Ultramicroporous Polyphenylenes via Diels–Alder Polycondensation Approach
Abstract
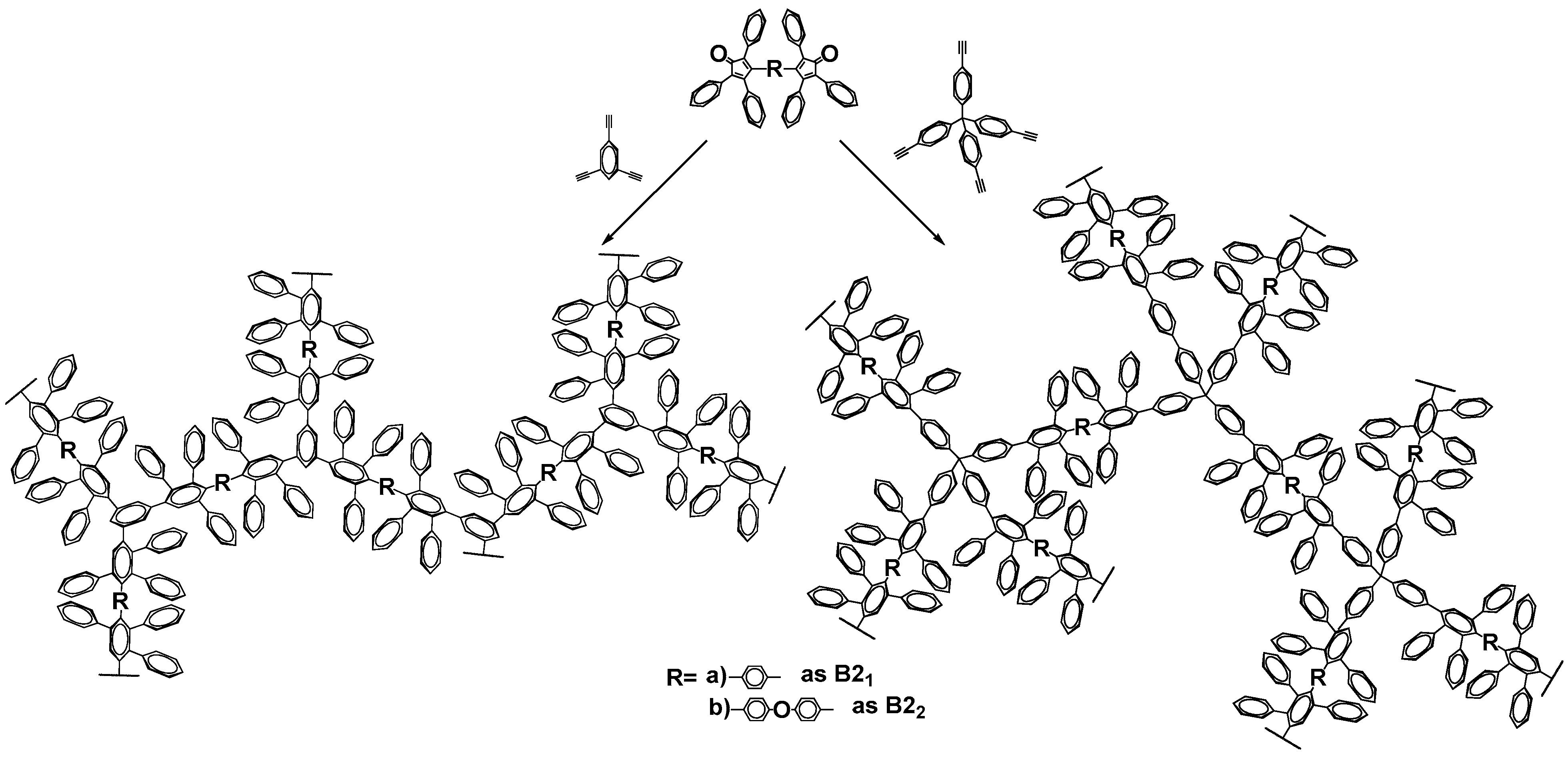
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthetic Procedures
3. Results and Discussion
3.1. Synthesis and Characterization of PPPhs
3.2. Gas Adsorption Properties of PPPhs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Ghanem, B.S.; Ali, Z.; Hazazi, K.; Han, Y.; Pinnau, I. Recent Progress on Polymers of Intrinsic Microporosity and Thermally Modified Analogue Materials for Membrane-Based Fluid Separations. Small Struct. 2021, 2, e2100049. [Google Scholar] [CrossRef]
- Foster, A.B.; Tamaddondar, M.; Luque-Alled, J.M.; Harrison, W.J.; Li, Z.; Gorgojo, P.; Budd, P.M. Understanding the Topology of the Polymer of Intrinsic Microporosity PIM-1: Cyclics, Tadpoles, and Network Structures and Their Impact on Membrane Performance. Macromolecules 2020, 53, 569–583. [Google Scholar] [CrossRef]
- Wang, Y.; Ghanem, B.S.; Han, Y.; Pinnau, I. State-of-the-art polymers of intrinsic microporosity for high-performance gas separation membranes. Curr. Opin. Chem. Eng. 2022, 35, 100755. [Google Scholar] [CrossRef]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Bildirir, H.; Gregoriou, V.G.; Avgeropoulos, A.; Scherf, U.; Chochos, C.L. Porous organic polymers as emerging new materials for organic photovoltaic applications: Current status and future challenges. Mater. Horiz. 2017, 4, 546–556. [Google Scholar] [CrossRef]
- Wong, Y.-L.; Tobin, J.M.; Xu, Z.; Vilela, F. Conjugated porous polymers for photocatalytic applications. J. Mater. Chem. A 2016, 4, 18677–18686. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Ban, D.-D.; Zhou, Y.-J.; Du, H.-J.; Zhao, A.-W.; Xie, L.-G.; Liu, G.-Q.; Chen, S.-R.; Mi, L.-W. Fluorescent porous organic polymers for detection and adsorption of nitroaromatic compounds. Sci. Rep. 2022, 12, 15876. [Google Scholar] [CrossRef]
- Marken, F.; Carta, M.; McKeown, N.B. Polymers of Intrinsic Microporosity in the Design of Electrochemical Multicom-ponent and Multiphase Interfaces. Anal. Chem. 2021, 93, 1213–1220. [Google Scholar] [CrossRef]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, Y.; Liu, Z. Design of porous organic polymer catalysts for transformation of carbon dioxide. Green Chem. Eng. 2022, 3, 96–110. [Google Scholar] [CrossRef]
- Modak, A.; Ghosh, A.; Bhaumik, A.; Chowdhury, B. CO2 hydrogenation over functional nanoporous polymers and metal-organic frameworks. Adv. Colloid Interface Sci. 2021, 290, e102349. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhao, H.; Cao, X.; Xiong, S.; Li, G.; Deng, J.; Yang, H.; Zhang, W.; Liu, Q. Enhancing Built-in Electric Field via Molecular Dipole Control in Conjugated Microporous Polymers for Boosting Charge Separation. ACS Appl. Mater. Interfaces 2022, 14, 35745–35754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Deng, Z.; Deng, J.; Au, C.-T.; Liao, Y.; Yang, H.; Liu, Q. Regulating the exciton binding energy of covalent triazine frameworks for enhancing photocatalysis. J. Mater. Chem. A 2022, 10, 22419–22427. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, H.; Cheng, Z.; Shi, Y.; Guo, Z.; Meng, N.; Thomas, A.; Liao, Y. Macroscale Conjugated Microporous Polymers: Controlling Versatile Functionalities Over Several Di-mensions. Adv. Mater. 2022, 34, e2104952. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of Intrinsic Microporosity (PIMs). Polymer 2020, 202, e122736. [Google Scholar] [CrossRef]
- Golubev, G.S.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. High free volume polymers for pervaporation. Curr. Opin. Chem. Eng. 2022, 36, e100788. [Google Scholar] [CrossRef]
- McKeown, N.B. The synthesis of polymers of intrinsic microporosity (PIMs). Sci. China Chem. 2017, 60, 1023–1032. [Google Scholar] [CrossRef]
- Kirk, R.A.; Putintseva, M.; Volkov, A.; Budd, P.M. The potential of polymers of intrinsic microporosity (PIMs) and PIM/graphene composites for pervaporation membranes. BMC Chem. Eng. 2019, 1, e18. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Volkova, Y.A.; Ponomarev, I.I.; Razorenov, D.Y.; Skupov, K.M.; Nikiforov, R.Y.; Chirkov, S.V.; Ryzhikh, V.E.; Belov, N.A.; Alentiev, A.Y. Polynaphthoylenebenzimidazoles for gas separation–Unexpected PIM relatives. Polymer 2022, 238, e124396. [Google Scholar] [CrossRef]
- Kathiresan, M. Metal-Ion/Metal Nanoparticle-Anchored Porous Organic Polymers as Efficient Catalysts for Organic Trans-formations–A Recent Overview. Chem. Asian J. 2023, 18, e202201299. [Google Scholar] [CrossRef]
- Giri, A.; Patra, A. Porous Organic Polymers: Promising Testbed for Heterogeneous Reactive Oxygen Species Mediated Photocatalysis and Nonredox CO 2 Fixation. Chem. Rec. 2022, 22, e202200071. [Google Scholar] [CrossRef]
- Yi, J.; Jeong, H.Y.; Shin, D.Y.; Kim, C.; Lee, S.J. Mn(III)-Porphyrin Containing Heterogeneous Catalyst based on Microporous Polymeric Constituents as a New Class of Catalyst Support. Chemcatchem 2018, 10, 3974–3977. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 2, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N.B. Triptycene Induced Enhancement of Membrane Gas Selectivity for Microporous Tröger’s Base Polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Litwiller, E.; Pinnau, I. Ultra-Microporous Triptycene-based Polyimide Membranes for High-Performance Gas Separation. Adv. Mater. 2014, 26, 3688–3692. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Env. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Yoon, S. A phenanthroline-based porous organic polymer for the iridium-catalyzed hydrogenation of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 14019–14026. [Google Scholar] [CrossRef]
- Masuda, S.; Mori, K.; Kuwahara, Y.; Yamashita, H. PdAg nanoparticles supported on resorcinol-formaldehyde polymers containing amine groups: The pro-motional effect of phenylamine moieties on CO2 transformation to formic acid. J. Mater. Chem. A 2019, 7, 16356–16363. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Padmanaban, S.; Park, K.; Jung, K.-D.; Yoon, S. An Efficient and Practical System for the Synthesis of N,N-Dimethylformamide by CO2 Hydrogenation using a Heterogeneous Ru Catalyst: From Batch to Continuous Flow. ChemSusChem 2020, 13, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, H.J.; Budd, P.M.; McKeown, N.B. Catalysis by microporous phthalocyanine and porphyrin network polymers. J. Mater. Chem. 2008, 18, 573–578. [Google Scholar] [CrossRef]
- Yuan, S.; Dorney, B.; White, D.; Kirklin, S.; Zapol, P.; Yu, L.; Liu, D.-J. Microporous polyphenylenes with tunable pore size for hydrogen storage. Chem. Commun. 2010, 46, 4547–4549. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Honsho, Y.; Seki, S.; Jiang, D. Light-Harvesting Conjugated Microporous Polymers: Rapid and Highly Efficient Flow of Light Energy with a Porous Polyphenylene Framework as Antenna. J. Am. Chem. Soc. 2010, 132, 6742–6748. [Google Scholar] [CrossRef]
- Kovalev, A.I.; Pastukhov, A.V.; Tkachenko, E.S.; Klemenkova, Z.S.; Kuvshinov, I.R.; Khotina, I.A. Polyphenylenes with Phen-1,3,5-triyl Branching Moieties Based on p-Diacetylbenzene: Synthesis and Study of Porosity and Heat Resistance. Polym. Sci. Ser. C 2020, 62, 205–213. [Google Scholar] [CrossRef]
- Lee, W.-E.; Han, D.-C.; Choi, H.-J.; Sakaguchi, T.; Lee, C.-L.; Kwak, G. Remarkable Change in Fluorescence Emission of Poly(diphenylacetylene) Film via in situ Desilylation Reaction: Correlation with Variations in Microporous Structure, Chain Conformation, and Lamellar Layer Distance. Macromol. Rapid Commun. 2011, 32, 1047–1051. [Google Scholar] [CrossRef]
- Sedláček, J.; Sokol, J.; Zedník, J.; Faukner, T.; Kubů, M.; Brus, J.; Trhlíková, O. Homo- and Copolycyclotrimerization of Ar-omatic Internal Diynes Catalyzed with Co2(CO)8: A Facile Route to Microporous Photoluminescent Polyphenylenes with Hyperbranched or Crosslinked Architecture. Macromol. Rapid Commun. 2018, 39, 1700518. [Google Scholar] [CrossRef]
- Miyake, J.; Watanabe, T.; Shintani, H.; Sugawara, Y.; Uchida, M.; Miyatake, K. Reinforced Polyphenylene Ionomer Membranes Exhibiting High Fuel Cell Performance and Mechanical Durability. ACS Mater. Au 2021, 1, 81–88. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Shen, P.; Chen, Z. Two-dimensional polyphenylene: Experimentally available porous graphene as a hydrogen purification mem-brane. Chem. Commun. 2010, 46, 3672–3674. [Google Scholar] [CrossRef]
- Zang, J.; Zhu, Z.; Mu, P.; Sun, H.; Liang, W.; Yu, F.; Chen, L.; Li, A. Synthesis and Properties of Conjugated Microporous Polymers Bearing Pyrazine Moieties with Macroscopically Porous 3D Networks Structures. Macromol. Mater. Eng. 2016, 301, 1104–1110. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, D.; Xue, Y.; Dai, L.; Chen, J.-F.; Cao, D. PAF-derived nitrogen-doped 3D Carbon Materials for Efficient Energy Conversion and Storage. Sci. Rep. 2015, 5, 8307. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Linares-Solano, A. Commentary on the paper “On the adsorption affinity coefficient of carbon dioxide in microporous car-bons” by E.S. Bickford et al. (Carbon 2004; 42: 1867–71). Carbon 2005, 43, 658–660. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Keshtov, M.L.; Keshtova, S.V.; Petrovskii, P.V.; Shchegolikhin, A.N.; Kirillov, A.A.; Kireev, V.V. New bis-tetraarylcyclopentadienones. Russ. Chem. Bull. 1998, 47, 318–320. [Google Scholar] [CrossRef]
- Galoppini, E.; Gilardi, R. Weak hydrogen bonding between acetylenic groups: The formation of diamondoid nets in the crystal structure of tetrakis(4-ethynylphenyl)methane. Chem. Commun. 1999, 2, 173–174. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.; Ma, M.; Tang, J.; Huang, F. Dendritic poly(silylene arylacetylene) resins based on 1,3,5-triethynylbenzene. Eur. Polym. J. 2020, 129, 109628. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Zinatullina, M.S.; Serkova, E.S.; Vlasov, P.S.; Peregudov, A.S.; Shifrina, Z.B. Hyperbranched pyridylphenylene polymers based on the first-generation dendrimer as a multifunc-tional monomer. RSC Adv. 2015, 5, 99510–99516. [Google Scholar] [CrossRef]
- Minelli, M.; Paul, D.R.; Sarti, G.C. On the interpretation of cryogenic sorption isotherms in glassy polymers. J. Membr. Sci. 2017, 540, 229–242. [Google Scholar] [CrossRef]
- Breunig, M.; Dorner, M.; Senker, J. Ultramicroporous polyimides with hierarchical morphology for carbon dioxide separation. J. Mater. Chem. A 2021, 9, 12797–12806. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Liu, H.; Li, T.; Ren, Y. Ultramicroporous Organophosphorus Polymers via Self-Accelerating P–C Coupling Reactions: Kinetic Effects on Crosslinking Environments and Porous Structures. J. Am. Chem. Soc. 2022, 144, 11748–11756. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, G. Porous Aromatic Frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef] [PubMed]


| Polymer a | Monomer A | Monomer B | C b, mol L−1 | Time, h | Mw c·10−3, g·mol−1 | Mw/Mn | Yield, % |
|---|---|---|---|---|---|---|---|
| 1 | A4 | B21 | 0.05 | 10 | Insoluble | - | 99.0 |
| 2 | A4 | B21 | 0.01 | 10 | 16.3 | 1.69 | 76.2 |
| 3 | A4 | B21 | 0.01 | 17 | 35.1 | 2.11 | 88.6 |
| 4 | A4 | B22 | 0.05 | 6 | Insoluble | - | 99.0 |
| 5 | A4 | B22 | 0.01 | 10 | 23.2 | 2.21 | 88.5 |
| 6 | A4 | B22 | 0.01 | 20 | 47.4 | 2.56 | 95.4 |
| 7 | A3 | B21 | 0.05 | 10 | Insoluble | - | 99.2 |
| 8 | A3 | B21 | 0.01 | 10 | 17.2 | 2.35 | 78.7 |
| 9 | A3 | B21 | 0.01 | 17 | 23.4 | 2.47 | 96.3 |
| 10 | A3 | B22 | 0.1 | 7 | Insoluble | - | 99.1 |
| 11 | A3 | B22 | 0.05 | 10 | 317.3 | 3.53 | 75.3 |
| 12 | A3 | B22 | 0.05 | 22 | 789.3 | 20.61 | 98.0 |
| Polymer | SBET a (N2), m2 g−1 | SDFT b (CO2), m2 g−1 | Vpore c (CO2), cm3 g−1 | D d, nm |
|---|---|---|---|---|
| 2 | 667 | 751 | 0.174 | 1.0 |
| 3 | 19 | 553 | 0.110 | 0.6 |
| 5 | 38 | 238 | 0.042 | 0.6 |
| 6 | 23 | 443 | 0.097 | 0.6 |
| 8 | 247 | 332 | 0.061 | 0.8–1.2 |
| 9 | 313 | 437 | 0.097 | 0.8–1.2 |
| 11 | 356 | 468 | 0.098 | 0.8–1.2 |
| 12 | 404 | 515 | 0.107 | 0.8–1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, S.A.; Kuchkina, N.V.; Mikhalchenko, A.V.; Krasnova, I.Y.; Khanin, D.A.; Skupov, K.M.; Shifrina, Z.B. Ultramicroporous Polyphenylenes via Diels–Alder Polycondensation Approach. Polymers 2023, 15, 2060. https://doi.org/10.3390/polym15092060
Sorokina SA, Kuchkina NV, Mikhalchenko AV, Krasnova IY, Khanin DA, Skupov KM, Shifrina ZB. Ultramicroporous Polyphenylenes via Diels–Alder Polycondensation Approach. Polymers. 2023; 15(9):2060. https://doi.org/10.3390/polym15092060
Chicago/Turabian StyleSorokina, Svetlana A., Nina V. Kuchkina, Alexander V. Mikhalchenko, Irina Yu. Krasnova, Dmitry A. Khanin, Kirill M. Skupov, and Zinaida B. Shifrina. 2023. "Ultramicroporous Polyphenylenes via Diels–Alder Polycondensation Approach" Polymers 15, no. 9: 2060. https://doi.org/10.3390/polym15092060






