Self-Luminous Wood Coatings with Carbon Dots/TiO2 Grafted Afterglow SrAl2O4: Eu, Dy Core-Shell Phosphors for Long-Lasting Formaldehyde Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
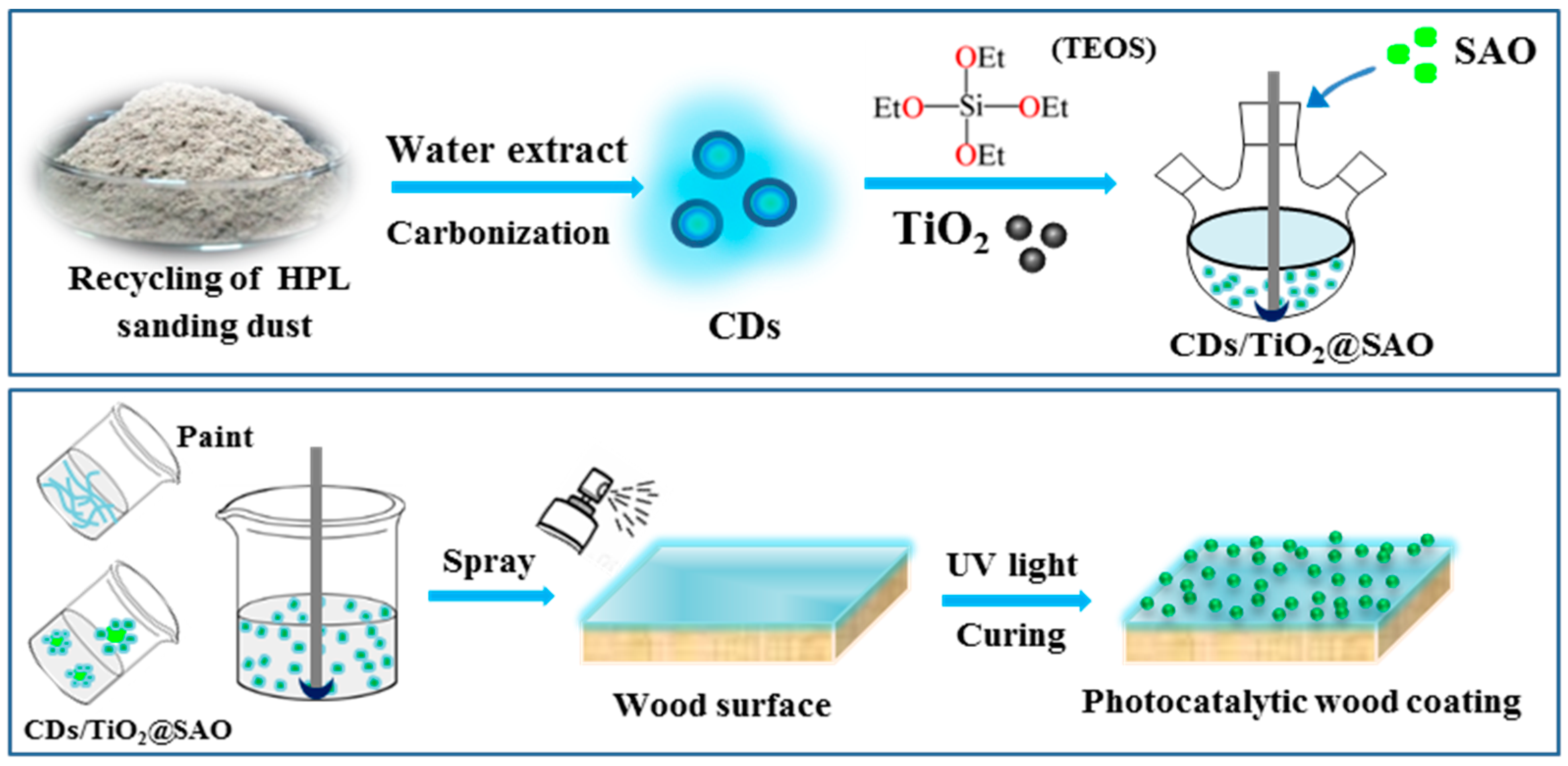
2.2. Synthesis of N,P-Doped CDs from Recycling Wood Sanding Dust
2.3. Formation of CDs/TiO2@SAO Composite Photocatalyst
2.4. Formation of Photocatalytic Wood Coatings with CDs/TiO2@SAO Composite
2.5. Material Characterization
2.6. Photocatalytic Activities Characterization
3. Results and Discussions
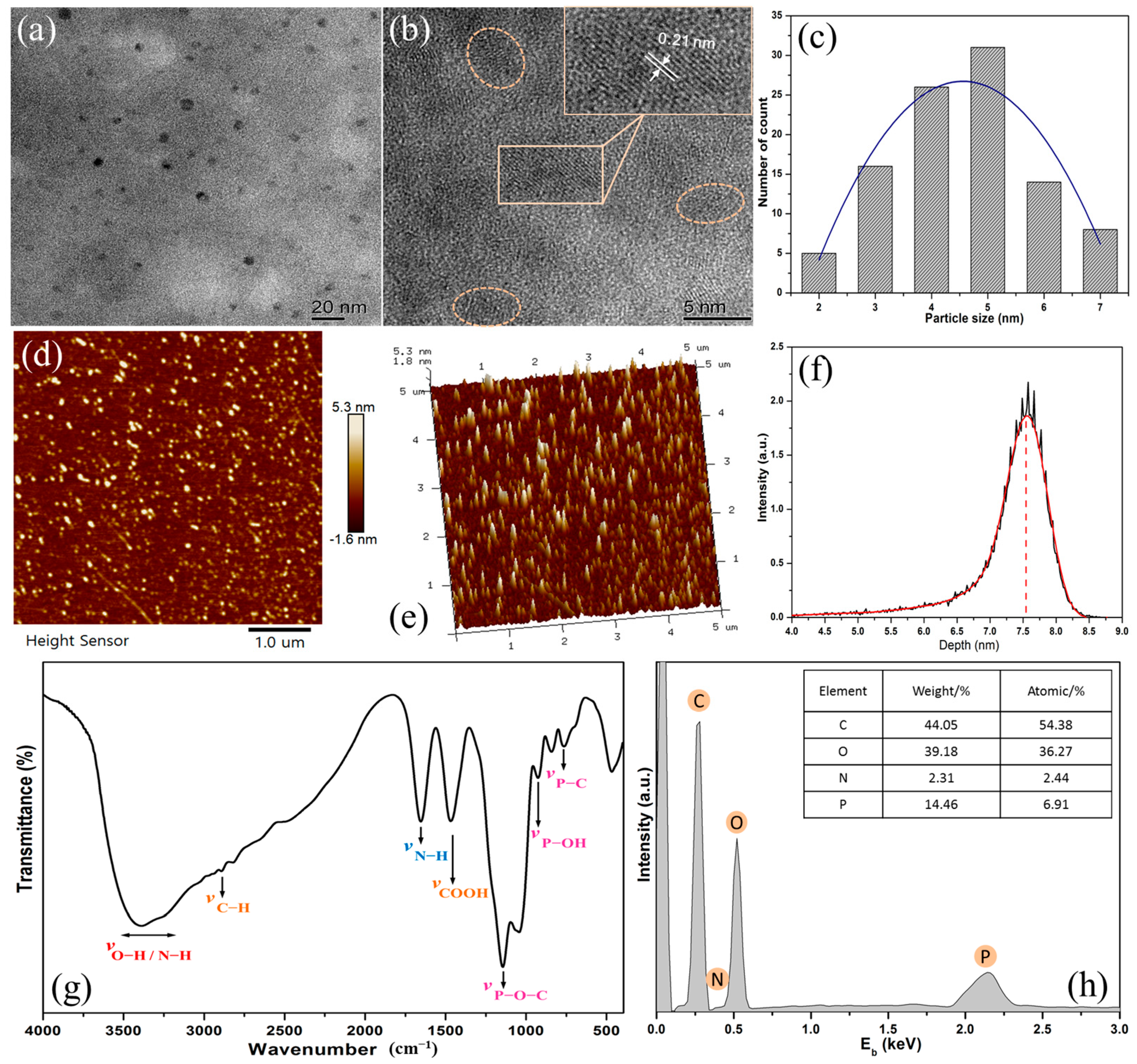
3.1. Characterization of Fluorescent N,P-Doped CDs
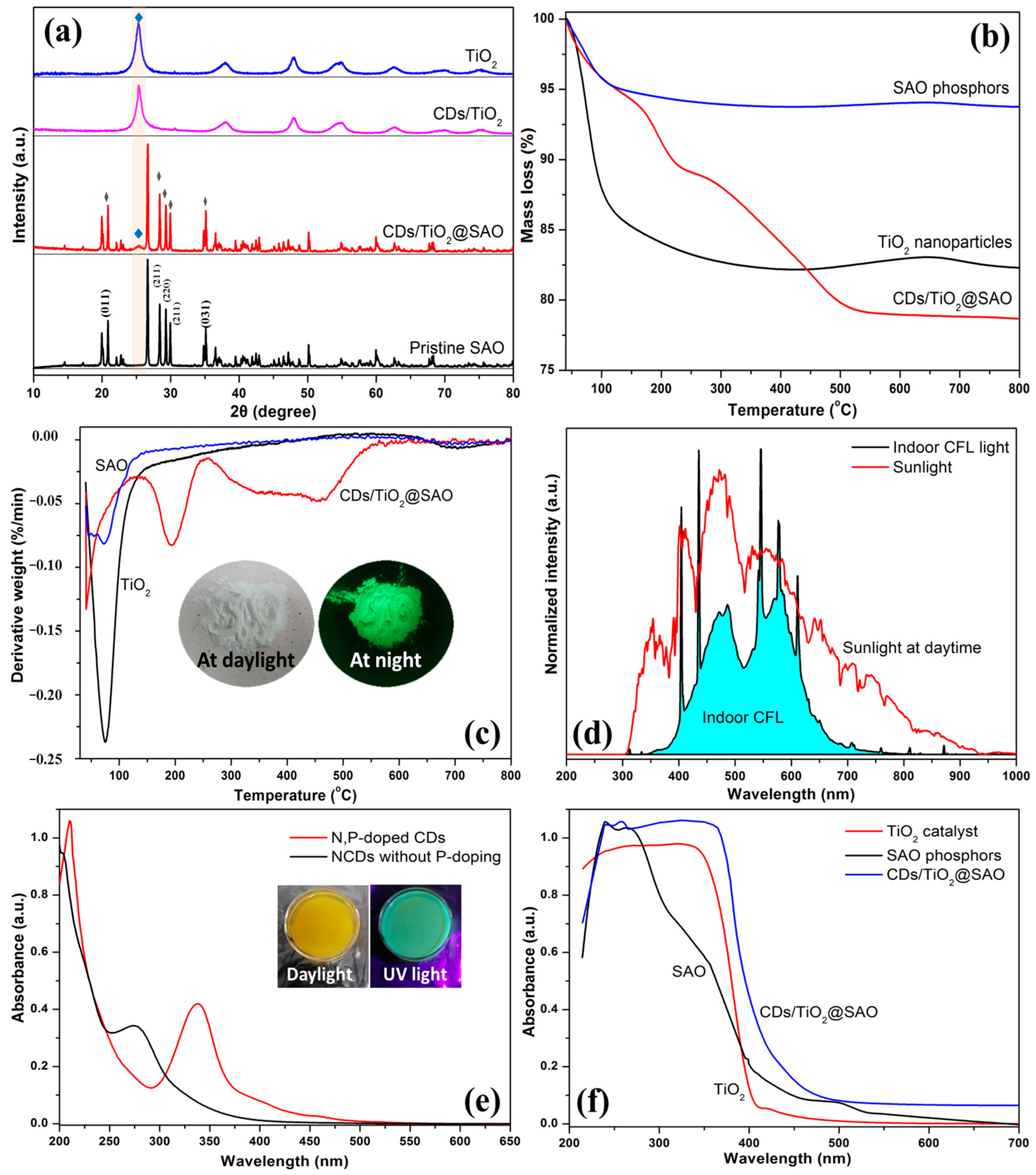
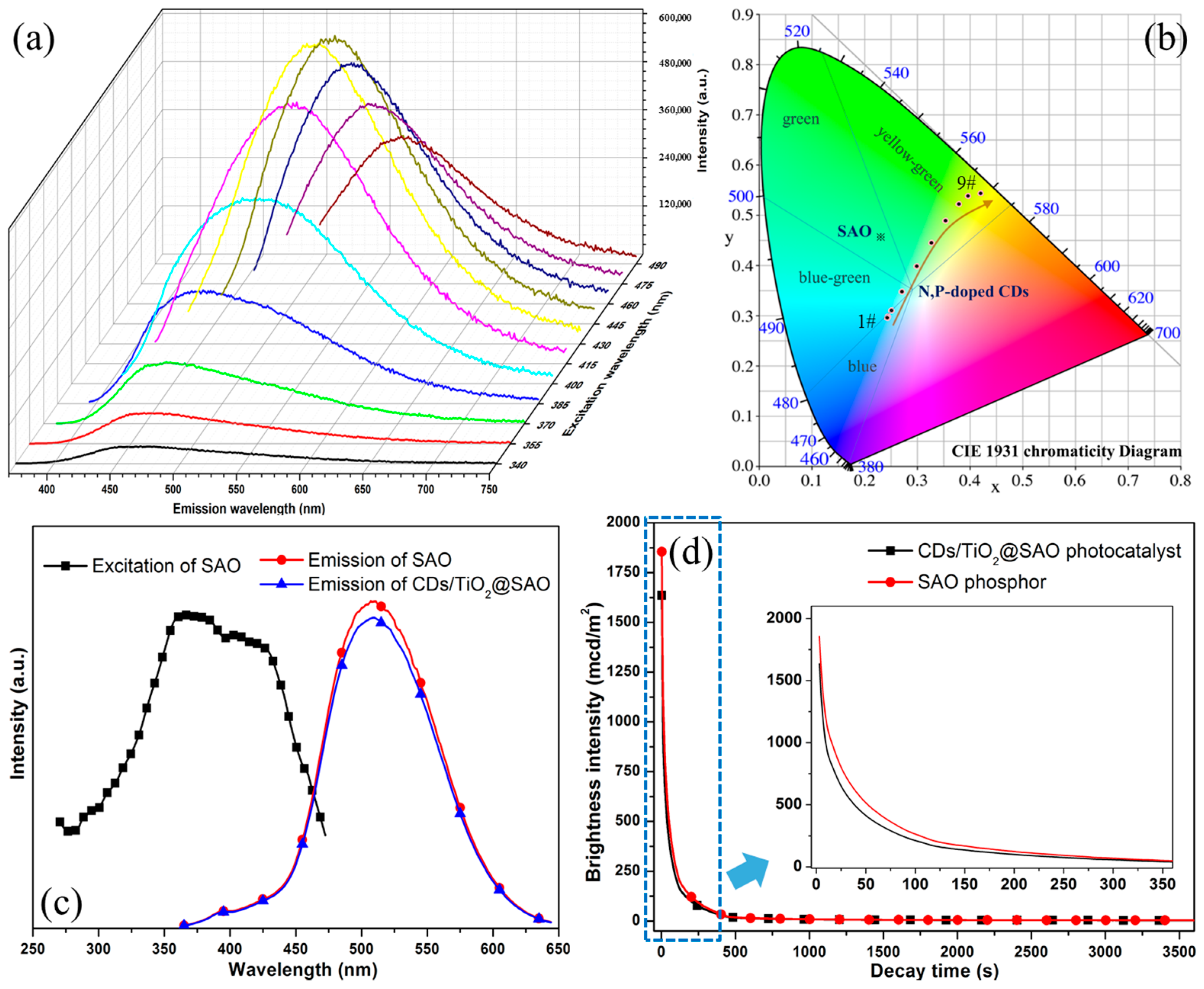
3.2. Characterization of CDs/TiO2@SAO Core-Shell Composite Photocatalyst
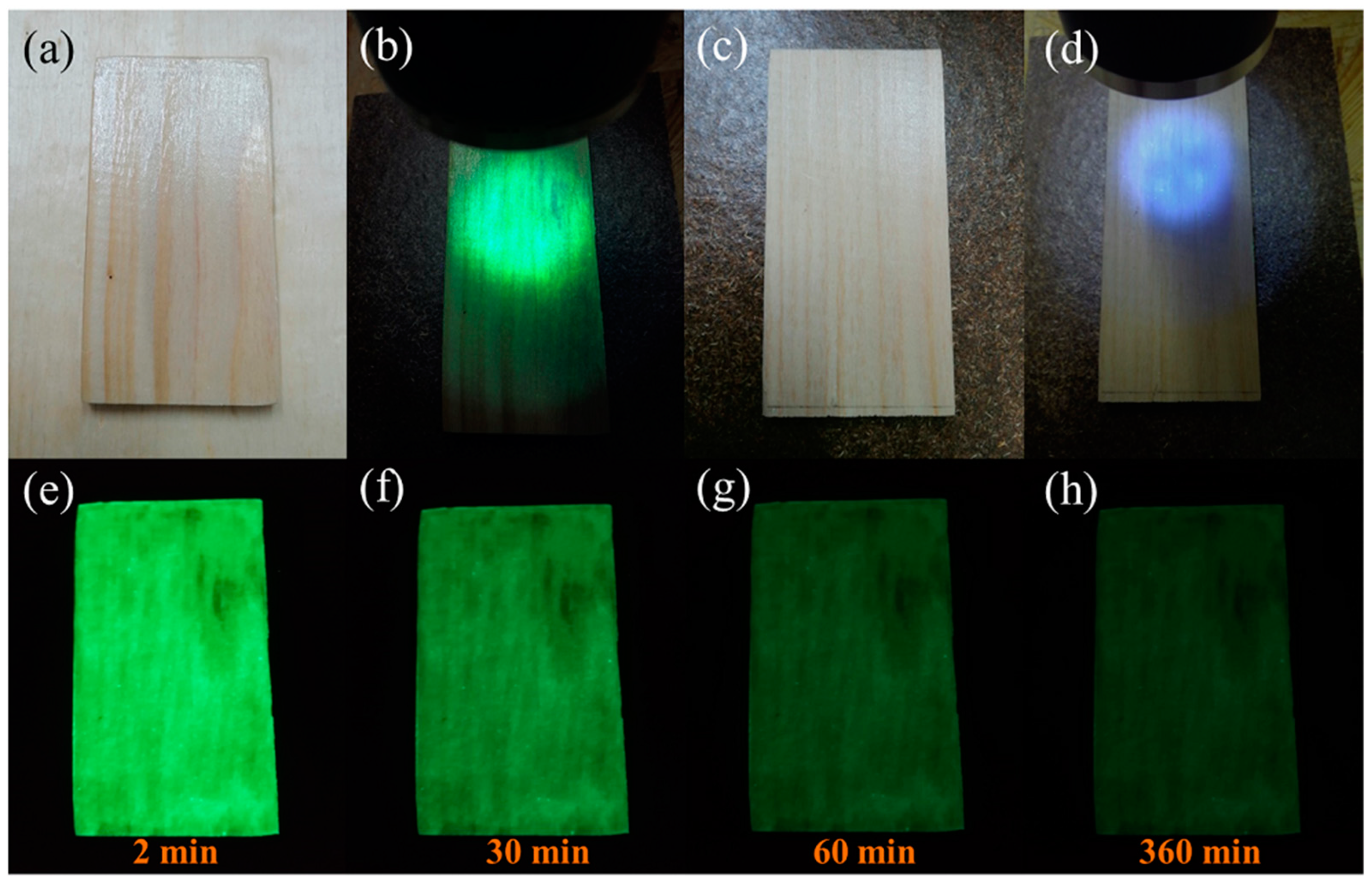
3.3. Morphologies of Wood Coatings with CDs/TiO2@SAO Composite Photocatalyst
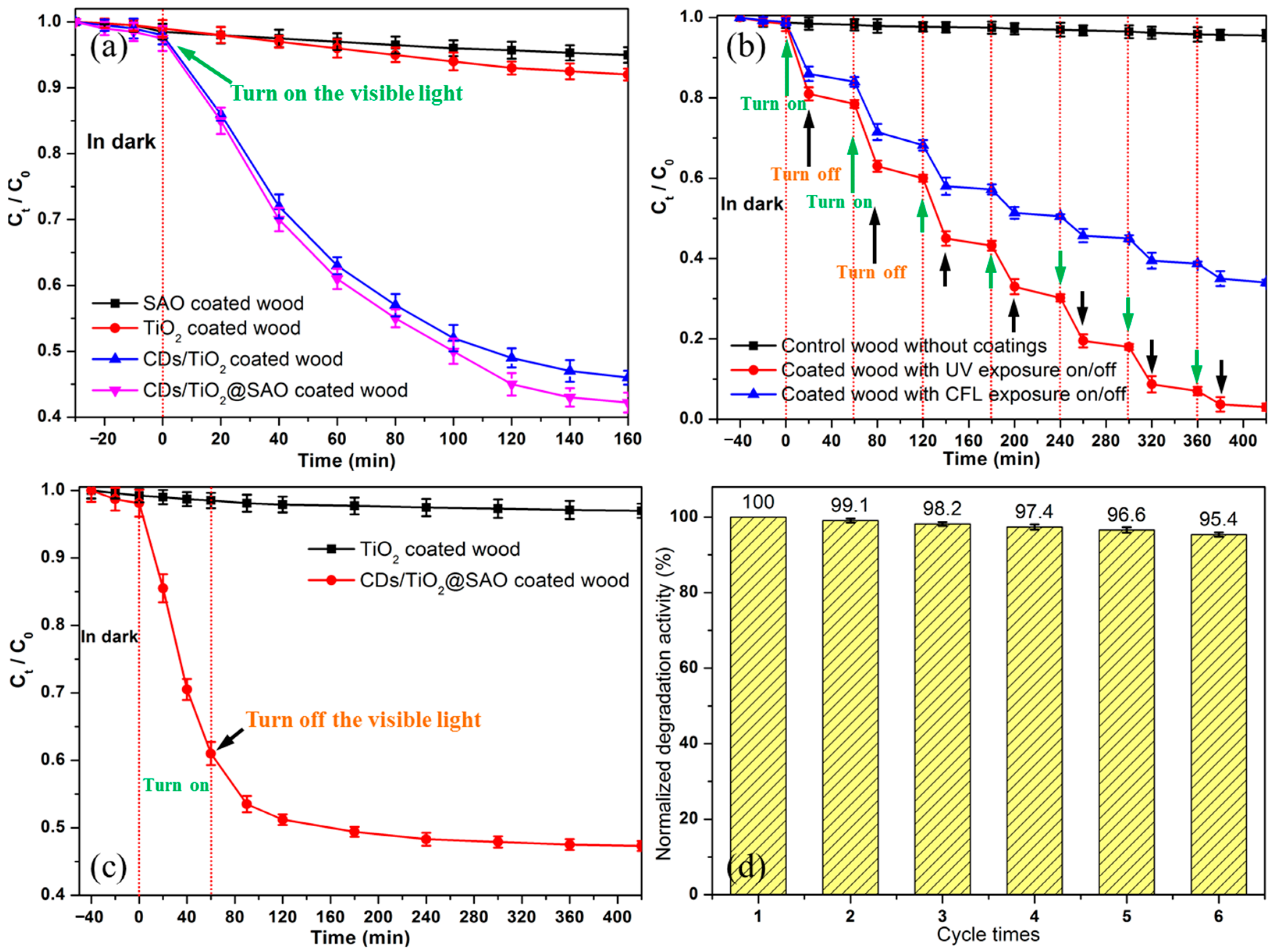
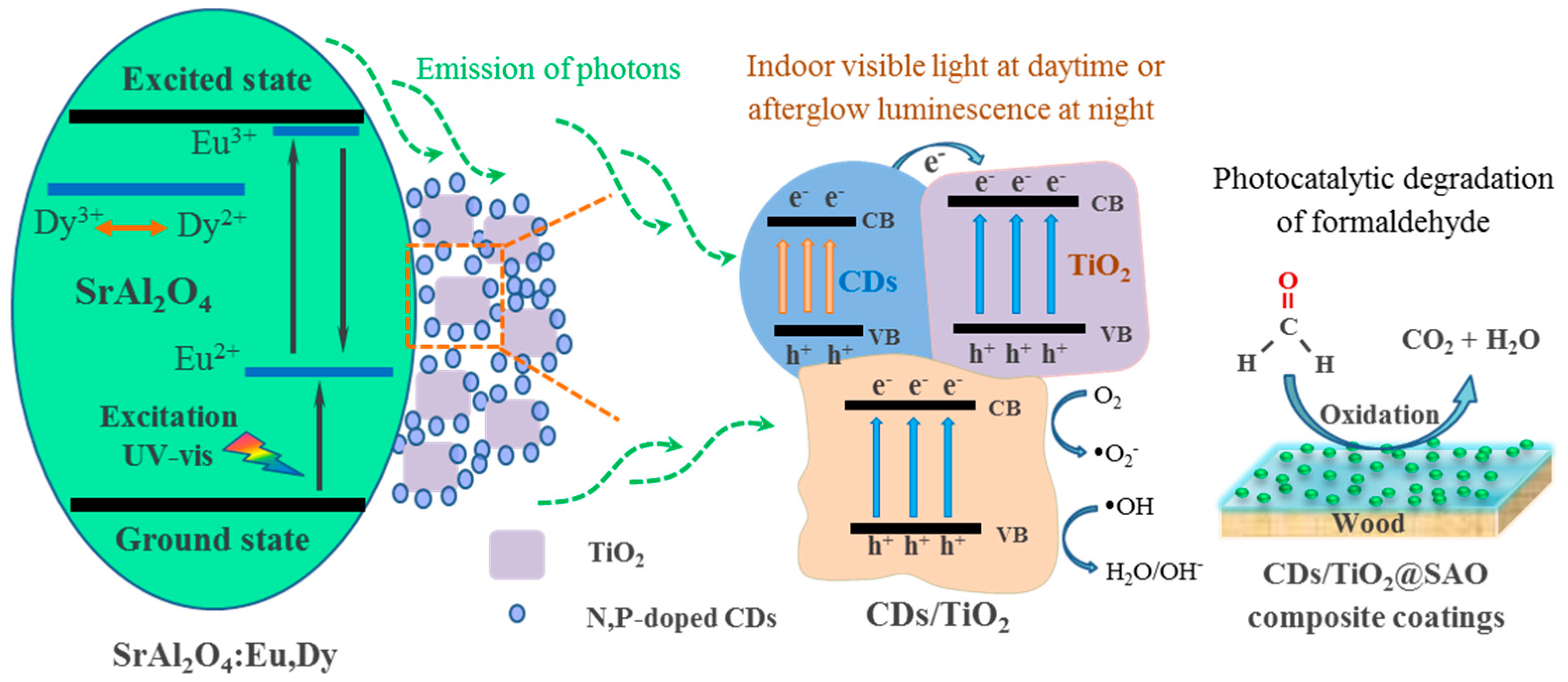
3.4. Photocatalytic Activity and Mechanism of Formaldehyde Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ikeda, K. Variations of formaldehyde and VOC levels during 3 years in new and older homes. Indoor Air 2006, 16, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Shi, G.; Wang, Z.; Rao, Z.; Xiao, W.; Xie, X.; Sun, J. Carbon quantum dots-TiO2 nanocomposite as an efficient photocatalyst for the photodegradation of aromatic ring-containing mixed VOCs: An experimental and DFT studies of adsorption and electronic structure of the interface. J. Hazard. Mater. 2021, 401, 123402. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Kim, J.T. Long-term impact of formaldehyde and VOC emissions from wood-based products on indoor environments and issues with recycled products. Indoor Built Environ. 2012, 21, 137–149. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Wang, L.; Liang, K.; Zhang, Y.; Cao, J. Early-stage emissions of formaldehyde and volatile organic compounds from building materials: Model development, evaluation, and applications. Environ. Sci. Technol. 2022, 56, 14680–14689. [Google Scholar] [CrossRef]
- Wolkoff, P.; Nielsen, G.D. Non-cancer effects of formaldehyde and relevance for setting an indoor air guideline. Environ. Int. 2010, 36, 788–799. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Dong, X.; Wang, Q.; Wang, Y.; Huang, J. The Research on formaldehyde concentration distribution in new decorated residential buildings. Procedia Eng. 2017, 205, 1535–1541. [Google Scholar] [CrossRef]
- Ogawa, M.; Kabe, I.; Terauchi, Y.; Tanaka, S. A strategy for the reduction of formaldehyde concentration in a hospital pathology laboratory. J. Occup. Health 2019, 61, 135–142. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Recent trend in risk assessment of formaldehyde exposures from indoor air. Arch. Toxicol. 2013, 87, 73–98. [Google Scholar] [CrossRef]
- Bouras, D.; Rasheed, M.; Barille, R.; Aldaraji, M.N. Efficiency of adding DD3+ (Li/Mg) composite to plants and their fibers during the process of filtering solutions of toxic organic dyes. Opt. Mater. 2022, 131, 112725. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Ji, Z.; Shao, D.; Sun, P.; Duan, J. High efficiency photocatalytic degradation of indoor formaldehyde with silver-doped ZnO/g-C3N4 composite catalyst under the synergistic effect of silver plasma effect and heterojunction. Opt. Mater. 2021, 111, 110721. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Zhao, R.; Chen, J.; Zhang, F.; Linhardt, R.J.; Zhong, W. Biotechnology progress for removal of indoor gaseous formaldehyde. Appl. Microbiol. Biot. 2020, 104, 3715–3727. [Google Scholar] [CrossRef] [PubMed]
- Moulis, F.; Krýsa, J. Photocatalytic degradation of several VOCs (n-hexane, n-butyl acetate and toluene) on TiO2 layer in a closed-loop reactor. Catal. Today 2013, 209, 153–158. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B-Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Luchechko, A.; Zhydachevskyy, Y.; Ubizskii, S.; Kravets, O.; Popov, A.I.; Rogulis, U.; Elsts, E.; Bulur, E.; Suchocki, A. Afterglow, TL and OSL properties of Mn2+-doped ZnGa2O4 phosphor. Sci. Rep. 2019, 9, 9544. [Google Scholar] [CrossRef]
- Li, J.; Ren, D.; Wu, Z.; Xu, J.; Bao, Y.; He, S.; Chen, Y. Flame retardant and visible light-activated Fe-doped TiO2 thin films anchored to wood surfaces for the photocatalytic degradation of gaseous formaldehyde. J. Colloid Interf. Sci. 2018, 530, 78–87. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Highly visible light active TiO2-xNx heterojunction photocatalysts. Chem. Mater. 2010, 22, 3843–3853. [Google Scholar] [CrossRef]
- Ferrighi, L.; Datteo, M.; Fazio, G.; Di Valentin, C. Catalysis under cover: Enhanced reactivity at the interface between (doped) graphene and anatase TiO2. J. Am. Chem. Soc. 2016, 138, 7365–7376. [Google Scholar] [CrossRef]
- Gan, J.; Wu, Y.; Yang, F.; Zhang, H.; Wu, X.; Wang, Y.; Xu, R. Wood-cellulose photoluminescence material based on carbon quantum dot for light conversion. Carbohyd. Polym. 2022, 290, 119429. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Zhang, Q.; Chmely, S.C.; Wu, Y.; Melcher, C.; Rajan, K.; Harper, D.P.; Wang, S.; Chen, Z. Recycling hot-water extractions of lignocellulosic biomass in bio-refinery for synthesis of carbon nanoparticles with amplified luminescence and its application in temperature sensing. Ind. Crops Prod. 2020, 145, 112066. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Zhang, Q.; Wu, Y.; Melcher, C.; Chmely, S.C.; Chen, Z.; Wang, S. Dual-emitting film with cellulose nanocrystal-assisted carbon dots grafted SrAl2O4,Eu2+,Dy3+ phosphors for temperature sensing. Carbohyd. Polym. 2019, 206, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhao, Y.; Sun, R.C.; Zhong, L.; Peng, X. Facile and high-yield synthesis of carbon quantum dots from biomass-derived carbons at mild condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Chen, Z.; Li, H. Carbon quantum dots modified BiOCl ultrathin nanosheets with enhanced molecular oxygen activation ability for broad spectrum photocatalytic properties and mechanism insight. ACS Appl. Mater. Inter. 2015, 7, 20111–20123. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yuan, Z.; Yang, G.; Tian, Z.; Jiang, Z.; Zhang, K.; Wang, C.; Chen, J. Enhancement of lignin-based carbon quantum dots from poplar pre-hydrolysis liquor on photocatalytic CO2 reduction via TiO2 nanosheets. Ind. Crops Prod. 2021, 160, 113161. [Google Scholar] [CrossRef]
- Martins, N.C.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B-Environ. 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Wang, X.; Wang, Y.; Zeng, Y.; Pui, D.Y.; Sun, J. Visible-light upconversion carbon quantum dots decorated TiO2 for the photodegradation of flowing gaseous acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Chen, Z.; Wang, S. Preparation and characterization of dual-functional coatings of nanofibrillated cellulose and modified SrAl2O4: Eu, Dy phosphors. Surf. Coat. Technol. 2018, 349, 318–327. [Google Scholar] [CrossRef]
- Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A.; Siva Kumar, N.; Elnour, A.Y.; Al-Zahrani, S.M. Strontium aluminate-based long afterglow PP composites: Phosphorescence, thermal, and mechanical characteristics. Polymers 2021, 13, 1373. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Li, Y.; Wu, B.; Luo, X.; Ouyang, S.; Luo, S.; Kheraif, A.A.; Lin, J. A g-C3N4@Au@ SrAl2O4:Eu2+,Dy3+ composite as an efficient plasmonic photocatalyst for round-the-clock environmental purification and hydrogen evolution. J. Mater. Chem. 2019, 7, 19173–19186. [Google Scholar] [CrossRef]
- Sikandar, M.A.; Ahmad, W.; Khan, M.H.; Ali, F.; Waseem, M. Effect of water resistant SiO2 coated SrAl2O4: Eu2+ Dy3+ persistent luminescence phosphor on the properties of Portland cement pastes. Constr. Build. Mater. 2019, 228, 116823. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.G.; Yamamoto, T.; Piasecki, M.; Popov, A.I. First-principles methods as a powerful tool for fundamental and applied research in the field of optical materials. In Phosphor Handbook; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–25. [Google Scholar]
- Zhang, L.; Lyu, S.; Chen, Z.; Wang, S. Fabrication flexible and luminescent nanofibrillated cellulose films with modified SrAl2O4: Eu, Dy phosphors via nanoscale silica and aminosilane. Nanomaterials 2018, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Yu, L.; Li, Z.; Sun, S. Preparation of high-quality biocompatible carbon dots by extraction with new thoughts on the luminescence mechanisms. Nanotechnology 2013, 24, 225601. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Li, Y. Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sensor. Actuat. B-Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Xiao, S.; Zhan, X.; Li, J. A robust superhydrophobic antibacterial Ag–TiO2 composite film immobilized on wood substrate for photodegradation of phenol under visible-light illumination. Ceram. Int. 2016, 42, 2170–2179. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Liu, W.; Gao, J.; Zheng, Y. Confined synthesis of phosphorus, nitrogen co-doped carbon dots with green luminescence and anion recognition performance. Polyhedron 2019, 171, 389–395. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodriguez, M.Á.; Fernandez, J.F. Long lasting phosphors: SrAl2O4: Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Mavengere, S.; Kim, J.S. Photocatalytic properties of g-C3N4–supported on the SrAl2O4: Eu, Dy/SiO2. Coatings 2020, 10, 917. [Google Scholar] [CrossRef]
- Liu, K.; Kong, F.; Zhu, C.; Jiang, G. Photocatalytic activity of phosphorus and nitrogen co-doped carbon quantum dots/TiO2 nanosheets. Nano 2020, 15, 2050151. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.; Liu, M.; Xue, C.; Chang, Q.; Wang, H.; Li, Y.; Song, Z.; Hu, S. Facile synthesis of carbon dots@2D MoS2 heterostructure with enhanced photocatalytic properties. Inorg. Chem. 2019, 58, 5746–5752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, G.; Meng, L.; Zhou, L.; Hu, L.; Nong, J.; Li, Y.; Wang, J.; Hu, K.; Yu, Q. Enhanced photoelectric performance of TiO2 nanotubes sensitized with carbon dots derived from bagasse. Chem. Phys. Lett. 2020, 749, 137428. [Google Scholar] [CrossRef]
- García, C.R.; Oliva, J.; Arroyo, A.; Garcia-Lobato, M.A.; Gomez-Solis, C.; Torres, L.D. Photocatalytic activity of bismuth doped SrAl2O4 ceramic powders. J. Photochem. Photobiol. A 2018, 351, 245–252. [Google Scholar] [CrossRef]
- Zargoosh, K.; Aliabadi, H.M. SrAl2O4: Eu2+: Dy3+/WO3/polyester nanocomposite as a highly efficient and environmentally friendly photocatalyst for removal of dyes from industrial wastes. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100273. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, Y.; Peng, L.; Chen, Z.; Lyu, S.; Wang, S. Self-Luminous Wood Coatings with Carbon Dots/TiO2 Grafted Afterglow SrAl2O4: Eu, Dy Core-Shell Phosphors for Long-Lasting Formaldehyde Removal. Polymers 2023, 15, 2077. https://doi.org/10.3390/polym15092077
Zhang L, Wang Y, Peng L, Chen Z, Lyu S, Wang S. Self-Luminous Wood Coatings with Carbon Dots/TiO2 Grafted Afterglow SrAl2O4: Eu, Dy Core-Shell Phosphors for Long-Lasting Formaldehyde Removal. Polymers. 2023; 15(9):2077. https://doi.org/10.3390/polym15092077
Chicago/Turabian StyleZhang, Longfei, Ying Wang, Limin Peng, Zhilin Chen, Shaoyi Lyu, and Siqun Wang. 2023. "Self-Luminous Wood Coatings with Carbon Dots/TiO2 Grafted Afterglow SrAl2O4: Eu, Dy Core-Shell Phosphors for Long-Lasting Formaldehyde Removal" Polymers 15, no. 9: 2077. https://doi.org/10.3390/polym15092077




