Part B: Improvement of the Optical Properties of Cellulose Nanocrystals Reinforced Thermoplastic Starch Bio-Composite Films by Ex Situ Incorporation of Green Silver Nanoparticles from Chaetomorpha linum
Abstract
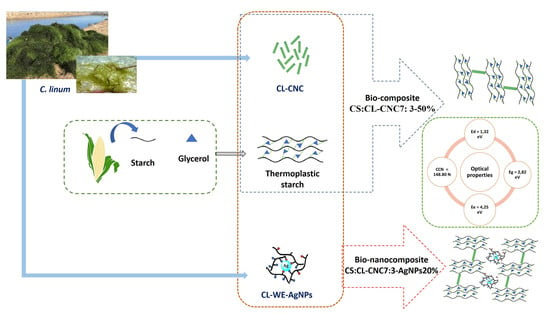
:1. Introduction
2. Experimental Work
2.1. Materials
2.1.1. Colloidal Silver Nanoparticle (AgNP) Synthesis
2.1.2. Development of Optical Bio-Nanocomposite Films
2.2. Characterization Methods
2.2.1. UV-Visible Analysis
2.2.2. FTIR Analysis
2.2.3. SEM and TEM Analyses
3. Results and Discussion
3.1. Impact of AgNPs Density on Optical Responses of Bio-Nanocomposite Films
3.1.1. Synthesis of Colloid Silver Nanoparticles (AgNPs)
3.1.2. Morphological Analysis of Synthesized AgNP and Its By-Products
3.1.3. FTIR Analysis of Synthesized AgNPs and Its By-Products
3.2. Characterization of Bio-Nanocomposite Films
3.2.1. Morphological Analysis
3.2.2. FTIR Analysis
3.3. Optical Properties of the Synthesized Bio-Nanocomposites Films: The Ability to Protect Films against UV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Ismadi; Damayanti, R.; Subiyanto, B.; Fatriasari, W.; Fudholi, A. A review on natural fibers for development of eco-friendly bio-composite: Characteristics, and utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Philip, C.S.; Nivetha, A.; Sakthivel, C.; Veena, C.G.; Prabha, I. Novel fabrication of cellulose sprinkled crystalline nanocomposites using economical fibrous sources: High performance, compatible catalytic and electrochemical properties. Microporous Mesoporous Mater. 2021, 318, 111021. [Google Scholar] [CrossRef]
- Irfan, M.; Qurashi, A.; Alam, M.W. Metal oxide nanostructures and nanocomposites for selective catalytic reduction of NOx: A review. Arab. J. Sci. Eng. 2010, 35, 79–92. [Google Scholar]
- Hassan, I.; Ai-Jawhari, H. Polymer Nanocomposite Matrices; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.; Ishak, M.; Zainudin, E.S.; Mahamud, A. Nanocellulose Reinforced Starch Polymer Composites: A Review of Preparation, Properties and Application. In Proceedings of the 5th International Conference on Applied Sciences and Engineering Application (ICASEA 2018), Cameron Highlands, Malaysia, 7–8 April 2018. [Google Scholar]
- Alsufyani, T.; M’sakni, N.H. Part A: Biodegradable Bio-Composite Film Reinforced with Cellulose Nanocrystals from Chaetomorpha linum into Thermoplastic Starch Matrices. Polymers 2023, 15, 1542. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.; Hall, W.P.; Lyandres, O.; Shah, N.; Zhao, J.; Duyne, R. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.; Starsich, F.; Dasargyri, A.; Wurnig, M.; Krumeich, F.; Boss, A.; Leroux, J.-C.; Pratsinis, S. Photothermal Killing of Cancer Cells by the Controlled Plasmonic Coupling of Silica-Coated Au/Fe2O3 Nanoaggregates. Adv. Funct. Mater. 2014, 24, 2818–2827. [Google Scholar] [CrossRef]
- Capadona, L.; Vinson, A.; Bartko, A.; Dickson, R. Photoactivated Fluorescence from Individual Silver Nanoclusters. Science 2001, 291, 103–106. [Google Scholar] [CrossRef]
- Marco, N.; Kristy, V.; Alison, C.; Muhsen, A.; Jinzhang, L.; Nunzio, M. Plasmonic effect of gold nanoparticles in organic solar cells. Sol. Energy 2014, 106, 23–37. [Google Scholar] [CrossRef]
- Sebastian, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. 2014, 53, 4756–4795. [Google Scholar]
- Heiz, U.; Landman, U. Nanocatalysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Guo, X.; Yan, L.; Lincoln, S.F. The formation and catalytic activity of silver nanoparticles in aqueous polyacrylate solutions. Front. Chem. Sci. Eng. 2016, 10, 432–439. [Google Scholar] [CrossRef]
- Kochylas, I.; Gardelis, S.; Likodimos, V.; Giannakopoulos, K.P.; Falaras, P.; Nassiopoulou, A.G. Improved Surface-Enhanced-Raman Scattering Sensitivity Using Si Nanowires/Silver Nanostructures by a Single Step Metal-Assisted Chemical Etching. Nanomaterials 2021, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Ong, B.S. Facile synthesis of silver nanoparticles useful for fabrication of high-conductivity elements for printed electronics. J. Am. Chem. Soc. 2005, 127, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castañon, G.-A.; Nino, N.; Martínez-Gutiérrez, F.; Martinez, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Kumar, A.; Madhu, G.; John, E.; Kuttinarayanan, S.; Nair, S.K. Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey. Green Process. Synth. 2020, 9, 268–274. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Singh, M. Green synthesis of silver nanoparticles: Methods, biological applications, delivery and toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61–94. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Xiaoyi, L.; Shaoqin, Z.; Horváth, P.G.; Bak, M.; Bejó, L.; Sipos, G.; Alpár, T. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023—A review on game changing materials. Heliyon 2022, 8, e12322. [Google Scholar] [CrossRef] [PubMed]
- Monowar, T.; Rahman, M.; Bhore, S.; Raju, G.; Sathasivam, K. Silver Nanoparticles Synthesized by Using the Endophytic Bacterium Pantoea ananatis are Promising Antimicrobial Agents against Multidrug Resistant Bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Islam, R.U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 2021, 11, 2625–2660. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Pandey, P.P. Preparation and Characterization of Polymer Nanocomposites. Soft Nanosci. Lett. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Bertrandie, J.; Han, J.; De Castro, C.; Yengel, E.; Gorenflot, J.; Anthopoulos, T.; Laquai, F.; Sharma, A.; Baran, D. The Energy Level Conundrum of Organic Semiconductors in Solar Cells. Adv. Mater. 2022, 34, e2202575. [Google Scholar] [CrossRef]
- Geoghegan, M.A.; Hadziioannou, G. Polymer Electronics; Oxford University Press: Oxford, UK, 2013; p. 256. [Google Scholar] [CrossRef]
- M’sakni, N.H.; Zayane, H.; Majdoub, H.; Morvan, C.; Roudesli, S.; Deratani, A. Extraction and characterization of polysaccharides from Mesembryanthemum crystallinum. e-Polymers 2005, 5, 63. [Google Scholar] [CrossRef]
- Virender, K.S.; Ria, A.Y.; Yekaterina, L. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- ElMetwally, M.A.; Amr, M.A.; Shalabya, I.B.; Mohamed, A.M. Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J. Mater. Res. Technol. 2018, 7, 419–431. [Google Scholar] [CrossRef]
- Soliman, T.; Abouhaswa, A. Synthesis and structural of Cd0.5Zn0.5F2O4 nanoparticles and its influence on the structure and optical properties of polyvinyl alcohol films. J. Mater. Sci. Mater. Electron. 2020, 31, 9666–9674. [Google Scholar] [CrossRef]
- Zeyada, H.; Elshabaan, M. Gamma-ray irradiation induced structural and optical constants changes of thermally evaporated neutral red thin films. J. Mater. Sci. 2012, 47, 493–502. [Google Scholar] [CrossRef]
- Ledinský, M.; Schönfeldová, T.; Holovský, J.; Aydin, E.; Hájková, Z.; Landová, L.; Neykova, N.; Fejfar, A.; De Wolf, S. Temperature Dependence of the Urbach Energy in Lead Iodide Perovskites. J. Phys. Chem. Lett. 2019, 10, 1368–1373. [Google Scholar] [CrossRef]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Abdelhamied, M.M.; Atta, A.K.; Abdelreheem, A.; Farag, A.T.M.; El Okr, M. Synthesis and Optical Properties of PVA/PANI/Ag Nanocomposite films. J. Mater. Sci. Mater. Electron. 2020, 31, 22629–22641. [Google Scholar] [CrossRef]
- Abdel Reheem, A.; Atta, A.; Afify, T. Optical and electrical properties of argon ion beam irradiated PVA/Ag nanocomposites. Surf. Rev. Lett. 2016, 24, 1750038. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Mohamed, L.; Abraheem, M.S.; Mansour, M.M.A.; Sherif, A.M. Biofabrication of Silver Nanoparticles Using Teucrium Apollinis Extract: Characterization, Stability, and Their Antibacterial Activities. Chemistry 2023, 5, 54–64. [Google Scholar] [CrossRef]
- Kocadağ Kocazorbaz, E. Green Synthesis, Optimization, and Characterization of Silver Nanoparticles from Euphorbia rigida Leaf Extract and Investigation of Their Antimicrobal Activities. Bilecik Şeyh Edebali Üniv. Fen Bilim. Derg. 2021, 8, 512–522. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Shakeel, A.; Saifullah; Mudasir, A.; Babu Lal, S.; Saiqa, I. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Kambala, V.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Mulenos, M.R.; Lujan, H.; Pitts, L.K.R.; Sayes, C.M. Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials 2020, 10, 1953. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Voss, C.; Rothen-Rutishauser, B.; Balog, S.; Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Chupin, G.; Bon, C.; Yannis, C.; Jonathan, P. Synthèse et détermination de la taille de nanoprismes d’argent. Bull. De L’union Des Phys. 2016, 110, 1339–1368. [Google Scholar]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Huipeng, Y.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Pawar, H.A.; Lalitha, K.G. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. 2014, 65, 167–175. [Google Scholar] [CrossRef]
- Subrhamanian, H.; Suriyamoorthy, P.; Rajasekar, D. Fourier transform infra-red spectroscopy analysis of Erythrina variegata L. J. Pharm. Sci. Res. 2017, 9, 2062–2067. [Google Scholar]
- Charles, E.; Lakshmi, P.K.; Selvaraj, M.; Vaidyanathan, S.; Srisudha, S.; Mary, M.B. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 224, 117382. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’Sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Kannan, R.; Arumugam, R.; Ramya, D.; Manivannan, D.K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl. Nanosci. 2012, 3, 229–233. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef]
- Zafar, S.; Zafar, A. Biosynthesis and Characterization of Silver Nanoparticles Using Phoenix dactylifera Fruits Extract and their In Vitro Antimicrobial and Cytotoxic Effects. Open Biotechnol. J. 2019, 13, 37–46. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Z.; Huang, G.; Cheng, H.; Han, L.; Zhao, S.; Chen, Y.; Meng, F. Purifying water with silver nanoparticles (AgNPs)-incorporated membranes: Recent advancements and critical challenges. Water Res. 2022, 222, 118901. [Google Scholar] [CrossRef] [PubMed]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A.; Mahardika, M.; Hafizulhaq, F. Characterization of the sonicated yam bean starch bionanocomposites reinforced by nanocellulose water hyacinth fiber (WHF): The effect of various fiber loading. J. Eng. Sci. Technol. 2018, 13, 2700–2715. [Google Scholar]
- Abral, H.; Hartono, J. Moisture absorption of starch based biocomposites reinforced with water hyacinth fibers. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012035. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Sreekala, M.S.; Kunaver, M.; Huskić, M.; Thomas, S. Morphology, transport characteristics and viscoelastic polymer chain confinement in nanocomposites based on thermoplastic potato starch and cellulose nanofibers from pineapple leaf. Carbohydr. Polym. 2017, 169, 176–188. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Lubis, M.; Harahap, M.; Ginting, M.H.S.; Sartika, M.; Azmi, H. Production of bioplastic from avocado seed starch reinforced with microcrystalline cellulose from sugar palm fibers. J. Eng. Sci. Technol. 2018, 13, 381–393. [Google Scholar]
- Jutarat, P.; Sudarat, C.; Suwat, M.; Areeya, H. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Kaewta, K.; Jariya, T. Studies on the structure and properties of thermoplastic starch/luffa fiber composites. Mater. Des. 2012, 40, 314–318. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, H.; Gao, M.; Zhang, M.; Liu, P.; Liu, X. Cellulose nanocrystals/silver nanoparticles: In-situ preparation and application in PVA films. Holzforschung 2020, 74, 523–528. [Google Scholar] [CrossRef]
- Nasher, M.A.; Youssif, M.I.; El-Ghamaz, N.A.; Zeyada, H.M. Structural, optical and electrical studies of Toluidine Blue thin films prepared by thermal evaporation technique. J. Lumin. 2018, 204, 428–435. [Google Scholar] [CrossRef]
- Davydov, A.S.; Dresner, S.B.; Knox, R.S. Theory of Molecular Excitons. Phys. Today 1972, 25, 55–57. [Google Scholar] [CrossRef]
- Buruiana, L.; Avram, E.; Popa, A.; Musteata, V.; Ioan, S. Electrical conductivity and optical properties of a new quaternized polysulfone. Polym. Bull. 2012, 68, 1641–1661. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.; Saber, D.; Rasheed, M.; Ahmed, H. Investigation of Metallic Silver Nanoparticles through UV-Vis and Optical Micrograph Techniques. Int. J. Electrochem. Sci. 2017, 12, 363–373. [Google Scholar] [CrossRef]
- Atta, A.; Abdel Reheem, A.; Abdeltwab, E. Ion Beam Irradiation Effects on Surface Morphology and Optical Properties of ZnO/PVA Composites. Surf. Rev. Lett. 2020, 27, 1950214. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.; Abdel Reheem, A.; Berber, M. Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers 2021, 13, 1225. [Google Scholar] [CrossRef]
- Abdulwahid, R.; Abdullah, O.; Aziz, S.B.; Hussen, S.; Muhammadsharif, F.; Yahya, M.Y. The study of structural and optical properties of PVA:PbO2 based solid polymer nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 12112–12118. [Google Scholar] [CrossRef]
- Isac, D.J. Urbach and Bandgap Energy along with Optical Constants Analysis of nanocrystalline LZYBCCO Ceramics. IJournals Int. J. Soc. Relev. Concern (IJSRC) 2015, 3, 1–11. [Google Scholar]
- Khairy, Y.; Yahia, I.S.; Elhosiny Ali, H. Facile synthesis, structure analysis and optical performance of manganese oxide-doped PVA nanocomposite for optoelectronic and optical cut-off laser devices. J. Mater. Sci. Mater. Electron. 2020, 31, 8072–8085. [Google Scholar] [CrossRef]
- Omed Gh, A.; Shujahadeen, B.A.; Mariwan, A.R. Structural and optical characterization of PVA:KMnO4 based solid polymer electrolyte. Results Phys. 2016, 6, 1103–1108. [Google Scholar] [CrossRef]
- Dejam, L.; Sabbaghzadeh, J.; Ghaderi, A.; Solaymani, S.; Matos, R.S.; Țălu, Ș.; da Fonseca Filho, H.D.; Sari, A.H.; Kiani, H.; Shayegan, A.H.S.; et al. Advanced nano-texture, optical bandgap, and Urbach energy analysis of NiO/Si heterojunctions. Sci. Rep. 2023, 13, 6518. [Google Scholar] [CrossRef]
- Awad, S.; El-Gamal, S.; El Sayed, A.M.; Abdel-Hady, E.E. Characterization, optical, and nanoscale free volume properties of Na-CMC/PAM/CNT nanocomposites. Polym. Adv. Technol. 2020, 31, 114–125. [Google Scholar] [CrossRef]
- Katerina Chryssou, M.S.a.E.L. A Study of the Optical Bandgap Energy and Urbach Energy Tail of Two White A4 Copy Paper Samples. Ann. Chem. Sci. Res. 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Abdul-Kader, A.M.; Turos, A.; Jagielski, J.; Nowicki, L.; Ratajczak, R.; Stonert, A.; AlMa’adeed, M. Hydrogen release in UHMWPE upon He-ion bombardment. Vacuum 2005, 78, 281–284. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.; Naqvi, A.; Virk, H.; De, U.; Avasthi, D.; Prasad, R. Study of optical band gap and carbon cluster sizes formed in 100 MeV Si8+ and 145 MeV Ne6+ ions irradiated polypropylene polymer. Indian J. Phys. 2009, 83, 969–976. [Google Scholar] [CrossRef]
- Fink, D.; Müller, M.; Chadderton, L.T.; Cannington, P.H.; Elliman, R.G.; McDonald, D.C. Optically absorbing layers on ion beam modified polymers: A study of their evolution and properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1988, 32, 125–130. [Google Scholar] [CrossRef]










| Sample Code | CL-CNC (g) | % NaOH (mL) | CS (g) | Distilled Water (mL) | Glycerol (mL) | CL-WE-AgNPs (mL) |
|---|---|---|---|---|---|---|
| CS:CL-CNC7: 3–50% [6] | 3.00 | 40 | 7.00 | 140 | 90.0 | - |
| CS:CL-CNC7:3-AgNPs 5% | 2.85 | 38 | 6.65 | 133 | 85.5 | 9 |
| CS:CL-CNC7:3-AgNPs 10%, | 2.70 | 36 | 6.30 | 126 | 81.0 | 18 |
| CS:CL-CNC7:3-AgNPs 15% | 2.55 | 34 | 5.95 | 119 | 76.5 | 27 |
| CS:CL-CNC7:3-AgNPs 20% | 2.40 | 32 | 5.60 | 112 | 72.0 | 36 |
| CS:CL-CNC7:3-AgNPs 40% | 1.80 | 24 | 4.20 | 84 | 54 | 72 |
| CS:CL-CNC7:3-AgNPs 50% | 1.50 | 20 | 3.50 | 70 | 45 | 90 |
| Absorption Edge (Ed) (eV) | Optical Bandgap (Eg) (eV) | Urbach Energy (Ee) (eV) | Carbon Cluster Number (N) | |
|---|---|---|---|---|
| CS:CL-CNC7:3–50% [6] | 2.47 | 3.12 | 6.89 | 121.56 |
| CS:CL-CNC-AgNPs 5% | 1.51 | 2.91 | 5.41 | 139.74 |
| CS:CL-CNC-AgNPs 10% | 1.44 | 2.88 | 5.21 | 142.67 |
| CS:CL-CNC-AgNPs 20% | 1.32 | 2.82 | 4.25 | 148.80 |
| CS:CL-CNC-AgNPs 40% | 1.20 | 2.76 | 3.65 | 155.35 |
| CS:CL-CNC-AgNPs 50% | 1.08 | 2.58 | 3.62 | 177.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’sakni, N.H.; Alsufyani, T. Part B: Improvement of the Optical Properties of Cellulose Nanocrystals Reinforced Thermoplastic Starch Bio-Composite Films by Ex Situ Incorporation of Green Silver Nanoparticles from Chaetomorpha linum. Polymers 2023, 15, 2148. https://doi.org/10.3390/polym15092148
M’sakni NH, Alsufyani T. Part B: Improvement of the Optical Properties of Cellulose Nanocrystals Reinforced Thermoplastic Starch Bio-Composite Films by Ex Situ Incorporation of Green Silver Nanoparticles from Chaetomorpha linum. Polymers. 2023; 15(9):2148. https://doi.org/10.3390/polym15092148
Chicago/Turabian StyleM’sakni, Nour Houda, and Taghreed Alsufyani. 2023. "Part B: Improvement of the Optical Properties of Cellulose Nanocrystals Reinforced Thermoplastic Starch Bio-Composite Films by Ex Situ Incorporation of Green Silver Nanoparticles from Chaetomorpha linum" Polymers 15, no. 9: 2148. https://doi.org/10.3390/polym15092148