Sorption of Total Petroleum Hydrocarbons in Microplastics
Abstract
1. Introduction
2. Materials and Methods
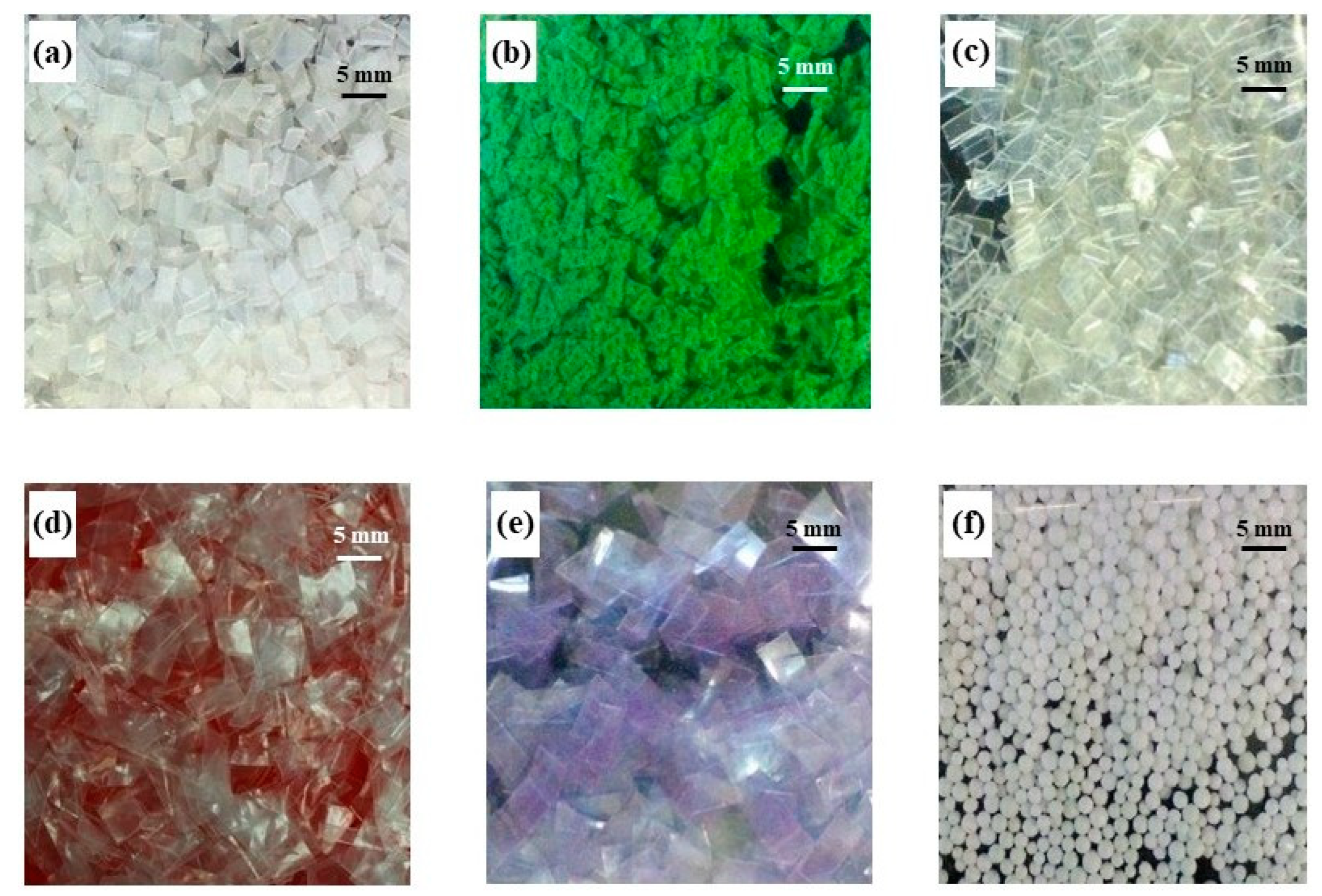
2.1. Preparation of Virgin Microplastics
2.2. Method for the Determination of the Concentration of Organic Compounds
2.2.1. Determination of the Concentration of Fuel Oil in the Liquid Phase
2.2.2. Determination of the Concentration of Fuel Oil on Microplastics
2.3. Determination of Surface Area, Average Pore Diameter, and Pore Volume of Virgin Microplastics
2.4. Evaluation of Sorption of Fuel Oil on Virgin Microplastics in Laboratory Conditions
2.5. Sorption Kinetics of Fuel Oil on Microplastics
2.6. Determination of the Concentration of Organic Compounds in Real Microplastics
2.6.1. Sampling of Microplastics
2.6.2. Extraction of Fuel Oil from Microplastics
3. Results and Discussion
3.1. Validation of the Method Used for the Determination of the Concentration of Organic Compounds in Emulsions
3.2. Sorption of Fuel Oil on Virgin Microplastics in Laboratory Conditions
3.3. Surface Area of Virgin Microplastics and Sorption of Fuel Oil
3.4. Sorption Kinetics of Fuel Oil on Virgin Microplastics
3.5. Sampling and Sorption Evaluation of Organic Compound on Real Microplastics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2021 An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2021. [Google Scholar]
- Aguirrezabal, I.U. Plásticos en México; Oficina Económica y Comercial de España en Ciudad de México. In Fecha: 01/07/2019 NIPO: 114-19-040-2. Fichas Sector México; ICEX España Exportación e Inversiones: Madrid, Spain, 2019. [Google Scholar]
- SEMARNAT. Diagnóstico Básico Para La Gestión Integral de Los Residuos 2020; SEMARNAT: Mexicali, Mexico, 2020.
- Bigdeli, M.; Mohammadian, A.; Pilechi, A.; Taheri, M. Lagrangian Modeling of Marine Microplastics Fate and Transport: The State of the Science. J. Mar. Sci. Eng. 2022, 10, 481. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic Interactions with Freshwater Microalgae: Hetero-Aggregation and Changes in Plastic Density Appear Strongly Dependent on Polymer Type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic Pollutants in Marine Plastic Debris from Canary Islands Beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef]
- Salman, M.S.; Sheikh, M.C.; Hasan, M.M.; Hasan, M.N.; Kubra, K.T.; Rehan, A.I.; Awual, M.E.; Rasee, A.I.; Waliullah, R.M.; Hossain, M.S.; et al. Chitosan-coated cotton fiber composite for efficient toxic dye encapsulation from aqueous media. Appl. Surf. Sci. 2023, 622, 157008. [Google Scholar] [CrossRef]
- Salman, M.S.; Hasan, M.N.; Hasan, M.M.; Kubra, K.T.; Sheikh, M.C.; Rehan, A.I.; Awual, M.E.; Hossain, M.S.; Awual, M.R. Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J. Mol. Struct. 2023, 1282, 135259. [Google Scholar] [CrossRef]
- Hasan, M.M.; Kubra, K.T.; Hasan, M.N.; Awual, M.E.; Salman, M.S.; Sheikh, M.C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.M.; Islam, M.S.; et al. Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater. J. Mol. Liq. 2023, 371, 121125. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The Ecological Impacts of Marine Debris: Unraveling the Demonstrated Evidence from What Is Perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef]
- van Raamsdonk, L.W.D.; van der Zande, M.; Koelmans, A.A.; Hoogenboom, P.L.A.; Peters, R.J.B.; Groot, M.J.; Peijnenburg, M.A.C.; Weesepoel, Y.J.A. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods 2020, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological Effects of Chemical Contaminants Adsorbed to Microplastics in the Clam Scrobicularia Plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef]
- le Bihanic, F.; Clérandeau, C.; Cormier, B.; Crebassa, J.C.; Keiter, S.H.; Beiras, R.; Morin, B.; Bégout, M.L.; Cousin, X.; Cachot, J. Organic Contaminants Sorbed to Microplastics Affect Marine Medaka Fish Early Life Stages Development. Mar. Pollut. Bull. 2020, 154, 111059. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarf, C. Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int. J. Environ. Res. Public. Health 2018, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent Organic Pollutants Carried by Synthetic Polymers in the Ocean Environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Liu, L.Y.; Zeng, E.Y. Polycyclic Aromatic Hydrocarbons Affiliated with Microplastics in Surface Waters of Bohai and Huanghai Seas, China. Environ. Pollut. 2018, 241, 834–840. [Google Scholar] [CrossRef]
- PEMEX. Informe de Sustentabilidad 2020; PEMEX: Mexico City, Mexico, 2020. [Google Scholar]
- ATSDR. Toxicological Profile for Total Petroleum Hydrocarbons; ATSDR: Atlanta, GA, USA, 1999.
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (PAHs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, H.J.; Kwon, J.H. Toxicity Cutoff of Aromatic Hydrocarbons for Luminescence Inhibition of Vibrio Fischeri. Ecotoxicol. Environ. Saf. 2013, 94, 116–122. [Google Scholar] [CrossRef]
- González-Soto, N.; Campos, L.; Navarro, E.; Bilbao, E.; Guilhermino, L.; Cajaraville, M.P. Effects of microplastics alone or with sorbed oil compounds from the water accommodated fraction of a North Sea crude oil on marine mussels (Mytilus galloprovincialis). Sci. Total Environ. 2022, 851, 157999. [Google Scholar] [CrossRef]
- Potter, B.B.; Wimsatt, J. Method 415.3, Rev. 1.2: Determination of Total Organic Carbon and Specific UV Absorbance at 254 Nm in Source Water and Drinking Water; EPA: Washington, DC, USA, 2009.
- Rios, L.M.; Jones, P.R.; Moore, C.; Narayan, U.V. Quantitation of Persistent Organic Pollutants Adsorbed on Plastic Debris from the Northern Pacific Gyre’s “Eastern Garbage Patch”. J. Environ. Monit. 2010, 12, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Rutherford, P.M.; Arocena, J.M.; Thring, R.W. A Proposed Method for Rapid and Economical Extraction of Petroleum Hydrocarbons from Contaminated Soils. Can. J. Soil Sci. 2011, 86, 725–728. [Google Scholar] [CrossRef]
- Fisner, M.; Majer, A.P.; Balthazar-Silva, D.; Gorman, D.; Turra, A. Quantifying Microplastic Pollution on Sandy Beaches: The Conundrum of Large Sample Variability and Spatial Heterogeneity. Environ. Sci. Pollut. Res. 2017, 24, 13732–13740. [Google Scholar] [CrossRef] [PubMed]
- Pons-Jiménez, M.; Guerrero-Peña, A.; Zavala-Cruz, J.; Alarcón, A. Extracción de Hidrocarburos y Compuestos Derivados del Petróleo en Suelos Con Características Físicas y Químicas Diferentes. Univ. Cienc. 2011, 27, 1–15. [Google Scholar]
- Naderi, M. Chapter Fourteen Surface Area Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 585–608. ISBN 9780123983077. [Google Scholar]
- Cifuentes Lemus, J.L.; Torres García, M.d.P.; Frías, M.M. El Océano y Sus Recursos II. Las Ciencias Del Mar: Oceanografía Geológica y Oceanografía Química. Available online: http://bibliotecadigital.ilce.edu.mx/sites/ciencia/volumen1/ciencia2/12/htm/oceano2.html (accessed on 26 August 2022).
- Wang, W.; Wang, J. Comparative Evaluation of Sorption Kinetics and Isotherms of Pyrene onto Microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, J.; Huang, D.; Wang, S.; Jiang, K.; Sun, W.; Chen, Z.; Cao, Z.; Ren, Y.; Wang, Q.; et al. Adsorption behavior of aniline pollutant on polystyrene microplastics. Chemosphere 2023, 323, 138187. [Google Scholar] [CrossRef]
- Heath, J.S.; Koblis, K.; Sager, S.L. Review of Chemical, Physical, and Toxicologic Properties of Components of Total Petroleum Hydrocarbons. J. Soil Contam. 2008, 2, 1–25. [Google Scholar] [CrossRef]
- Tang, G.; Liu, M.; Zhou, Q.; He, H.; Chen, K.; Zhang, H.; Hu, J.; Huang, Q.; Luo, Y.; Ke, H.; et al. Microplastics and Polycyclic Aromatic Hydrocarbons (PAHs) in Xiamen Coastal Areas: Implications for Anthropogenic Impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar] [CrossRef]
- Liu, L.; Fokkink, R.; Koelmans, A.A. Sorption of Polycyclic Aromatic Hydrocarbons to Polystyrene Nanoplastic. Environ. Toxicol. Chem. 2016, 35, 1650–1655. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- Endo, S.; Koelmans, A.A. Sorption of Hydrophobic Organic Compounds to Plastics in the Marine Environment: Equilibrium. Handb. Environ. Chem. 2019, 78, 185–204. [Google Scholar] [CrossRef]
- Goedecke, C.; Stollin, U.M.; Hering, S.; Richter, J.; Piechotta, C.; Paul, A.; Braun, U. A First Pilot Study on the Sorption of Environmental Pollutants on Various Microplastic Materials. J. Environ. Anal. Chem. 2017, 4, 1000191. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, M.; Yin, X.; Wang, L.; Lou, Y.; Qu, L.; Liu, X.; Zhu, H.; Qiu, Y. Sorption of 3,6-Dibromocarbazole and 1,3,6,8-Tetrabromocarbazole by Microplastics. Mar. Pollut. Bull. 2019, 138, 458–463. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced Adsorption of Oxytetracycline to Weathered Microplastic Polystyrene: Kinetics, Isotherms and Influencing Factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A Combined Experimental and Modeling Study to Evaluate PH-Dependent Sorption of Polar and Non-Polar Compounds to Polyethylene and Polystyrene Microplastics. Environ. Sci. Eur. 2018, 30, 30. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G. Sorption Behaviors of Phenanthrene, Nitrobenzene, and Naphthalene on Mesoplastics and Microplastics. Environ. Sci. Pollut. Res. Int. 2019, 26, 12563–12573. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The Partition Behavior of Perfluorooctanesulfonate (PFOS) and Perfluorooctanesulfonamide (FOSA) on Microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of Non-Polar Organic Compounds by Micro-Sized Plastic Particles in Aqueous Solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Seltenrich, N. New Link in the Food Chain? Marine Plastic Pollution and Seafood Safety. Environ. Health Perspect. 2015, 123, A34–A41. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, M.; Han, P.; Liang, G.; Zhang, T.; Liu, G. Comparative Analysis on the Sorption Kinetics and Isotherms of Fipronil on Nondegradable and Biodegradable Microplastics. Environ. Pollut. 2019, 254, 112927. [Google Scholar] [CrossRef]
- Kook, H.; Cha, M.; Park, C. Transport of Emerging Organic Ultraviolet (UV) Filters in Ceramic Membranes: Role of Polyethylene (PE) Microplastics. Chemosphere 2022, 309, 136570. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive Sorption of Persistent Organic Pollutants onto Microplastics in the Marine Environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption Properties of Tylosin on Four Different Microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef]
- Zhao, L.; Rong, L.; Xu, J.; Lian, J.; Wang, L.; Sun, H. Sorption of Five Organic Compounds by Polar and Nonpolar Microplastics. Chemosphere 2020, 257, 127206. [Google Scholar] [CrossRef]
- Bouhroum, R.; Boulkamh, A.; Asia, L.; Lebarillier, S.; ter Halle, A.; Syakti, A.D.; Doumenq, P.; Malleret, L.; Wong-Wah-chung, P. Concentrations and Fingerprints of PAHs and PCBs Adsorbed onto Marine Plastic Debris from the Indonesian Cilacap Coast and The North Atlantic Gyre. Reg. Stud. Mar. Sci. 2019, 29, 100611. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic Micropollutants in Marine Plastics Debris from the Open Ocean and Remote and Urban Beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]


| Model | Equation | Linear Equation |
|---|---|---|
| Pseudo first-order model | ||
| Pseudo second-order model | ||
| Intraparticle diffusion (ID) | - |
| Plastic Type | Initial Concentration of Fuel Oil in Emulsions | |||
|---|---|---|---|---|
| 126.35 ± 10.12 mg/L | 14.38 ± 2.20 mg/L | 1.93 ± 0.39 mg/L | 0.23 ± 0.10 mg/L | |
| PS | 2894.48 ± 484.76 | 1935.44 ± 2.28 | 224.55 ± 3.9 | 62.80 ± 0.08 |
| HDPE | 1744.11 ± 119.36 | 1970.01 ± 13.15 | 207.90 ± 25.43 | 59.08 ± 1.97 |
| PET | 1844.93 ± 502.15 | 1892.01 ± 19.0 | 186.13 ± 9.05 | 50.78 ± 4.13 |
| PP | 2255.91 ± 264.34 | 1964.56 ± 17.55 | 239.52 ± 21.74 | 63.38 ± 1.23 |
| LDPE | 2914.39 ± 359.49 | 2014.64 ± 34.67 | 223.71 ± 1.29 | 64.89 ± 0.40 |
| PVC | 2543.01 ± 422.39 | 1704.27 ± 3.22 | 198.57 ± 13.11 | 55.90 ± 3.24 |
| Plastic Type | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (10−2 cm3/g) |
|---|---|---|---|
| PS | 17.45 | 5.57 | 1.42 |
| HDPE | 2.85 | 11.66 | 2.43 |
| PET | 6.55 | 4.91 | 0.83 |
| PP | 11.23 | 5.05 | 0.96 |
| LDPE | 13.25 | 4.25 | 0.80 |
| PVC | 7.73 | 4.94 | 1.41 |
| Plastic Type | qe (mg/kg) | Pseudo First-Order | Pseudo Second-Order | Intraparticle Diffusion (ID) | |||
|---|---|---|---|---|---|---|---|
| k (1/h) | R2 | k (kg/mg h) | R2 | k (mg/ kg h) | R2 | ||
| PS | 261.42 | 0.02 | 0.95 | 0.0038 | 0.99 | 12.00 | 0.64 |
| PP | 308.73 | 0.02 | 0.88 | 0.0032 | 0.99 | 15.00 | 0.67 |
| LDPE | 315.52 | 0.02 | 0.91 | 0.0031 | 0.99 | 9.00 | 0.69 |
| Beach | Tamiahua | Tuxpan | La Barra de Sontecomapan | Coatzacoalcos |
|---|---|---|---|---|
| qe * (mg/kg) | 35,258.81 | 16,133.76 | 1660.85 | 8776.99 |
| Standard deviation (mg/kg) | 12,921.23 | 2980.46 | 1114.73 | 3088.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Salas, A.A.; Velasco-Pérez, M.; Mendoza-Muñoz, N.; Vázquez-Morillas, A.; Beltrán-Villavicencio, M.; Alvarez-Zeferino, J.C.; Ojeda-Benítez, S. Sorption of Total Petroleum Hydrocarbons in Microplastics. Polymers 2023, 15, 2050. https://doi.org/10.3390/polym15092050
Cruz-Salas AA, Velasco-Pérez M, Mendoza-Muñoz N, Vázquez-Morillas A, Beltrán-Villavicencio M, Alvarez-Zeferino JC, Ojeda-Benítez S. Sorption of Total Petroleum Hydrocarbons in Microplastics. Polymers. 2023; 15(9):2050. https://doi.org/10.3390/polym15092050
Chicago/Turabian StyleCruz-Salas, Arely Areanely, Maribel Velasco-Pérez, Nayely Mendoza-Muñoz, Alethia Vázquez-Morillas, Margarita Beltrán-Villavicencio, Juan Carlos Alvarez-Zeferino, and Sara Ojeda-Benítez. 2023. "Sorption of Total Petroleum Hydrocarbons in Microplastics" Polymers 15, no. 9: 2050. https://doi.org/10.3390/polym15092050
APA StyleCruz-Salas, A. A., Velasco-Pérez, M., Mendoza-Muñoz, N., Vázquez-Morillas, A., Beltrán-Villavicencio, M., Alvarez-Zeferino, J. C., & Ojeda-Benítez, S. (2023). Sorption of Total Petroleum Hydrocarbons in Microplastics. Polymers, 15(9), 2050. https://doi.org/10.3390/polym15092050












