid. Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barbee, M.H.; Wright, Z.M.; Allen, B.P.; Taylor, H.F.; Patteson, E.F.; Knight, A.S. Protein-mimetic self-assembly with synthetic macromolecules. Macromolecules 2021, 54, 3585–3612. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Sasaki, M.; Hiroiwa, A.; Yoshimoto, N.; Miyagawa, K.; Umakoshi, H.; Kuboi, R. Relationship between the mobility of phosphocholine headgroups of liposomes and the hydrophobicity at the membrane interface: A characterization with spectrophotometric measurements. Colloids Surf. B Biointerfaces 2011, 88, 221–230. [Google Scholar] [CrossRef]
- Jan, N.; Madni, A.; Rahim, M.A.; Khan, N.U.; Jamshaid, T.; Khan, A.; Jabar, A.; Khan, S.; Shah, H. In vitro anti-leukemic assessment and sustained release behaviour of cytarabine loaded biodegradable polymer based nanoparticles. Life Sci. 2021, 267, 118971. [Google Scholar] [CrossRef]
- Oseni, B.A.; Azubuike, C.P.; Okubanjo, O.O.; Igwilo, C.I.; Panyam, J. Encapsulation of Andrographolide in poly(lactide-co-glycolide) Nanoparticles: Formulation Optimization and in vitro Efficacy Studies. Front. Bioeng. Biotechnol. 2021, 9, 639409. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.-M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Bonaccorso, A.; Carbone, C.; Lupo, G.; Anfuso, C.D.; Giurdanella, G.; Caggia, C.; Randazzo, C.; Russo, N.; Luca, R.G.; et al. Melatonin loaded hybrid nanomedicine: DoE approach, optimization and in vitro study on diabetic retinopathy model. Int. J. Pharm. 2022, 627, 122195. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ishihara, K.; Yusa, S.-I. Separated micelles formation of pH-responsive random and block copolymers containing phosphorylcholine groups. Polymers 2022, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Le Meins, J.-F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Dąbczyński, P.; Kostruba, A.; Budkowski, A. Temperature- and pH-Responsive Schizophrenic Copolymer Brush Coatings with Enhanced Temperature Response in Pure Water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef] [PubMed]
- Bütün, V.; Liu, S.; Weaver, J.V.M.; Bories-Azeau, X.; Cai, Y.; Armes, S.P. A brief review of ‘schizophrenic’ block copolymers. React. Funct. Polym. 2006, 66, 1381–5148. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. Thermo- and pH-responsive poly[(diethylene glycol methyl ether methacrylate)-co-(2-diisopropylamino ethyl methacrylate)] hyperbranched copolymers: Self-assembly and drug-loading. Polym. Chem. 2023, 14, 587–599. [Google Scholar] [CrossRef]
- Gordon, V.D.; O’Halloran, T.J.; Shindell, O. Membrane adhesion and the formation of heterogeneities: Biology, biophysics, and biotechnology. Phys. Chem. Chem. Phys. 2015, 17, 15522–15533. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Bedu-Addo, F.K.; Huang, L. Interaction of PEG-phospholipid conjugates with phospholipid: Implications in liposomal drug delivery. Adv. Drug. Deliv. Rev. 1995, 16, 235–247. [Google Scholar] [CrossRef]
- Hristova, K.; Kenworthy, A.; McIntosh, T.J. Effect of Bilayer Composition on the Phase Behavior of Liposomal Suspensions Containing Poly(ethy1ene glycol)-Lipids. Macromolecules 1995, 28, 7693–7699. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. DPPC/poly(2-methyl-2-oxazoline)-grad-poly(2-phenyl-2-oxazoline) chimeric nanostructures as potential drug nanocarriers. J. Nanopart Res. 2013, 15, 1685. [Google Scholar] [CrossRef]
- Fang, R.H.; Aryal, S.; Hu, C.M.; Zhang, L. Quick synthesis of lipid-polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir 2010, 26, 16958–16962. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell. Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2018, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control. Release 2020, 324, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Selianitis, D.; Pispas, S. P(MMA-co-HPMA)-b-POEGMA copolymers: Synthesis, micelle formation in aqueous media and drug encapsulation. Polym. Int. 2021, 70, 1508–1522. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. PDMAEMA-b-PLMA-b-POEGMA triblock terpolymers via RAFT polymerization and their self-assembly in aqueous solutions. Polym. Chem. 2017, 8, 4538–4547. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. PLMA-b-POEGMA amphiphilic block copolymers: Synthesis and self-assembly in aqueous media. Polym. Chem. 2017, 55, 155–163. [Google Scholar] [CrossRef]
- Góis, J.R.; Rocha, N.; Popov, A.V.; Guliashvili, T.; Matyjaszewski, K.; Serra, A.C.; Coelho, J.F.J. Synthesis of well-defined functionalized poly(2-(diisopropylamino)ethyl methacrylate) using ATRP with sodium dithionite as a SARA agent. Polym. Chem. 2014, 5, 3919–3928. [Google Scholar] [CrossRef]
- Avanti Polar Lipids. Available online: https://avantilipids.com/product/850365 (accessed on 17 June 2022).
- Avanti Polar Lipids. Available online: https://avantilipids.com/product/850375 (accessed on 17 June 2022).




| Statistical (Random) Copolymers | |||
|---|---|---|---|
| P(OEGMA ¹ 950-co-DIPAEMA ²) | 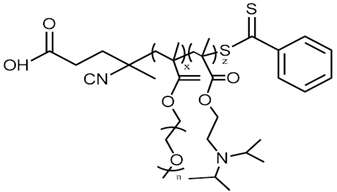 | ||
| Mw 4 (×104) (g/mol) | Mw/Mn 4 | %PDIPAEMA 3 | |
| Copolymer 1 | 1.10 | 1.16 | 37 |
| Copolymer 2 | 1.24 | 1.13 | 70 |
| Sample | Weight Ratio | Ι (kHz) | Rh (nm) | PDI | Rg/Rh | I1/I3 |
|---|---|---|---|---|---|---|
| DSPC:1 | 9:1 | 4210 | 62 | 0.31 | 1.4 | 1.65 |
| DSPC:1 | 7:3 | 21,645 | 103 | 0.20 | 1.3 | 1.78 |
| DSPC:1 | 5:5 | 3023 | 58 | 0.31 | 1.4 | 1.68 |
| DSPC:DOPC:1 | 9:1 | 4230 | 95 | 0.40 | 1.5 | 1.62 |
| DSPC:DOPC:1 | 7:3 | 16,419 | 99 | 0.27 | 1.1 | 1.63 |
| DSPC:DOPC:1 | 5:5 | 192 | 57 | 0.52 | 1.2 | 1.69 |
| DSPC:2 | 9:1 | 1087 | 58 (76% wP) 1 | 0.42 | - | 1.68 |
| DSPC:2 | 7:3 | 413 | 23 | 0.46 | - | 1.67 |
| DSPC:2 | 5:5 | 499 | 33 | 0.27 | - | 1.58 |
| DSPC:DOPC:2 | 9:1 | 3970 | 69 | 0.28 | 1.4 | 1.61 |
| DSPC:DOPC:2 | 7:3 | 4730 | 94 (92% wP) 2 | 0.33 | - | 1.50 |
| DSPC:DOPC:2 | 5:5 | 493 | 108 (44% wP) 3 | 0.46 | - | 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllopoulou, E.; Selianitis, D.; Pippa, N.; Gazouli, M.; Valsami, G.; Pispas, S. Development of Hybrid DSPC:DOPC:P(OEGMA950-DIPAEMA) Nanostructures: The Random Architecture of Polymeric Guest as a Key Design Parameter. Polymers 2023, 15, 1989. https://doi.org/10.3390/polym15091989
Triantafyllopoulou E, Selianitis D, Pippa N, Gazouli M, Valsami G, Pispas S. Development of Hybrid DSPC:DOPC:P(OEGMA950-DIPAEMA) Nanostructures: The Random Architecture of Polymeric Guest as a Key Design Parameter. Polymers. 2023; 15(9):1989. https://doi.org/10.3390/polym15091989
Chicago/Turabian StyleTriantafyllopoulou, Efstathia, Dimitriοs Selianitis, Natassa Pippa, Maria Gazouli, Georgia Valsami, and Stergios Pispas. 2023. "Development of Hybrid DSPC:DOPC:P(OEGMA950-DIPAEMA) Nanostructures: The Random Architecture of Polymeric Guest as a Key Design Parameter" Polymers 15, no. 9: 1989. https://doi.org/10.3390/polym15091989
APA StyleTriantafyllopoulou, E., Selianitis, D., Pippa, N., Gazouli, M., Valsami, G., & Pispas, S. (2023). Development of Hybrid DSPC:DOPC:P(OEGMA950-DIPAEMA) Nanostructures: The Random Architecture of Polymeric Guest as a Key Design Parameter. Polymers, 15(9), 1989. https://doi.org/10.3390/polym15091989









