One-Step Bulk-Suspension Polymerization of Polyethylene Glycol-Based Copolymer Microspheres for Phase Change Textiles
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of SMA-g-PEG Microspheres
2.3. Characterization of SMA-g-PEG
2.4. Determination of PEG Grafting Rate
2.5. Process Performance
3. Results and Discussion
3.1. Factors Influencing the Phase Change Properties of SMA-g-PEG
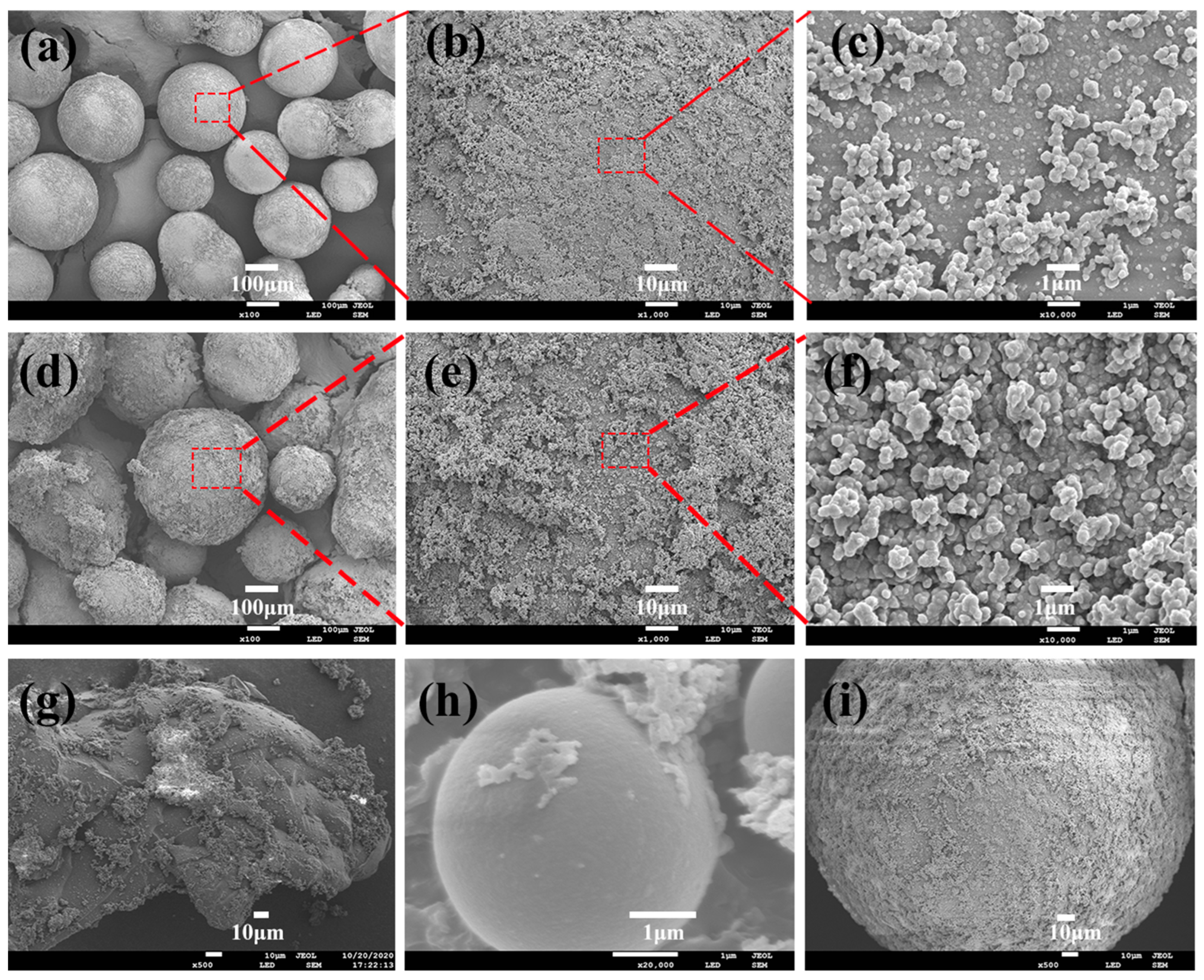
3.2. Structure and Morphology Analysis of SMA-g-PEG Microsphere
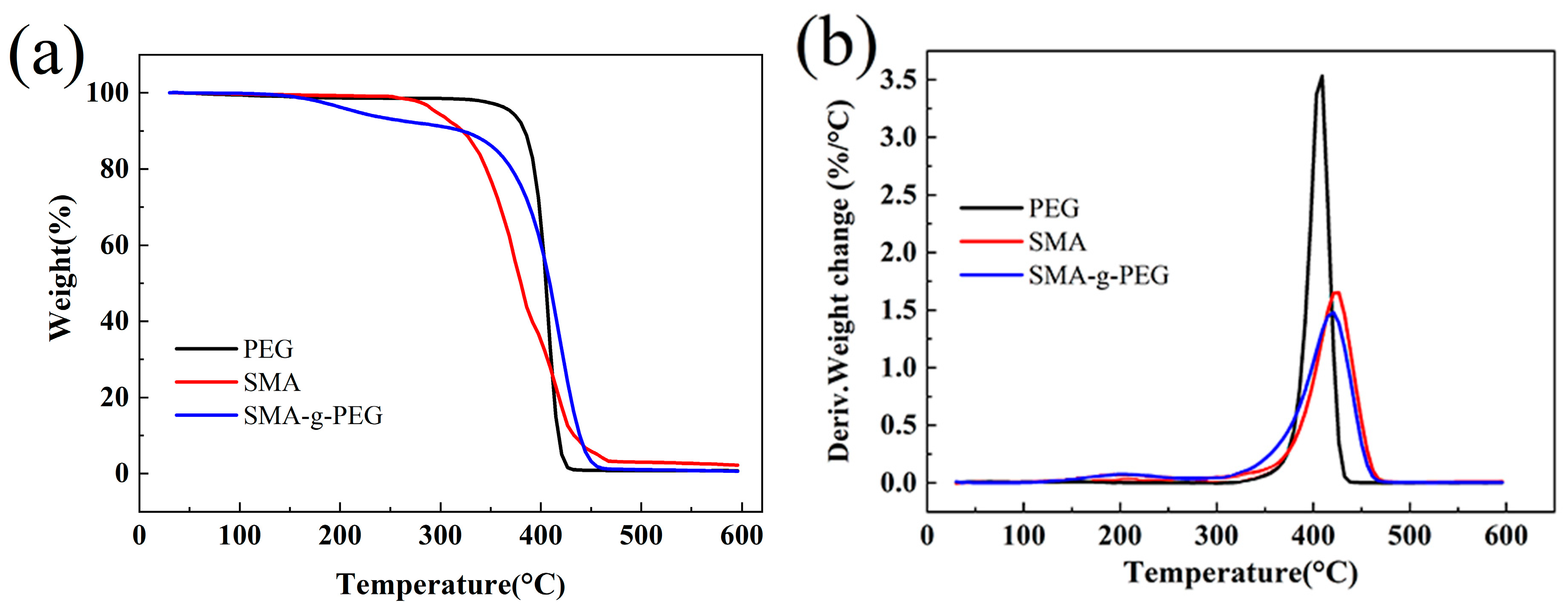
3.3. TGA Analysis of SMA-g-PEG
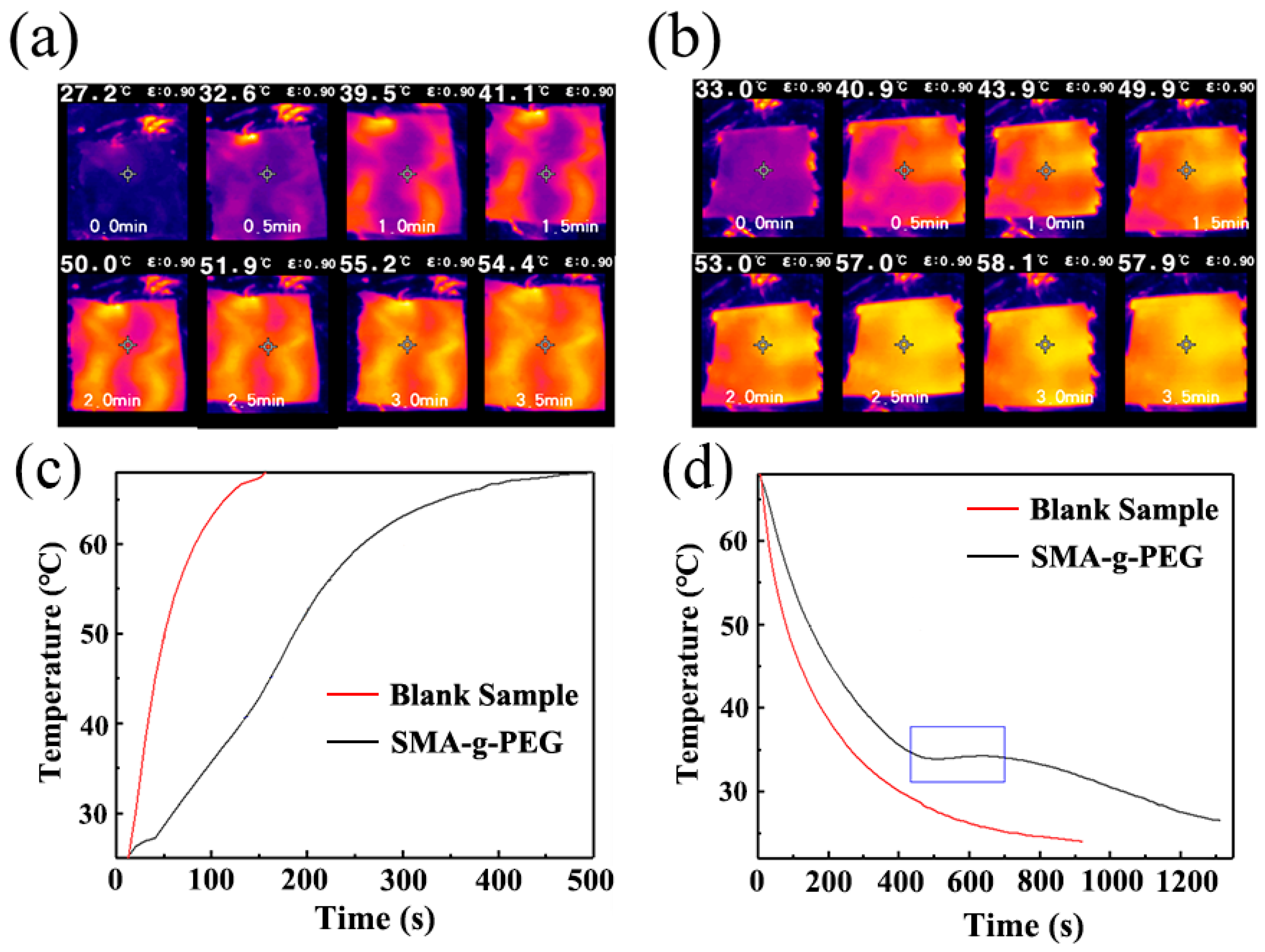
3.4. Thermal Reliability of SMA-g-PEG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEG | polyethylene glycol |
| SMA | polystyrene/maleic anhydride |
| SMA-g-PEG | polystyrene/maleic anhydride grafted polyethylene glycol |
| S-SPCM | solid–solid phase change material |
| PCMs | phase change materials |
| PS | polystyrene |
| MAH | maleic anhydride |
| PVA | poly (vinyl alcohol) |
| AIBN | 2-methylpropionitrile |
| KOH | potassium hydroxide |
References
- Wang, X.; Li, W.; Luo, Z.; Wang, K.; Shah, S.P. A critical review on phase change materials (PCM) for sustainable and energy efficient building: Design, characteristic, performance and application. Energy Build. 2022, 260, 111923. [Google Scholar] [CrossRef]
- Hassan, F.; Jamil, F.; Hussain, A.; Ali, H.M.; Janjua, M.M.; Khushnood, S.; Farhan, M.; Altaf, K.; Said, Z.; Li, C. Recent advancements in latent heat phase change materials and their applications for thermal energy storage and buildings: A state of the art review. Sustain. Energy Technol. Assess. 2021, 49, 101646. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage–A review. Appl. Energy 2018, 235, 846–873. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Yang, K.; Venkataraman, M.; Zhang, X.; Wiener, J.; Zhu, G.; Yao, J.; Militky, J. Review: Incorporation of organic PCMs into textiles. J. Mater. Sci. 2022, 57, 798–847. [Google Scholar] [CrossRef]
- King, M.F.L.; Rao, P.N.; Sivakumar, A.; Mamidi, V.K.; Richard, S.; Vijayakumar, M.; Arunprasath, K.; Kumar, P.M. Thermal performance of a double-glazed window integrated with a phase change material (PCM). Mater. Today Proc. 2021, 50, 1516–1521. [Google Scholar] [CrossRef]
- Malaquias, A.F.; Neves, S.; Campos, J. Incorporation of phase change materials in fire protective clothing considering the presence of water. Int. J. Therm. Sci. 2023, 183, 107870. [Google Scholar] [CrossRef]
- Niles, N.; Vijayarajah, V. A study of the effect of PEG-1000 phase change materials coating on selected fabric properties. J. Ind. Text. 2022, 52. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2018, 147, 841–855. [Google Scholar] [CrossRef]
- Yan, D.; Zhao, S.; Ge, C.; Gao, J.; Gu, C.; Fan, Y. PBT/adipic acid modified PEG solid-solid phase change composites. J. Energy Storage 2022, 52, 104753. [Google Scholar] [CrossRef]
- Deshpande, M.; Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Microwave Assisted Preparation of Poly(ethylene) glycol/Lignin Blends for Thermal Energy Storage. J. Energy Storage 2021, 35, 102338. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Huang, C.; Su, F.; Wu, B.; Sun, Z.; Xu, F.; Sun, L.; Xia, Y.; Peng, H.; et al. Construction of double cross-linking PEG/h-BN@GO polymeric energy-storage composites with high structural stability and excellent thermal performances. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 638, 128193. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Darkwa, J. The micro-/nano-PCMs for thermal energy storage systems: A state of art review. Int. J. Energy Res. 2019, 43, 5572–5620. [Google Scholar] [CrossRef]
- Meng, Y.; Majoinen, J.; Zhao, B.; Rojas, O.J. Form-stable phase change materials from mesoporous balsa after selective removal of lignin. Compos. Part B Eng. 2020, 199, 108296. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Alkan, C. Thermal energy storage properties of polyethylene glycol grafted styrenic copolymer as novel solid-solid phase change materials. Int. J. Energy Res. 2020, 44, 3976–3989. [Google Scholar] [CrossRef]
- Niu, Z.; Qi, S.; Shuaib, S.S.A.; Yuan, W. Flexible, stimuli-responsive and self-cleaning phase change fiber for thermal energy storage and smart textiles. Compos. Part B Eng. 2021, 228, 109431. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Alkan, C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 2017, 161, 219–225. [Google Scholar] [CrossRef]
- Louis, A.; Rebert, E. Making Styrene/Maleic Anhydride Copolymers by Suspension Polymerization. U.S. Patent 4145375, 20 March 1979. [Google Scholar]
- Ikutegbe, C.A.; Farid, M.M. Application of phase change material foam composites in the built environment: A critical review. Renew. Sustain. Energy Rev. 2020, 131, 110008. [Google Scholar] [CrossRef]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Cai, Y.; Wang, R.; Li, T. Form-stable phase change composites: Preparation, performance, and applications for thermal energy conversion, storage and management. Energy Storage Mater. 2021, 42, 380–417. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, J.; Zhang, T.; Xu, L.; Li, Q.; Ren, H.; Shi, Z.; Zhou, F. Performance analysis on the concentrated photovoltaic /thermal air collector with phase change material and vacuum double-glazing for temperature regulation. Renew. Energy 2023, 207, 27–39. [Google Scholar] [CrossRef]
- Xing, C.-M.; Yang, W.-T. A Novel, Facile Method for the Preparation of Uniform, Reactive Maleic Anhydride/Vinyl Acetate Copolymer Micro- and Nanospheres. Macromol. Rapid Commun. 2004, 25, 1568–1574. [Google Scholar] [CrossRef]
- Chen, R.; Yi, C.; Wu, H.; Guo, S. Solid state mechano-chemical grafting copolymerization of hydroxyethyl cellulose with acrylic acid. J. Appl. Polym. Sci. 2009, 112, 3537–3542. [Google Scholar] [CrossRef]
- Sclavons, M.; Franquinet, P.; Carlier, V.; Verfaillie, G.; Fallais, I.; Legras, R.; Laurent, M.; Thyrion, F. Quantification of the maleic anhydride grafted onto polypropylene by chemical and viscosimetric titrations, and FTIR spectroscopy. Polymer 2000, 41, 1989–1999. [Google Scholar] [CrossRef]
- Su, J.-C.; Liu, P.-S. A novel solid–solid phase change heat storage material with polyurethane block copolymer structure. Energy Convers. Manag. 2006, 47, 3185–3191. [Google Scholar] [CrossRef]
- Günther, E.; Mehling, H.; Hiebler, S. Modeling of subcooling and solidification of phase change materials. Model. Simul. Mater. Sci. Eng. 2007, 15, 879. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Hikosaka, M.; Toda, A. Power law of nucleation rate of folded-chain single crystals of polyethylene. Colloid Polym. Sci. 2001, 279, 382–386. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Fu, Y. Melt grafting of maleic anhydride onto polypropylene with assistance of α-methylstyrene. Polym. Eng. Sci. 2011, 52, 814–819. [Google Scholar] [CrossRef]
- Park, B.-D.; Lee, Y.-S. The effect of PEG groups on swelling properties of PEG-grafted-polystyrene resins in various solvents. React. Funct. Polym. 2000, 44, 41–46. [Google Scholar] [CrossRef]
- Rowe, A.A.; Tajvidi, M.; Gardner, D.J. Thermal stability of cellulose nanomaterials and their composites with polyvinyl alcohol (PVA). J. Therm. Anal. Calorim. 2016, 126, 1371–1386. [Google Scholar] [CrossRef]
- Jarm, V.; Bogdanić, G. Investigation of the decomposition of copolymers of styrene and maleic anhydride using thermogravimetric analysis. Thermochim. Acta 1990, 171, 39–47. [Google Scholar] [CrossRef]
- Ansari, J.A.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Ikutegbe, C.A.; Farid, M.M. A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor. Energies 2021, 14, 7867. [Google Scholar] [CrossRef]
- Min, Z.; Wang, Q.; Xie, Y.; Xie, J.; Zhang, B. Influence of polyethylene glycol (PEG) chain on the performance of epoxy asphalt binder and mixture. Constr. Build. Mater. 2020, 272, 121614. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; He, Y. Novel smart textile with ultraviolet shielding and thermo-regulation fabricated via electrospinning. J. Energy Storage 2021, 42, 103094. [Google Scholar] [CrossRef]





| Sample | PS: PEG =mol: mol | Stirring Rate (rpm) | Reaction Time (h) | Reaction Temperature (°C) |
|---|---|---|---|---|
| A | 0.5:1/1:1/2:1 yield (%):45.2/54.1/48.9 | 250 | 6.5 | 70 |
| B | 1:1 | 200/250/300 yield (%):42.9/54.1/10.9 | 6.5 | 70 |
| C | 1:1 | 250 | 5/6/6.5/7/7.5 yield (%):38.6/39.9/54.1/5.9/1.0 | 70 |
| D | 1:1 | 250 | 6.5 | 60/65/70/75/80 yield (%):20.7/36.4/54.1/37.1/20.3 |
| Melting | Solidifying | Yield (%) | Grafting Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | |||
| Sample A: PS: PEG (mol: mol) | ||||||||
| 0.5:1 | 43.9 | 49.7 | 47.3 | 32.4 | 29.1 | 44.5 | 45.2 | 29.5 |
| 1:1 | 42.1 | 48.9 | 79.3 | 29.6 | 24.7 | 76.9 | 54.1 | 52.5 |
| 2:1 | 41.9 | 47.3 | 66.2 | 33.1 | 28.3 | 65.7 | 48.9 | 43.5 |
| Sample B: Stirring rate | ||||||||
| 200 rpm | 38.7 | 47.5 | 54.3 | 29.2 | 25.6 | 54.1 | 42.9 | 35.8 |
| 250 rpm | 42.1 | 48.9 | 79.3 | 29.6 | 24.7 | 76.9 | 54.1 | 52.5 |
| 300 rpm | 53.5 | 56.3 | 6.3 | 31.2 | 27.3 | 6.0 | 10.9 | 4.0 |
| Sample C: Time | ||||||||
| 5 h | 39.3 | 47.0 | 49.3 | 33.7 | 29.6 | 48.7 | 38.6 | 32.2 |
| 6 h | 41.2 | 50.2 | 57.0 | 33.5 | 29.8 | 54.3 | 39.9 | 35.9 |
| 6.5 h | 42.1 | 48.9 | 79.3 | 29.6 | 24.7 | 76.9 | 54.1 | 52.5 |
| 7 h | 41.2 | 45.3 | 10.8 | 28.9 | 25.8 | 10.6 | 5.9 | 7.0 |
| 7.5 h | 42.4 | 46.3 | 4.8 | 29.9 | 26.0 | 4.7 | 1.0 | 3.1 |
| Sample D: Temperature | ||||||||
| 60 °C | 41.3 | 49.9 | 40.0 | 33.9 | 29.5 | 39.7 | 20.7 | 26.3 |
| 65 °C | 43.3 | 48.6 | 50.0 | 34.3 | 29.6 | 49.9 | 36.4 | 33.0 |
| 70 °C | 42.1 | 48.9 | 79.3 | 29.6 | 24.7 | 76.9 | 54.1 | 52.5 |
| 75 °C | 40.3 | 47.2 | 66.4 | 34.2 | 29.5 | 66.3 | 37.1 | 43.9 |
| 80 °C | 42.0 | 47.4 | 24.1 | 30.9 | 27.3 | 23.9 | 20.3 | 15.8 |
| Sample | Melting | Solidifying | ||||
|---|---|---|---|---|---|---|
| Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | Onset Temperature (°C) | Peak Temperature (°C) | Latent Heat (J/g) | |
| PEG | 37.5 | 52.0 | 151.1 | 32.7 | 32.5 | 145.2 |
| SMA | 27.9 | 28.5 | 0.2 | 0 | 0 | 0 |
| SMA-g-PEG | 36.5 | 48.9 | 79.3 | 39.6 | 24.7 | 76.9 |
| SMA-g-PEG after 1000th cycles | 36.2 | 49.6 | 65.7 | 38.9 | 24.5 | 63.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Zeng, J.; Tang, S.; Bai, Z.; Jiang, J.; Zhang, H.; Wang, Y. One-Step Bulk-Suspension Polymerization of Polyethylene Glycol-Based Copolymer Microspheres for Phase Change Textiles. Polymers 2023, 15, 2090. https://doi.org/10.3390/polym15092090
Zhou G, Zeng J, Tang S, Bai Z, Jiang J, Zhang H, Wang Y. One-Step Bulk-Suspension Polymerization of Polyethylene Glycol-Based Copolymer Microspheres for Phase Change Textiles. Polymers. 2023; 15(9):2090. https://doi.org/10.3390/polym15092090
Chicago/Turabian StyleZhou, Guohang, Jiexiang Zeng, Song Tang, Zijian Bai, Jianyu Jiang, Hong Zhang, and Yan Wang. 2023. "One-Step Bulk-Suspension Polymerization of Polyethylene Glycol-Based Copolymer Microspheres for Phase Change Textiles" Polymers 15, no. 9: 2090. https://doi.org/10.3390/polym15092090




