The Effect of α-Olefin–Maleic Anhydride Copolymer on the Rheological and Crystalline Properties and Microcellular Foaming Behavior of Polyamide 6
Abstract
:1. Introduction
2. Experiment
2.1. Materials
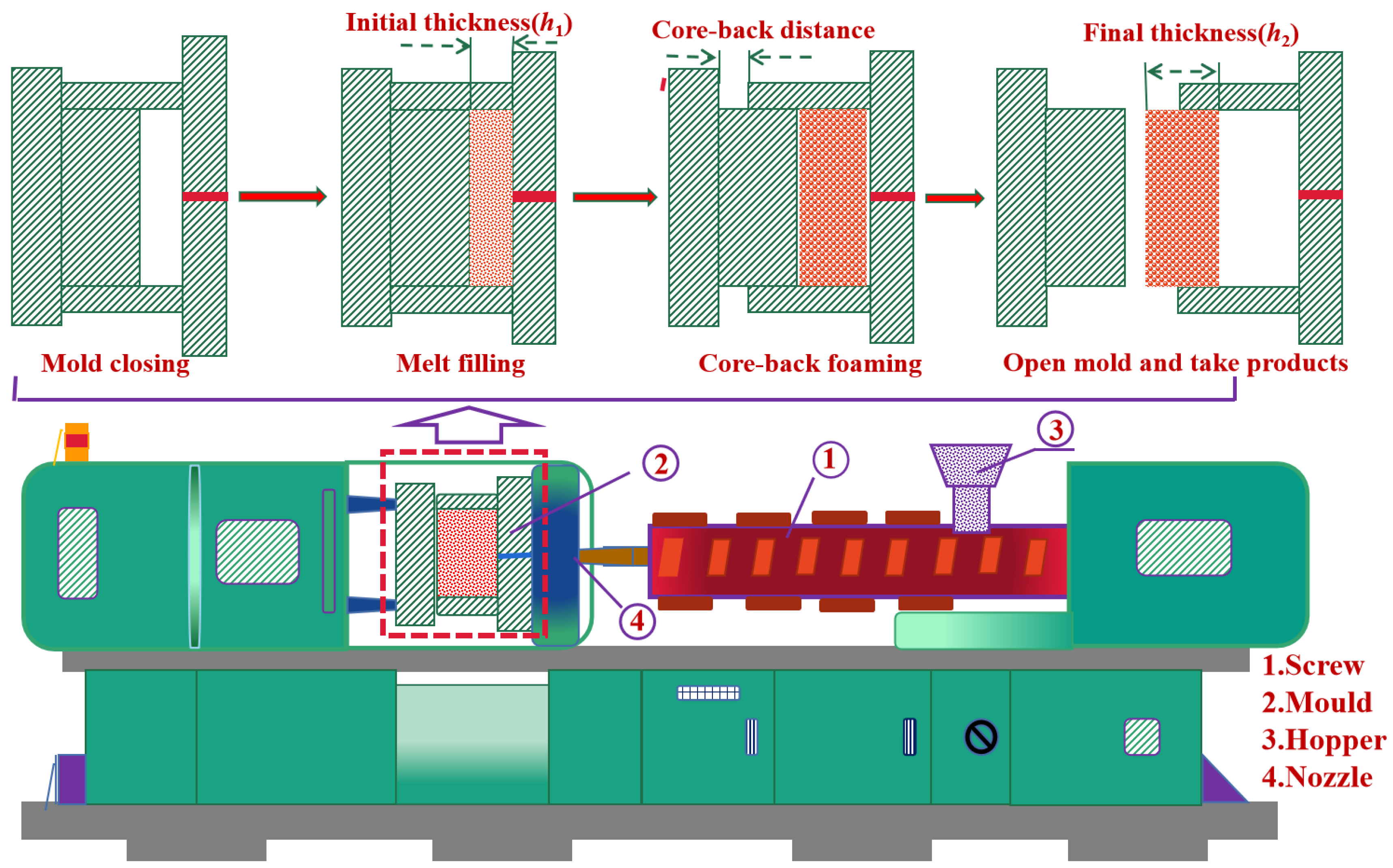
2.2. Sample Preparation
2.3. Characterizations
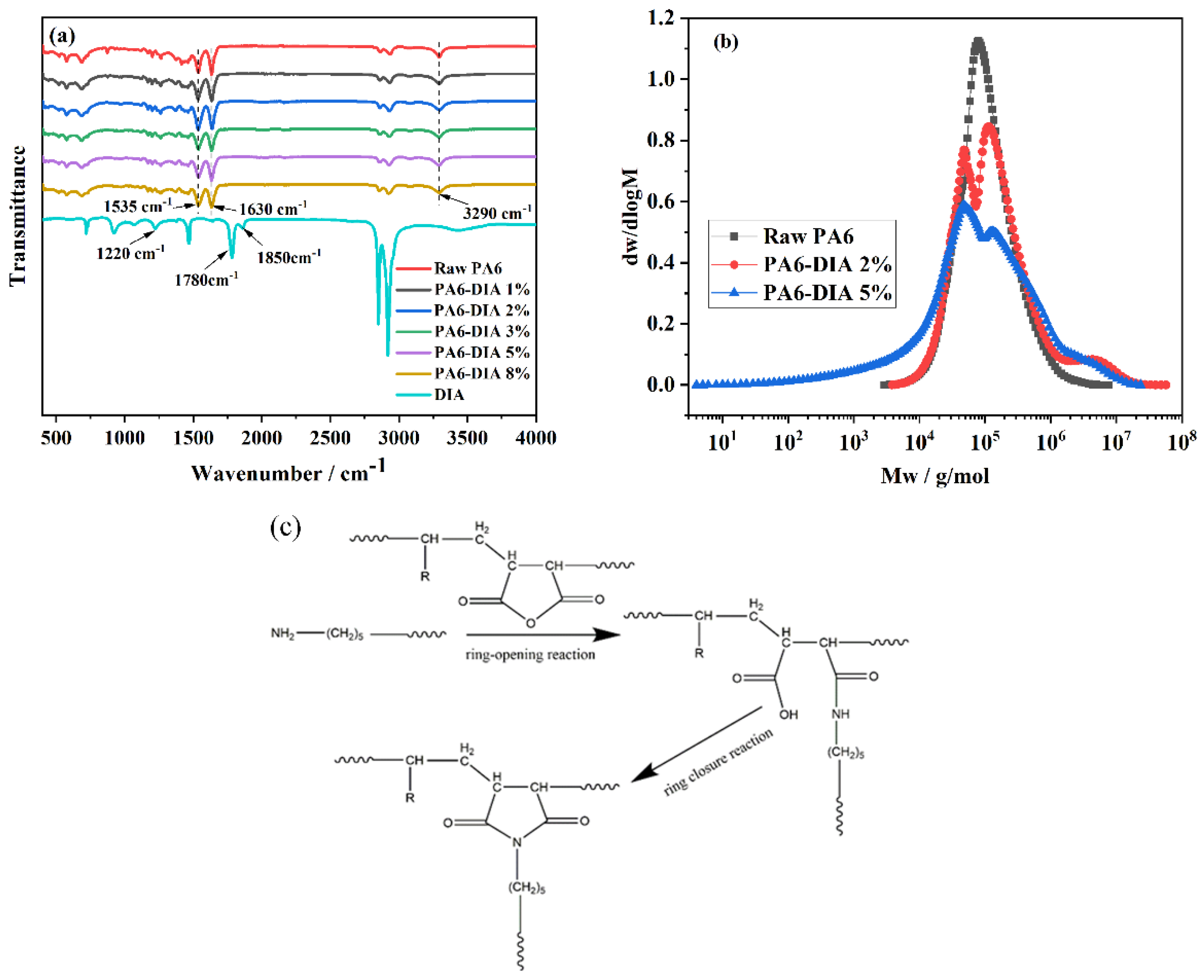
2.3.1. Fourier Transform Infrared Spectra (FTIR)
2.3.2. Gel Permeation Chromatography (GPC)
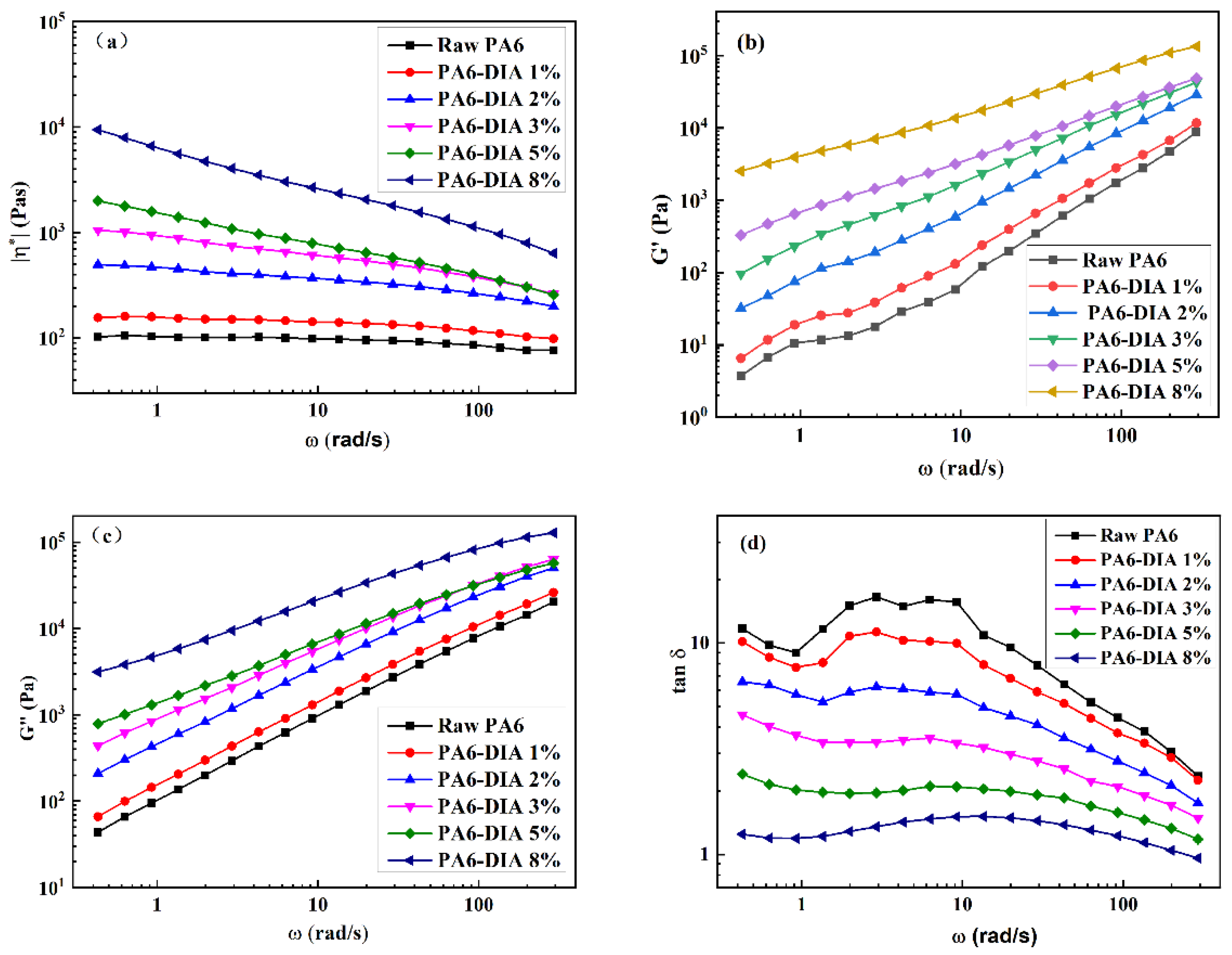
2.3.3. Rheological Behavior
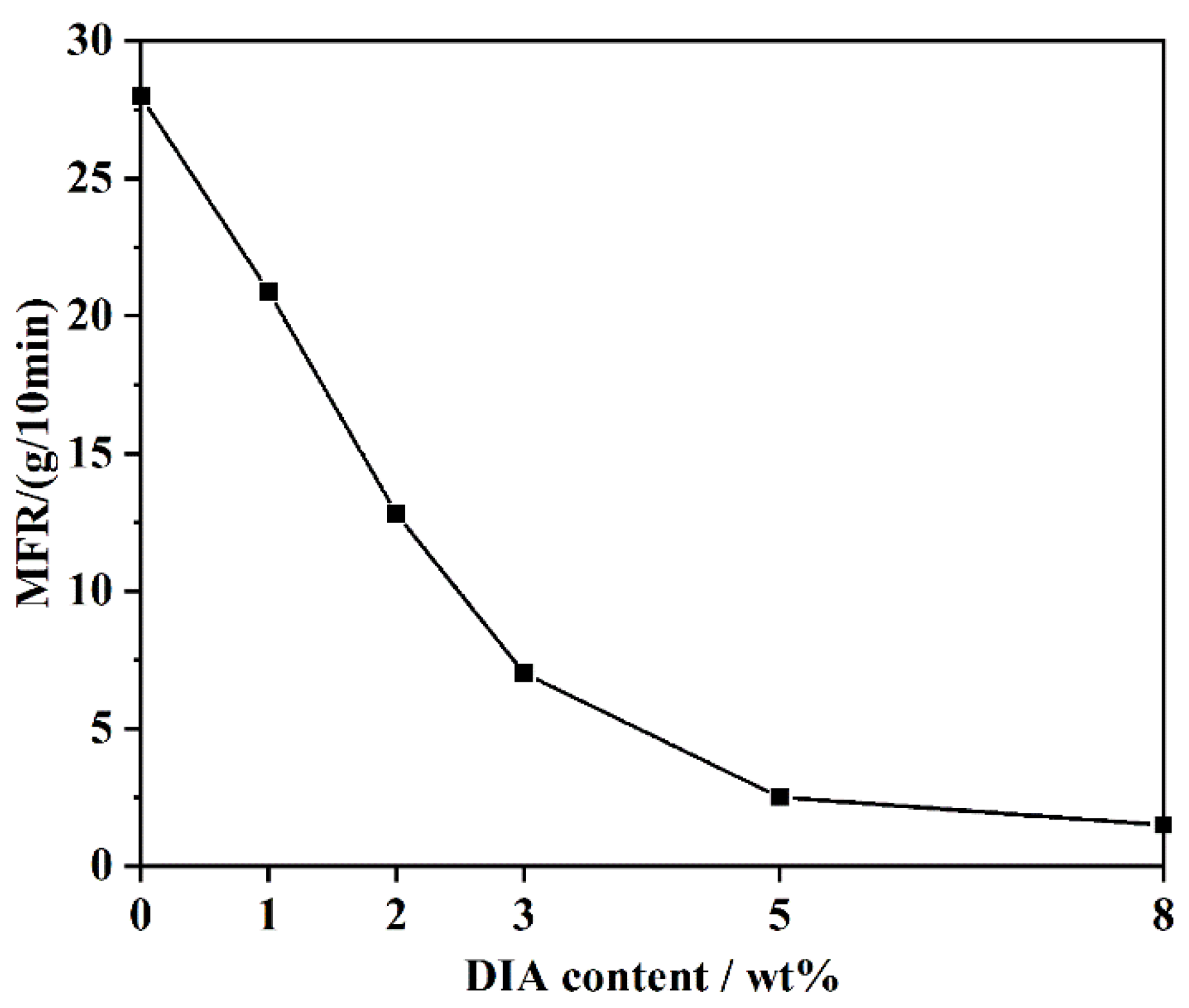
2.3.4. Melt Flow Rate (MFR)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. X-ray Diffraction (XRD)
2.3.7. Hydrophilic and Water Absorption Test
2.3.8. Scanning Electron Microscopy (SEM)
2.3.9. Mechanical Properties
3. Results and Discussion
3.1. Chain Extension Characterization
3.2. Rheological Properties
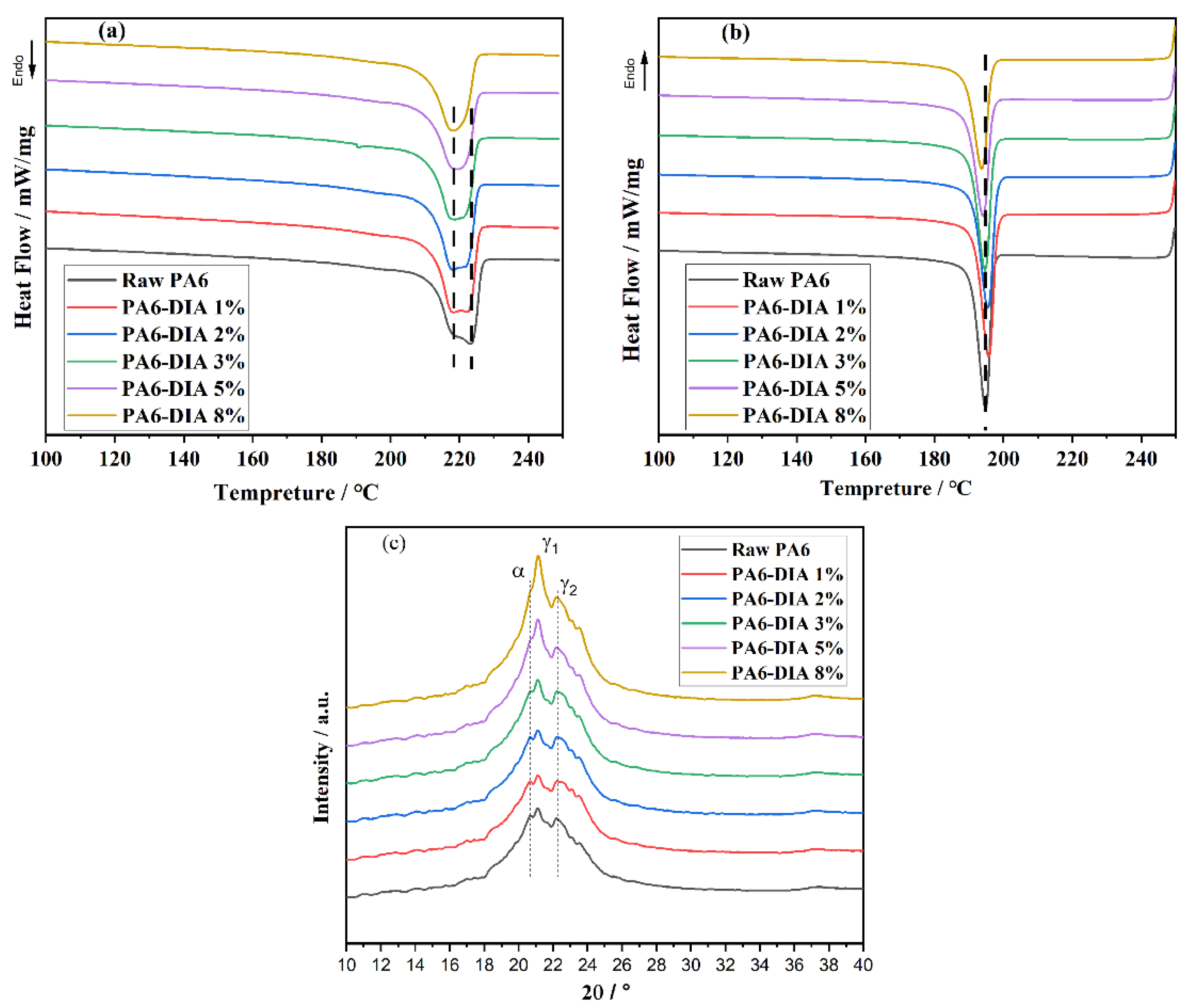
3.3. Crystallization Properties and Crystal Structure
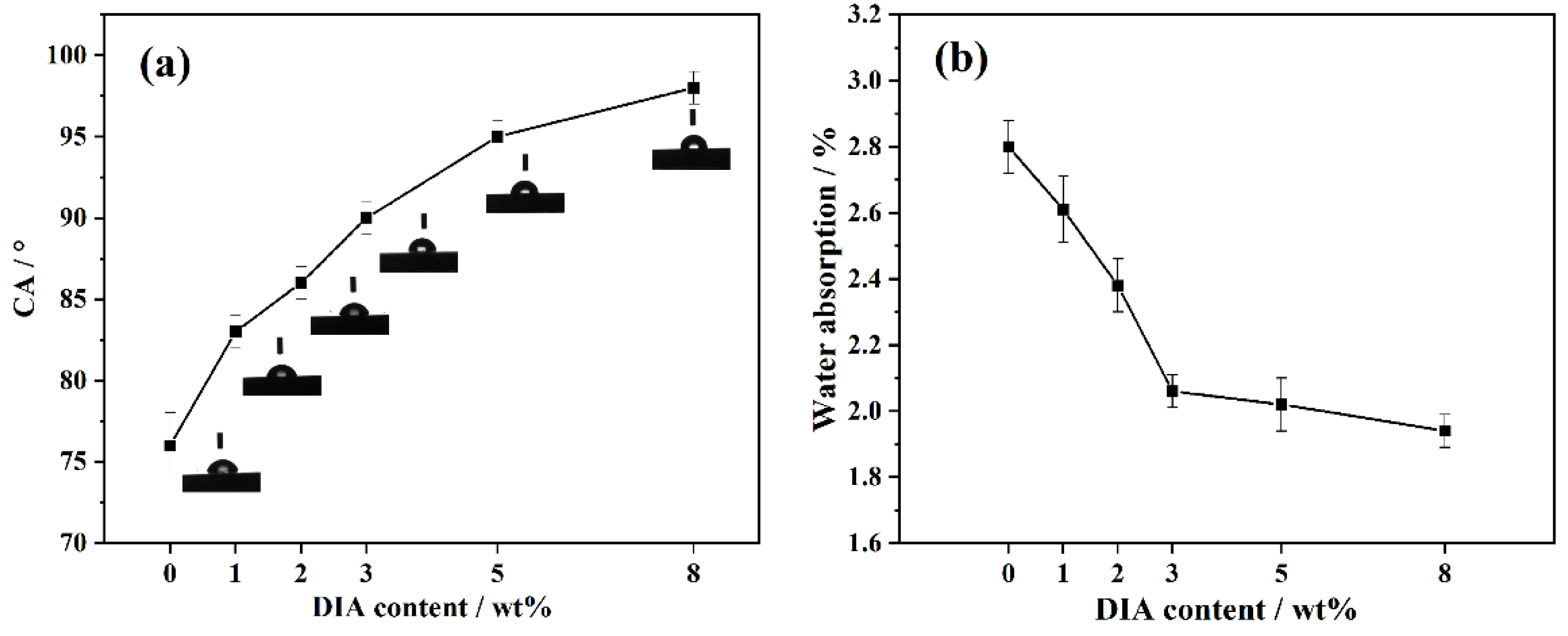
3.4. Water Absorbency
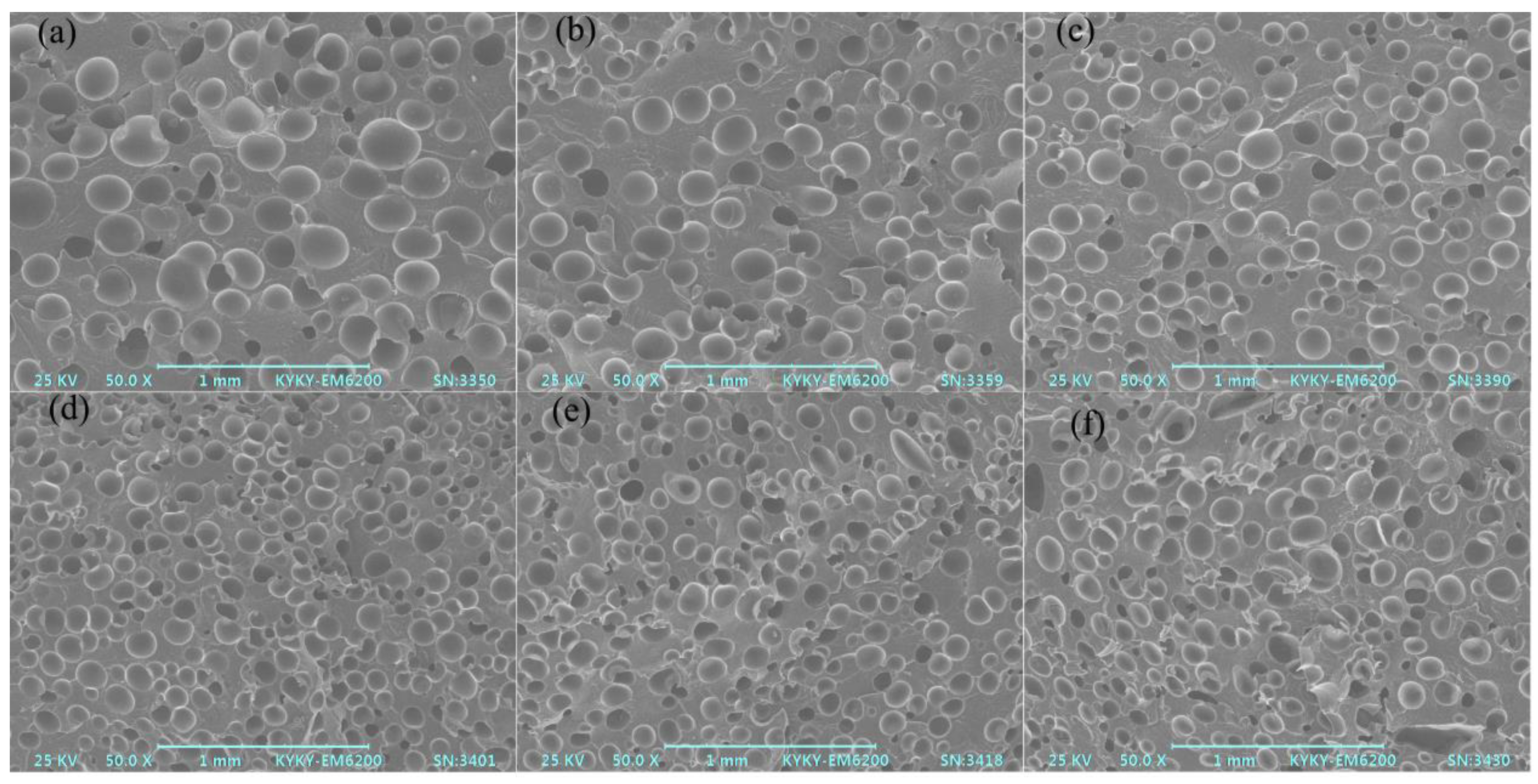
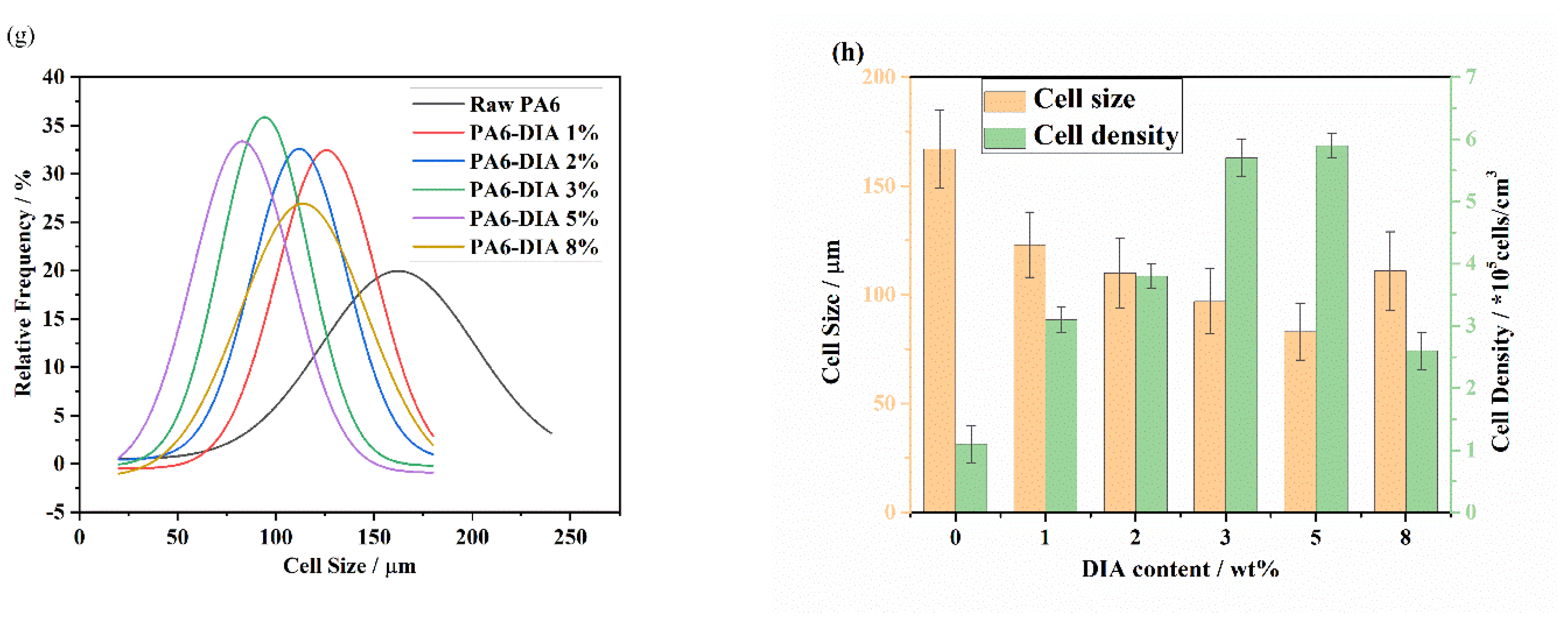
3.5. Foaming Performance of Different PA6 Samples
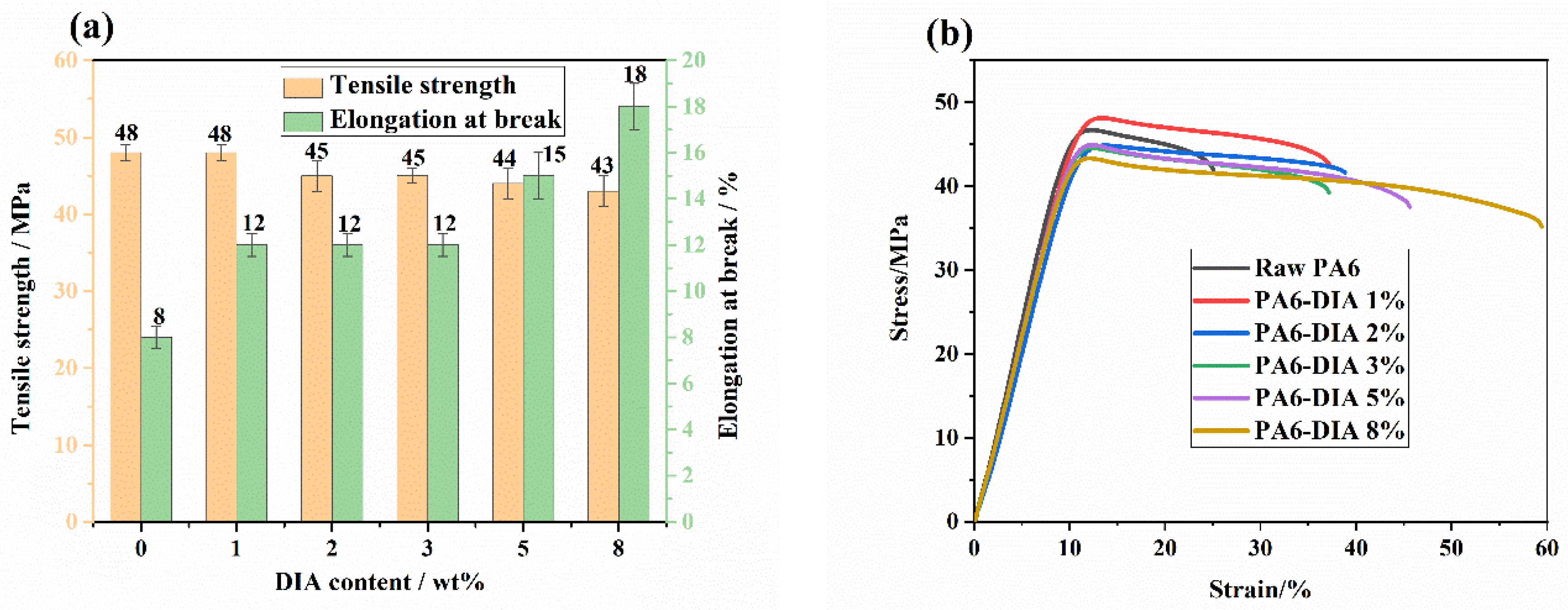
3.6. Mechanical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, B.; Cai, F.P.; Qin, X.Z.; Wang, B.; Jiang, G.L.; Gao, J.H. The influence of extrusion process on micromorphology of PA6/POE/POE-g-MA ternary blends: A quantitative analysis. J. Elastom. Plast. 2021, 2, 110–122. [Google Scholar]
- Thaysa, R.M.F.; Matheus, A.L.; Filipe, L.D.; Aline, B.D.S. Effect of hollow glass microspheres addition on density reduction and mechanical properties of PA6/glass fibers composites. Polimeros 2022, 32, e2022001. [Google Scholar]
- Jiang, T.H.; Zhang, H.; Zeng, X.B.; Zhang, C.; Gong, W.; He, L. The effect of injection process for microcellular foaming on the cell morphology and surface quality of Polyamide 6. Mater. Res. Express 2021, 8, 045311. [Google Scholar] [CrossRef]
- Sridhar, A.; Adusumalli, R.B.; Doddipatla, P.; Venkateshan, K.C. Comparative study between dry and wet properties of thermoplastic PA6/PP novel matrix-based carbon fibre composites. Sci. Eng. Compos. Mater. 2021, 28, 579–591. [Google Scholar] [CrossRef]
- Han, S.; Jiang, C.; Mi, J.G.; Wang, Y.Q.; Chen, S.H.; Wang, X.D. Influence of fibrillated poly(tetrafluoroethylene) on microcellular foaming of polyamide 6 in the presence of supercritical CO2. J. Appl. Polym. Sci. 2021, 43, e51258. [Google Scholar] [CrossRef]
- Mohsenzadeh, R. Experimental studies on mechanical properties and toughening mechanisms of PA6/zeolite nanocomposites. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 23, 7233–7240. [Google Scholar] [CrossRef]
- Ryu, Y.; Sohn, J.S.; Yun, C.S.; Cha, S.W. Shrinkage and warpage minimization of glass-fiber-reinforced polyamide 6 parts by microcellular foam injection molding. Polymers 2020, 12, 889. [Google Scholar] [CrossRef]
- Surace, R.; Pagano, C.; Bellantone, V.; Gatti, S.; Castellani, L.; Vighi, M.; Stoclet, G.; Sechi, S.; Fassi, I.; Baldi, F. Injection vs micro-injection molding of nano-particle filled polyamide 6: Moldability and structuring. Polymer 2021, 230, 124035. [Google Scholar] [CrossRef]
- Tuna, B.; Benkreira, H. Chain extension of recycled PA6. Polym. Eng. Sci. 2018, 58, 1037–1042. [Google Scholar] [CrossRef]
- Buccella, M.; Dorigato, A.; Pasqualini, E. Chain extension behavior and thermo-mechanical properties of polyamide 6 chemically modified with 1,1′-carbonyl-bis-caprolactam. Polym. Eng. Sci. 2013, 54, 158–165. [Google Scholar] [CrossRef]
- Xu, Z.J.; Lin, X.C.; Luo, C.Y.; Xiao, W.D. The application of a tri-functional epoxy resin as a crosslinking agent in extruded polyamide-6 foam. Polym. Sci. Ser. B 2019, 61, 574–581. [Google Scholar]
- Xu, M.L.; Lu, J.W.; Zhao, J.C.; Wei, L.F.; Liu, T.; Zhao, L.; Park, C.B. Rheological and foaming behaviors of long-chain branched polyamide 6 with controlled branch length. Polymer 2021, 224, 123730. [Google Scholar] [CrossRef]
- Xu, M.L.; Lu, J.W.; Qiao, Y.N.; Wei, L.F.; Liu, T.; Lee, P.C.; Zhao, L.; Park, C.B. Toughening mechanism of long chain branched polyamide 6. Mater. Design. 2020, 196, 109173. [Google Scholar] [CrossRef]
- Azdast, T.; Hasanzadeh, R. Increasing cell density/decreasing cell size to produce microcellular and nanocellular thermoplastic foams: A review. J. Cell. Plast. 2020, 57, 769. [Google Scholar] [CrossRef]
- Xu, M.L.; Chen, Y.C.; Liu, T.; Zhao, L.; Park, C.B. Determination of modified polyamide 6′s foaming windows by bubble growth simulations based on rheological measurements. J. Appl. Polym. Sci. 2019, 136, 48138. [Google Scholar] [CrossRef]
- Lu, C.X.; Chen, T.; Zhao, X.Z.; Ren, X.C.; Cai, X.F. Chemical modification of polyamide-6 by chain extension with 2,2′-bis(2-oxazoline). J. Polym. Sci. Pol. Phys. 2010, 45, 1976–1982. [Google Scholar] [CrossRef]
- Duin, M.; Borggreve, R.J.M. Blends of polyamides and maleic-anhydride-containing polymers: Interfacial chemistry and properties. In Reactive Modifiers for Polymers, 3rd ed.; Al-Malaika, S., Ed.; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Han, S.; Jiang, C.; Yu, K.S.; Mi, J.G.; Chen, S.H.; Wang, X.D. Influence of crystallization on microcellular foaming behavior of PA6 in a supercritical CO2-assisted route. J. Appl. Polym. Sci. 2020, 137, e49183. [Google Scholar] [CrossRef]
- Pang, Y.Y.; Cao, Y.Y.; Zheng, W.G.; Park, C.B. A comprehensive review of cell structure variation and general rules for polymer microcellular foams. Chem. Eng. J. 2022, 430, 132662. [Google Scholar] [CrossRef]
- Alfonsius, B.A.; Savvas, G.H.; Shivendar, K.G.; Henry, H. Effects of molecular structure on the rheology and processability of blow-molding high-density polyethylene resins. Adv. Polym. Tech. 2001, 20, 1–13. [Google Scholar]
- Sifri, R.J.; Padilla-Velez, O.; Coates, G.W.; Fors, P.B. Controlling the shape of molecular weight distributions in coordination polymerization and its impact on physical properties. J. Am. Chem. Soc. 2020, 142, 1443–1448. [Google Scholar] [CrossRef]
- Di, Y.W.; Iannace, S.; Maio, E.D.; Nicolais, L. Reactively modified poly (lactic acid): Properties and foam processing. Macromol. Mater. Eng. 2005, 290, 1083–1090. [Google Scholar] [CrossRef]
- Ozmen, C.S.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stabil. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Gentekos, D.T.; Sifri, R.J.; Fors, B.F. Controlling polymer properties through the shape of the molecular-weight distribution. Nat. Rev. Mater. 2019, 4, 761–774. [Google Scholar] [CrossRef]
- Song, J.S.; Mi, J.G.; Zhou, H.F.; Wang, X.D.; Zhang, Y.X. Chain extension of poly (butylene adipate-co-terephthalate) and its microcellular foaming behaviors. Polym. Degrad. Stabil. 2018, 157, 143–152. [Google Scholar] [CrossRef]
- Chandra, A.; Gong, S.Q.; Yuan, M.J. Microstructure and crystallography in microcellular injection-molded polyamide-6 nanocomposite and neat resin. Polym. Eng. Sci. 2005, 45, 52–61. [Google Scholar] [CrossRef]
- Xu, M.L.; Yan, H.C.; He, Q.J.; Wan, C.; Liu, T.; Zhao, L.; Park, C.B. Chain extension of polyamide 6 using multifunctional chain extenders and reactive extrusion for melt foaming. Eur. Polym. J. 2017, 96, 210–220. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Rheology and extrusion foaming of chain-branched Poly (lactic acid). Polym. Eng. Sci. 2010, 50, 629–642. [Google Scholar] [CrossRef]
| Sample | Mn (g/mol) | Mw (g/mol) | Mp (g/mol) | PDI |
|---|---|---|---|---|
| Raw PA6 | 61,869 | 131,064 | 93,713 | 2.118 |
| PA6–DIA2% | 61,303 | 274,100 | 135,869 | 4.471 |
| PA6–DIA5% | 8909 | 247,413 | 209,476 | 27.77 |
| Samples | Raw PA6 | PA6–DIA1% | PA6–DIA2% | PA6–DIA3% | PA6–DIA5% | PA6–DIA8% |
|---|---|---|---|---|---|---|
| Tm1, Tm2 (°C) | 222.5, 218 | 222, 218 | 222, 218 | 221, 218 | 219 | 218 |
| Tc (°C) | 195 | 195.5 | 195.4 | 195 | 194 | 194 |
| Xc (%) | 13 | 10 | 9 | 7 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Jiang, T.; Zeng, X.; Zhu, N.; Shen, C.; Gong, W.; Zhang, C.; He, L. The Effect of α-Olefin–Maleic Anhydride Copolymer on the Rheological and Crystalline Properties and Microcellular Foaming Behavior of Polyamide 6. Polymers 2023, 15, 2056. https://doi.org/10.3390/polym15092056
Li S, Jiang T, Zeng X, Zhu N, Shen C, Gong W, Zhang C, He L. The Effect of α-Olefin–Maleic Anhydride Copolymer on the Rheological and Crystalline Properties and Microcellular Foaming Behavior of Polyamide 6. Polymers. 2023; 15(9):2056. https://doi.org/10.3390/polym15092056
Chicago/Turabian StyleLi, Shengnan, Tuanhui Jiang, Xiangbu Zeng, Nenggui Zhu, Chao Shen, Wei Gong, Chun Zhang, and Li He. 2023. "The Effect of α-Olefin–Maleic Anhydride Copolymer on the Rheological and Crystalline Properties and Microcellular Foaming Behavior of Polyamide 6" Polymers 15, no. 9: 2056. https://doi.org/10.3390/polym15092056
APA StyleLi, S., Jiang, T., Zeng, X., Zhu, N., Shen, C., Gong, W., Zhang, C., & He, L. (2023). The Effect of α-Olefin–Maleic Anhydride Copolymer on the Rheological and Crystalline Properties and Microcellular Foaming Behavior of Polyamide 6. Polymers, 15(9), 2056. https://doi.org/10.3390/polym15092056





