Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Anion-Exchange Membranes
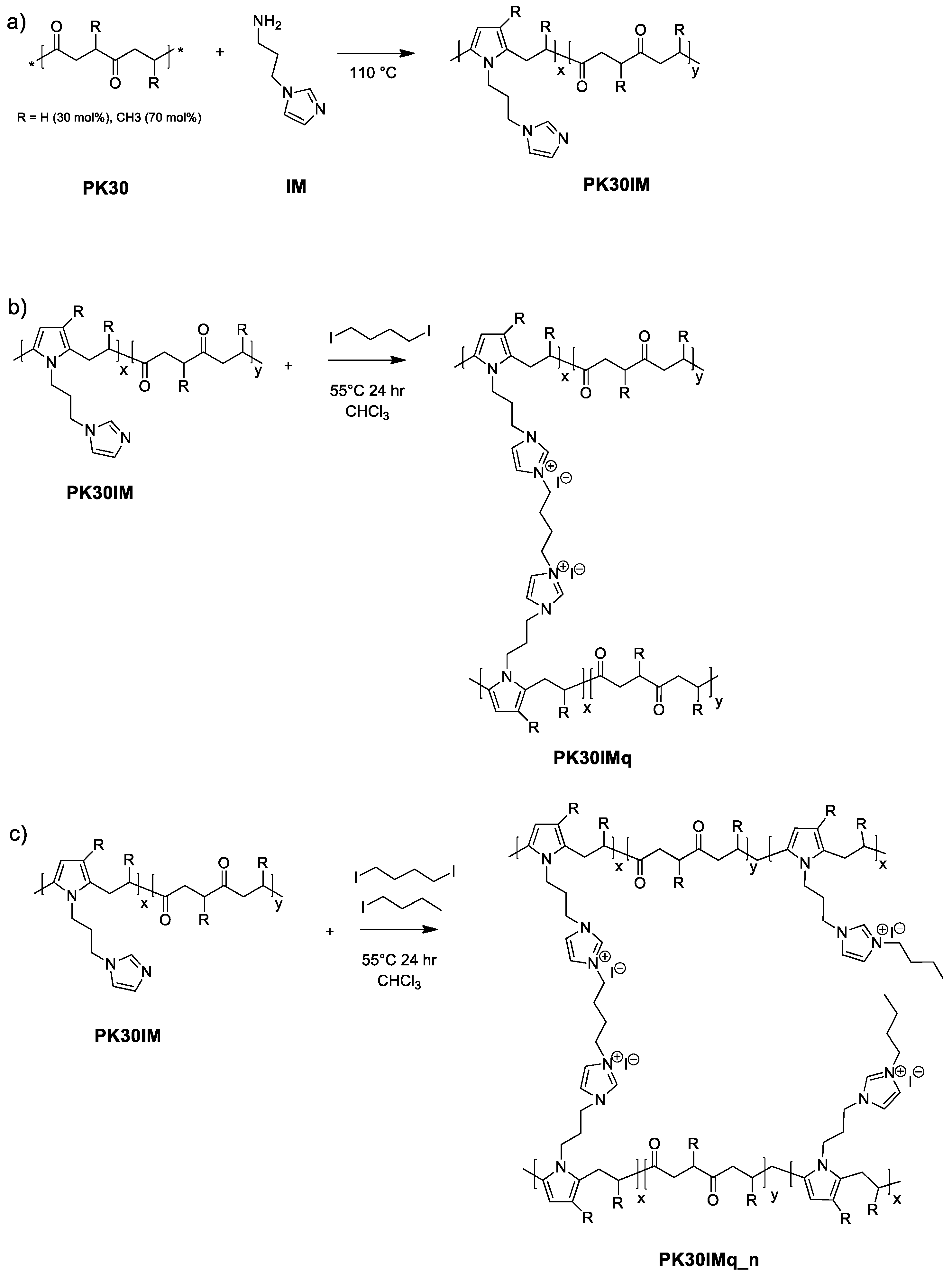
2.2.1. Polyketone Functionalization with 1-(3-aminopropyl)imidazole
2.2.2. Membrane Preparation
2.3. Instruments and Methods
2.4. Characterization of the Anion-Exchange Membranes
2.4.1. Water Uptake (WU)
2.4.2. Ion-Exchange Capacity (IEC)
2.5. Electrolytic Cell Tests
3. Results and Discussion
3.1. Preparation of the PK30IMq and PK30IMq_n
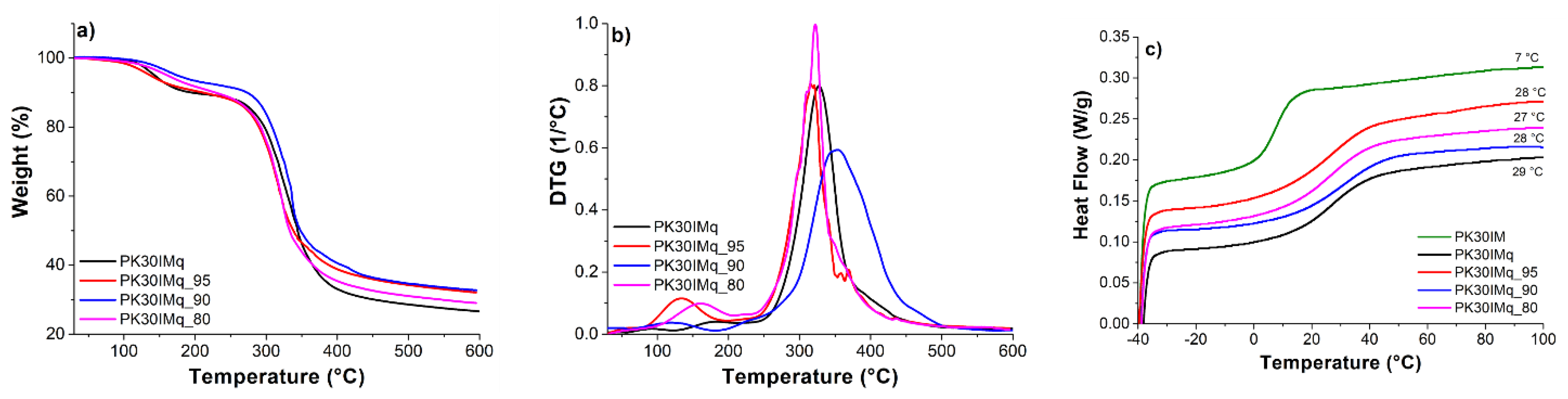
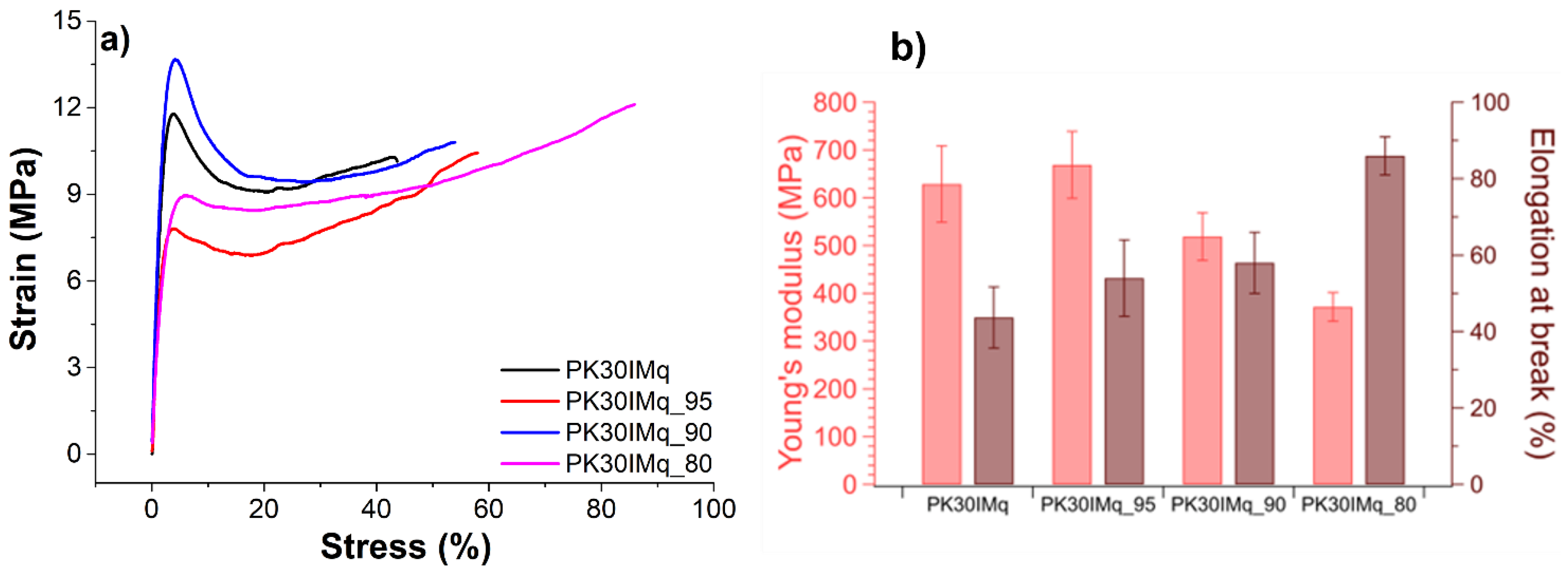
3.2. Water Uptake and Ion-Exchange Capacity
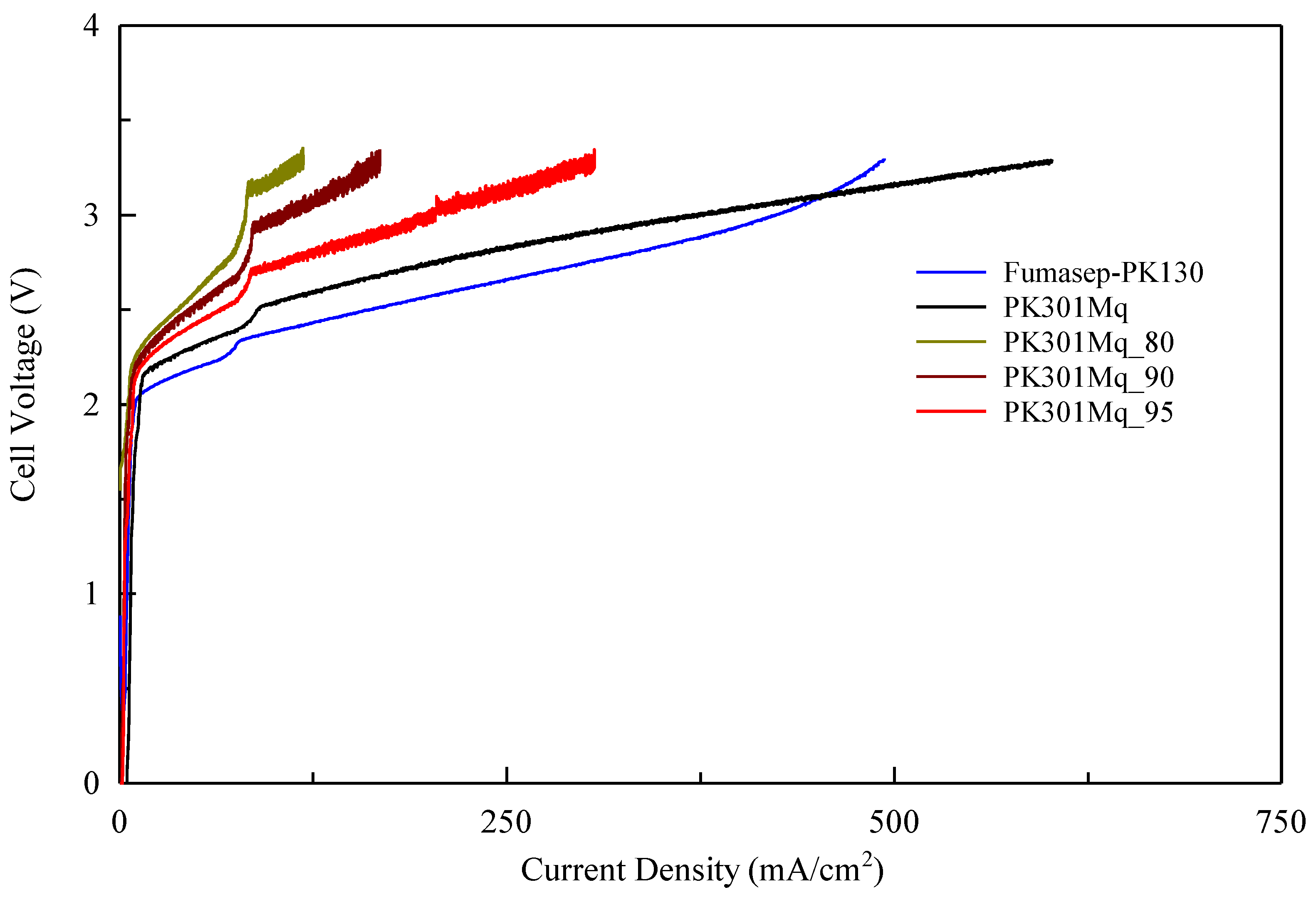
3.3. Electrolytic Cell Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A review on anion exchange membranes for fuel cells: Anion-exchange polyelectrolytes and synthesis strategies. Int. J. Hydrogen Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog Polym Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Faraj, M.; Boccia, M.; Miller, H.; Martini, F.; Borsacchi, S.; Geppi, M.; Pucci, A. New LDPE based anion-exchange membranes for alkaline solid polymeric electrolyte water electrolysis. Int. J. Hydrogen Energy 2012, 37, 14992–15002. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. A review of alkaline solid polymer membrane in the application of AEM electrolyzer: Materials and characterization. Int. J. Energy Res. 2021, 45, 18337–18354. [Google Scholar] [CrossRef]
- Hua, D.; Huang, J.; Fabbri, E.; Rafique, M.; Song, B. Development of Anion Exchange Membrane Water Electrolysis and the Associated Challenges: A Review. ChemElectroChem 2023, 10, e202200999. [Google Scholar] [CrossRef]
- Yang, B.; Cunman, Z. Progress in constructing high-performance anion exchange Membrane: Molecular design, microphase controllability and In-device property. Chem. Eng. J. 2023, 457, 141094. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Ge, J.; Xing, W.; Zhu, J. Challenges and Strategies of Anion Exchange Membranes in Hydrogen-electricity Energy Conversion. Chem. A Eur. J. 2023, e202203173. [Google Scholar] [CrossRef]
- Faraj, M.; Elia, E.; Boccia, M.; Filpi, A.; Pucci, A.; Ciardelli, F. New anion conducting membranes based on functionalized styrene-butadiene-styrene triblock copolymer for fuel cells applications. J. Polym. Sci. A Polym. Chem. 2011, 49, 3437–3447. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Kulkarni, P.V.; Kariduraganavar, M.Y. Ion Exchange Membranes, Methods and Processes for Production Thereof and Uses in Specific Applications. US6814865B1, 19 April 2004. [Google Scholar]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zheng, X.; Sun, W.; Dou, S.X.; Ma, T.; Pan, H. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Sata, T.; Yamane, Y.; Matsusaki, K. Preparation and properties of anion exchange membranes having pyridinium or pyridinium derivatives as anion exchange groups. J. Polym. Sci. A Polym. Chem. 1998, 36, 49–58. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, Y.; Yang, Z.; Wu, B.; Hu, M.; Liu, X.; Hou, J.; Xu, T. Hyper-branched anion exchange membranes with high conductivity and chemical stability. Chem. Commun. 2016, 52, 10141–10143. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Sun, L.; Wei, C.; Zhang, H.; Wu, L.; Ge, X.; Xu, T. A π-Conjugated Anion-Exchange Membrane with an Ordered Ion-Conducting Channel via the McMurray Coupling Reaction. Angew. Chem. 2023, 135, e202215017. [Google Scholar] [CrossRef]
- Espiritu, R.; Golding, B.T.; Scott, K.; Mamlouk, M. Degradation of radiation grafted anion exchange membranes tethered with different amine functional groups via removal of vinylbenzyl trimethylammonium hydroxide. J. Power Sour. 2018, 375, 373–386. [Google Scholar] [CrossRef]
- Sata, T. Ion Exchange Membranes; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kim, J.J.; Park, E.J.; Hwang, T.S. Synthesis of Polyketone Anion Ion Exchange Fibers by Paal-Knorr Reaction and Its Physico-Chemical Properties. Macromol. Res. 2020, 28, 465–471. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric amines by chemical modifications of alternating aliphatic polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Vavasori, A.; Ronchin, L. Polyketones: Synthesis and Applications. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 1–41. [Google Scholar] [CrossRef]
- Ataollahi, N.; Tomasino, E.; Cotini, O.; di Maggio, R. Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica. Energies 2022, 15, 1953. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Giannetti, A.; Lima, G.M.R.; Orozco, F.; Picchioni, F.; Mattoli, V.; Bose, R.K.; Pucci, A. Thermally Switchable Electrically Conductive Thermoset rGO/PK Self-Healing Composites. Polymers 2021, 13, 339. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Gabbani, A.; Mazzotta, A.; Ruggeri, M.; Orozco, F.; Cappello, V.; Gemmi, M.; Bose, R.K.; Picchioni, F.; Pineider, F.; et al. Rapid self-healing in IR-responsive plasmonic indium tin oxide/polyketone nanocomposites. J. Mater. Chem. A 2022, 10, 12957–12967. [Google Scholar] [CrossRef]
- Mul, W.P.; Dirkzwager, H.; Broekhuis, A.A.; Heeres, H.J.; van der Linden, A.J.; Orpen, A.G. Highly active, recyclable catalyst for the manufacture of viscous, low molecular weight, CO-ethene-propene-based polyketone, base component for a new class of resins. Inorg. Chim. Acta 2002, 327, 147–159. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, R.-Y.; Liu, Z.; Yang, M.-B.; Yang, W. Electrospun Modified Polyketone-Based Anion Exchange Membranes with High Ionic Conductivity and Robust Mechanical Properties. ACS Appl. Energy Mater. 2021, 4, 5187–5200. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Zhang, Z.-M.; Zhou, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Imidazole-functionalized polyketone-based polyelectrolytes with efficient ionic channels and superwettability for alkaline polyelectrolyte fuel cells and multiple liquid purification. J. Mater. Chem. A 2021, 9, 14827–14840. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Rakhshani, S.; Araneo, R.; Pucci, A.; Rinaldi, A.; Giuliani, C.; Pozio, A. Synthesis and Characterization of a Composite Anion Exchange Membrane for Water Electrolyzers (AEMWE)”. Membranes 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]


| Sample | PK30IM mg | 1,4-Diiodiobutane mg (%) | 1-Iodiobutane mg (%) |
|---|---|---|---|
| PK30IMq | 500 | 116 (100) | 0 (0) |
| PK30IMq_95 | 500 | 92 (95) | 27 (5) |
| PK30IMq_90 | 500 | 104 (90) | 13 (10) |
| PK30IMq_80 | 500 | 110 (80) | 6 (20) |
| Sample | Iodine Content (wt.%) |
|---|---|
| PK30IMq | 25 |
| PK30IMq_80 | 20 |
| PK30IMq_90 | 22 |
| PK30IMq_95 | 31 |
| Sample | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|
| PK30IMq | 629 ± 80 | 44 ± 8 |
| PK30IMq_95 | 372 ± 30 | 86 ± 5 |
| PK30IMq_90 | 669 ± 70 | 54 ± 10 |
| PK30IMq_80 | 519 ± 50 | 58 ± 8 |
| Sample | WU (%) | IEC (meq/g) |
|---|---|---|
| PK30IMq | 38 ± 2 | 2.22 ± 0.02 |
| PK30IMq_80 | 33 ± 5 | 1.92 ± 0.01 |
| PK30IMq_90 | 35 ± 1 | 2.1 ± 0.3 |
| PK30IMq_95 | 37 ± 2 | 1.9 ± 0.2 |
| Sample | Resistance (Ω) | ASR (Ω*cm2) | Ionic Conductivity (mS/cm) |
|---|---|---|---|
| PK30IMq | 0.65 | 1.30 | 9.69 |
| PK30IMq_80 | 2.88 | 5.76 | 3.47 |
| PK30IMq_90 | 1.82 | 3.64 | 3.76 |
| PK30IMq_95 | 1.57 | 3.14 | 3.28 |
| Fumasep-PK-130 | 0.71 | 1.42 | 9.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racchi, O.; Baldassari, R.; Araya-Hermosilla, E.; Mattoli, V.; Minei, P.; Pozio, A.; Pucci, A. Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis. Polymers 2023, 15, 2027. https://doi.org/10.3390/polym15092027
Racchi O, Baldassari R, Araya-Hermosilla E, Mattoli V, Minei P, Pozio A, Pucci A. Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis. Polymers. 2023; 15(9):2027. https://doi.org/10.3390/polym15092027
Chicago/Turabian StyleRacchi, Ottavia, Rebecca Baldassari, Esteban Araya-Hermosilla, Virgilio Mattoli, Pierpaolo Minei, Alfonso Pozio, and Andrea Pucci. 2023. "Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis" Polymers 15, no. 9: 2027. https://doi.org/10.3390/polym15092027
APA StyleRacchi, O., Baldassari, R., Araya-Hermosilla, E., Mattoli, V., Minei, P., Pozio, A., & Pucci, A. (2023). Polyketone-Based Anion-Exchange Membranes for Alkaline Water Electrolysis. Polymers, 15(9), 2027. https://doi.org/10.3390/polym15092027









