Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Choline Acrylate
2.2.2. Measurement of Solvent Density
2.2.3. Preparation of Dispersions and Regeneration of Cellulose
2.2.4. Rheological Studies
2.2.5. Wide-Angle X-ray Diffraction Study
2.2.6. Microscopic Investigation
2.2.7. Application of ChA/Water for 3D Printing with BC
2.2.8. MD Simulations and Model Systems
2.2.9. Mechanical Measurements
3. Results and Discussion
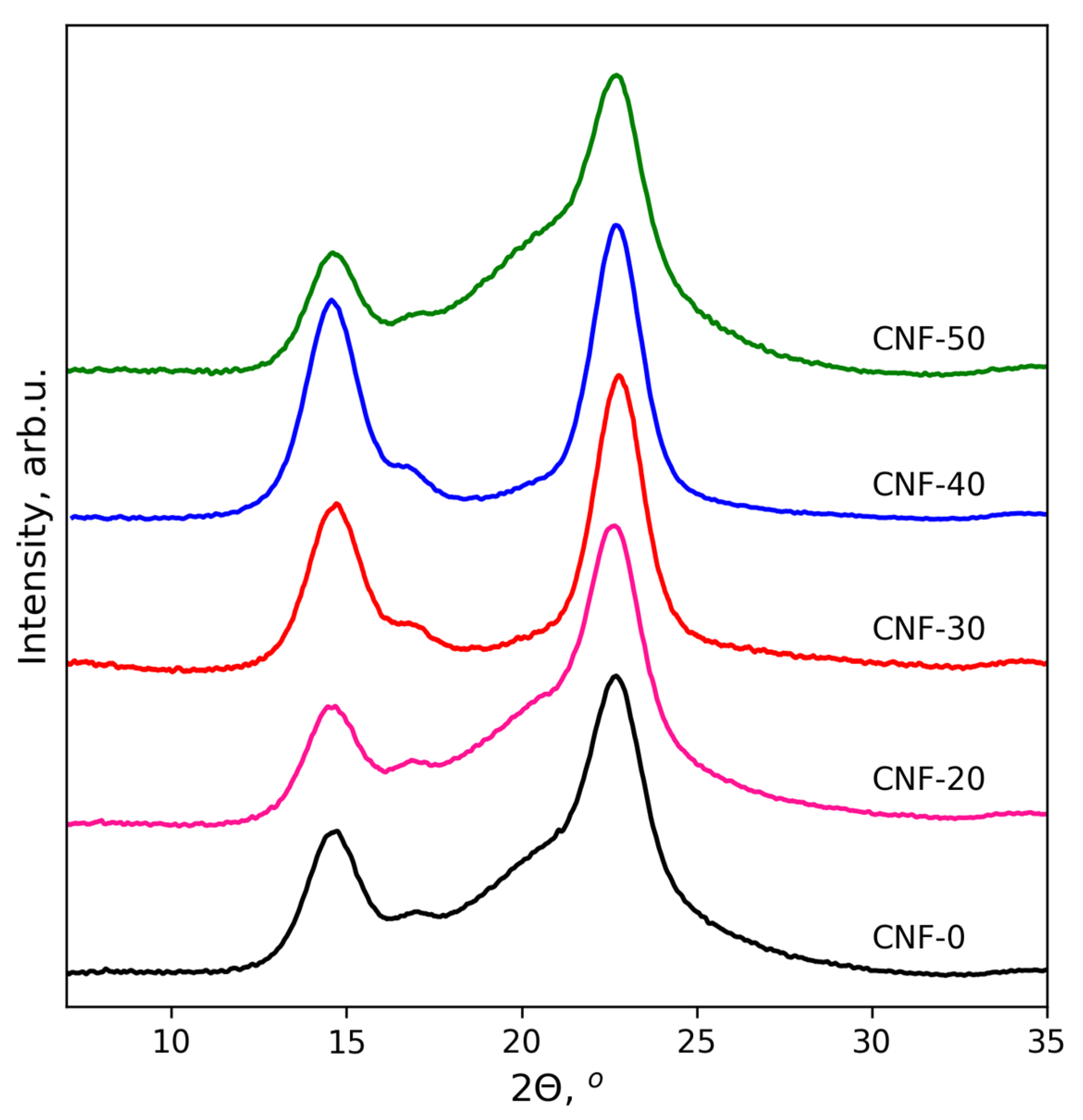
3.1. Wide-Angle X-ray Diffraction Study
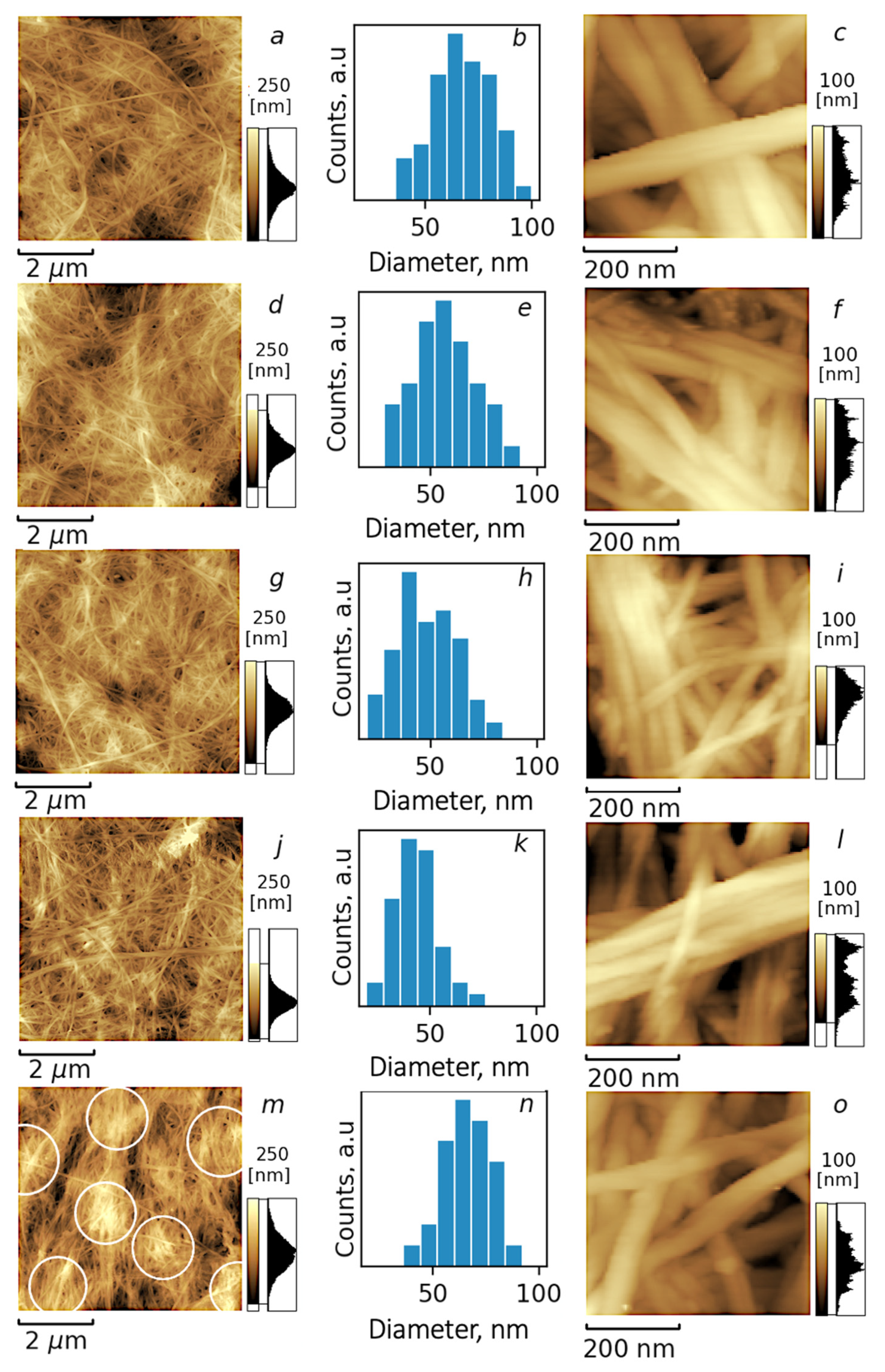
3.2. Microscopic Investigation
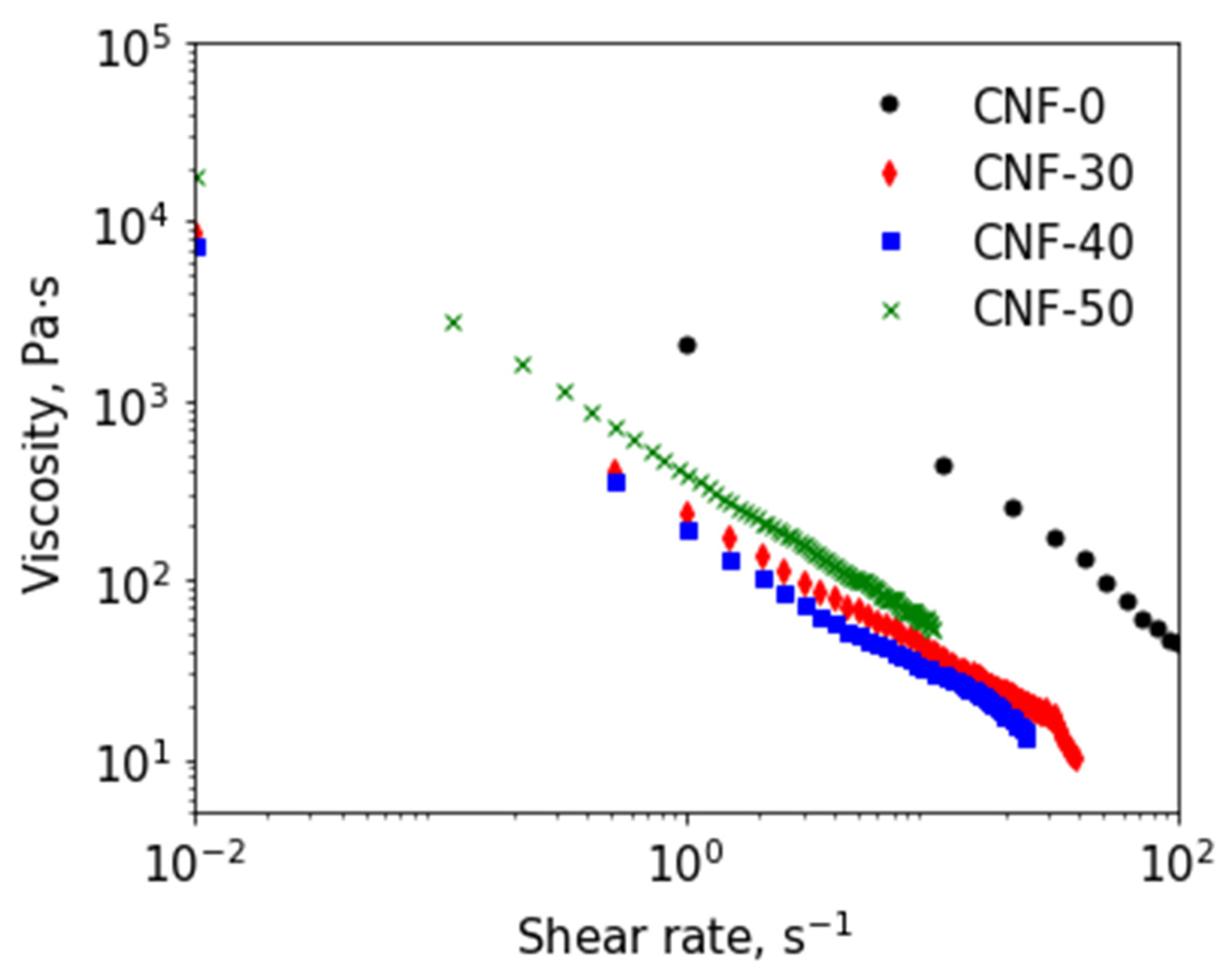
3.3. Rheological Properties of BC Nanofiber Dispersions in ChA/Water
3.4. Molecular Dynamics Simulations
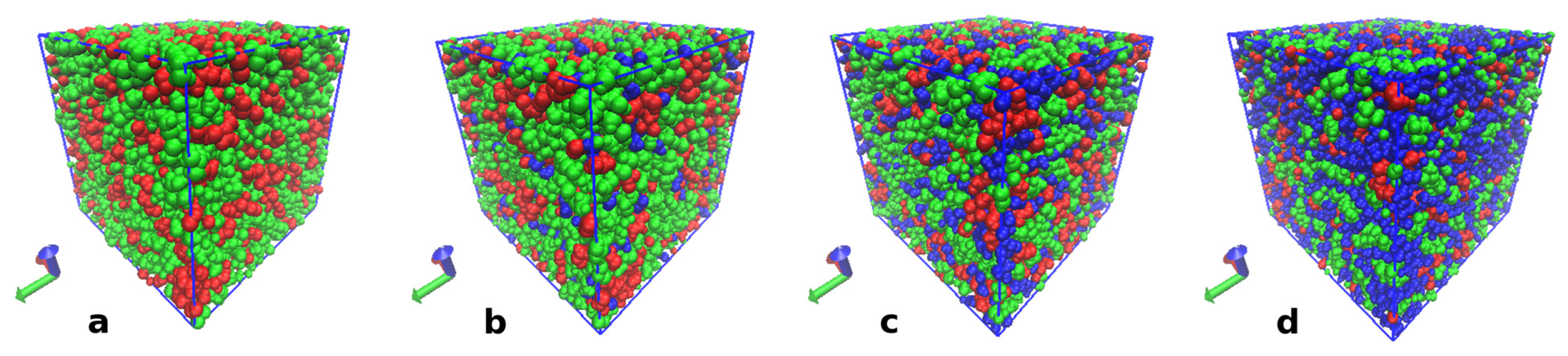
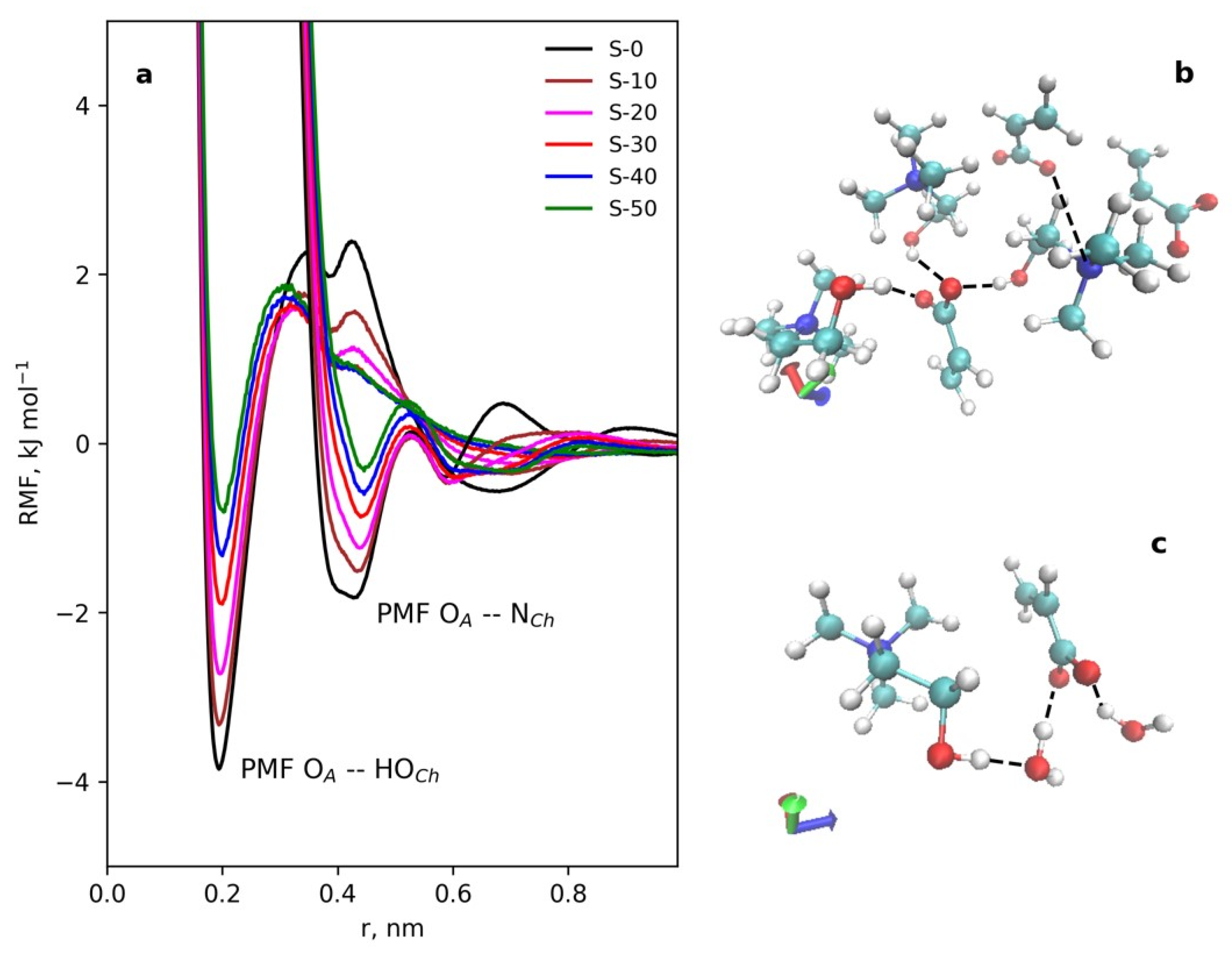
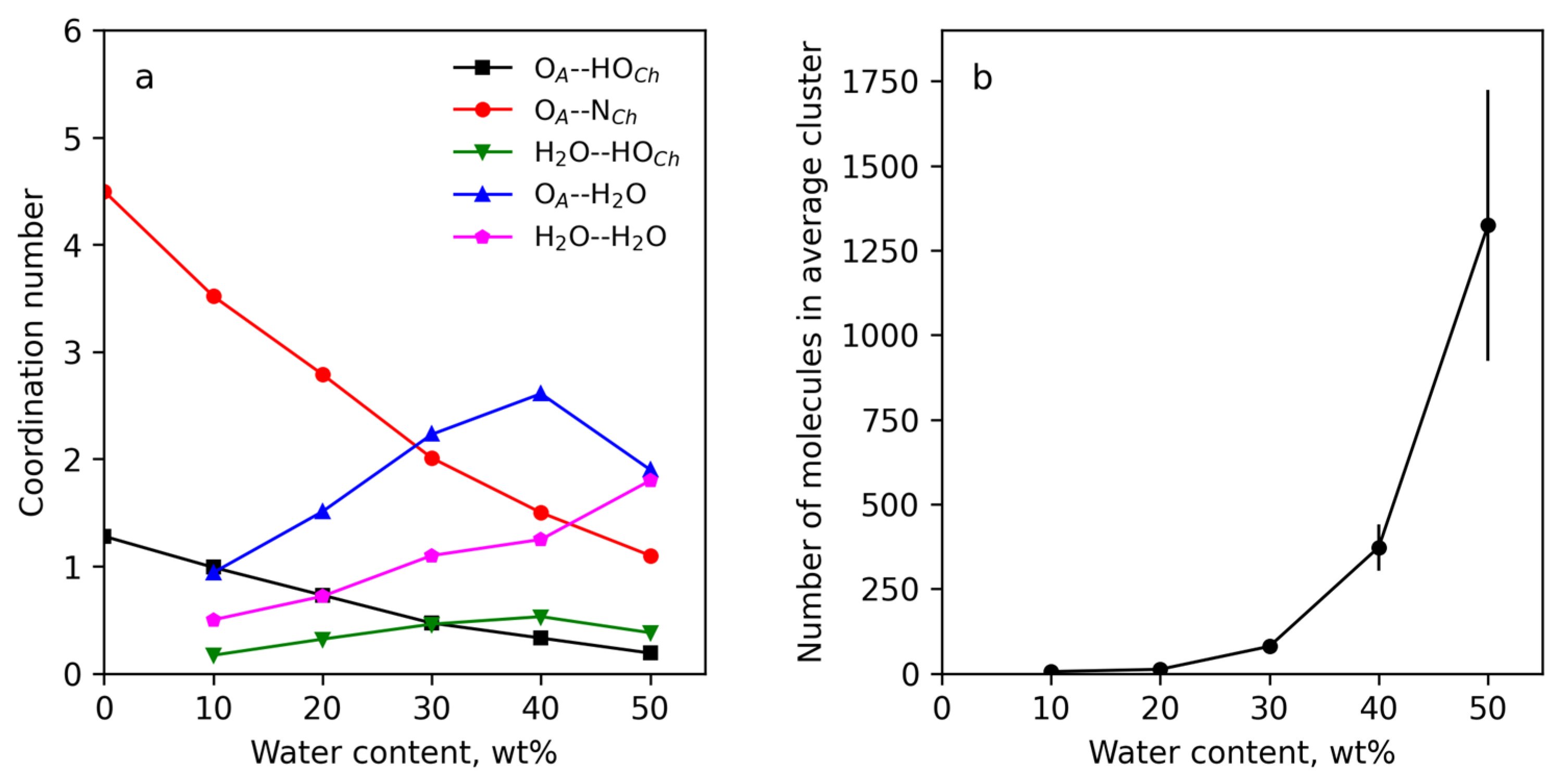
3.4.1. IL Structure and Effect of Water
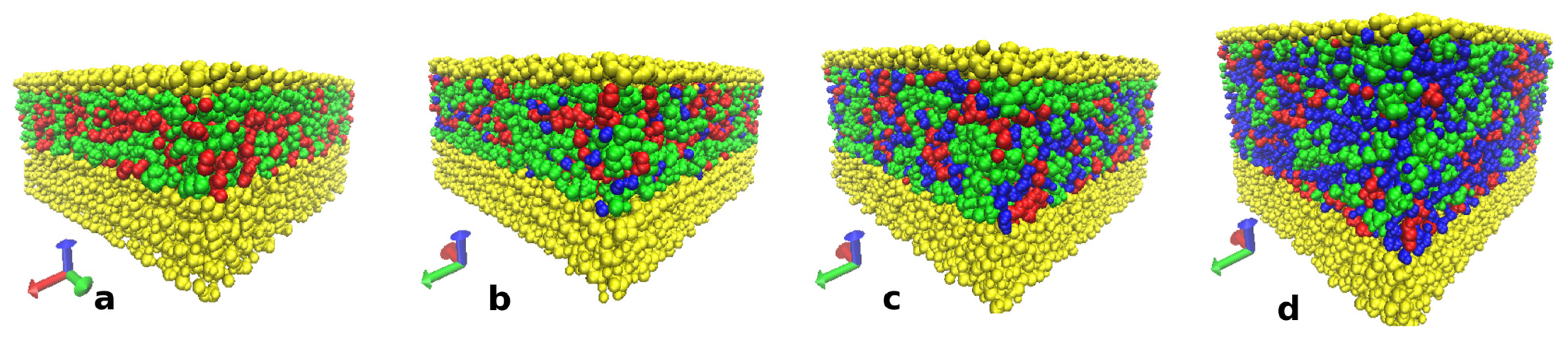
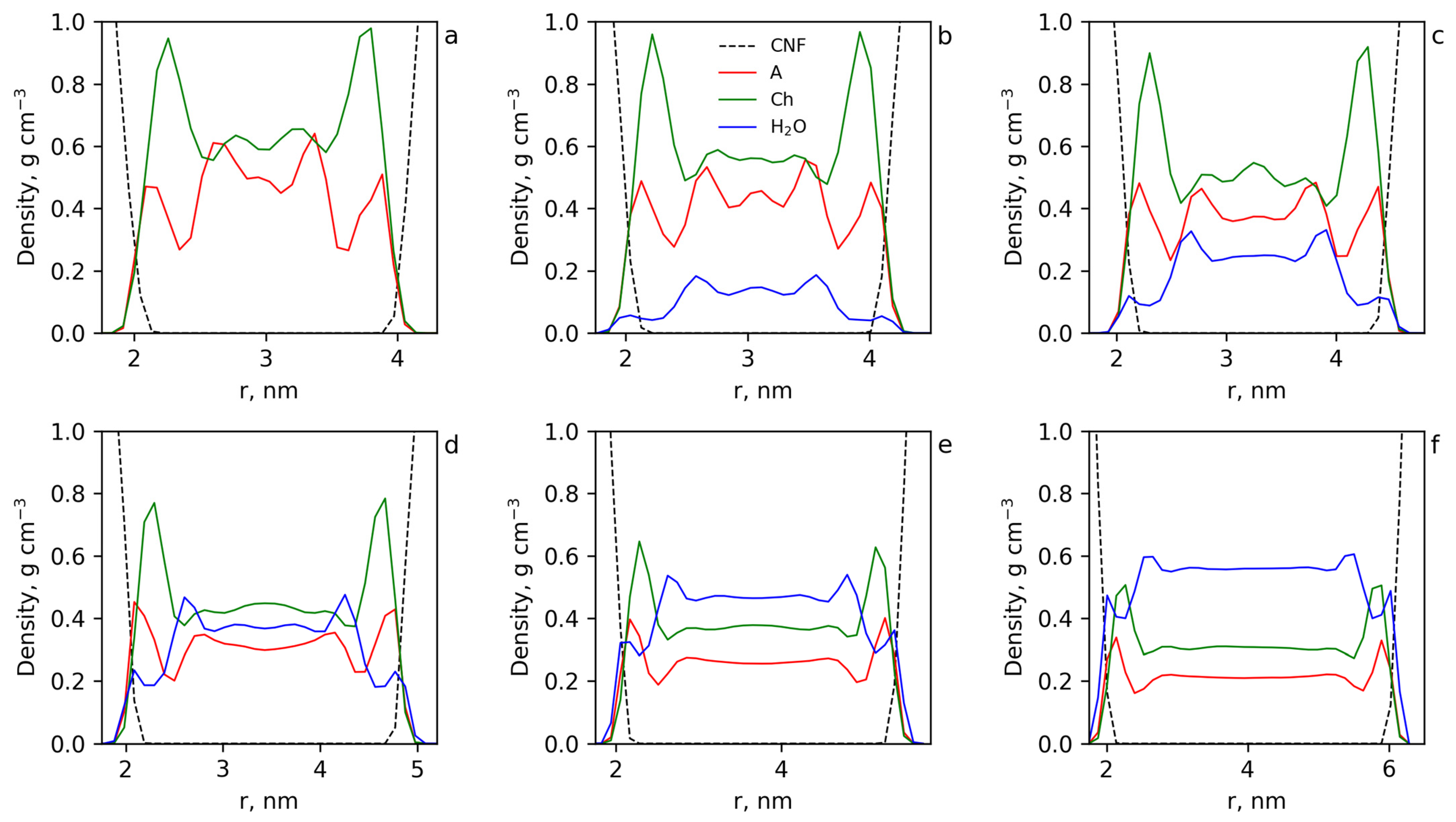
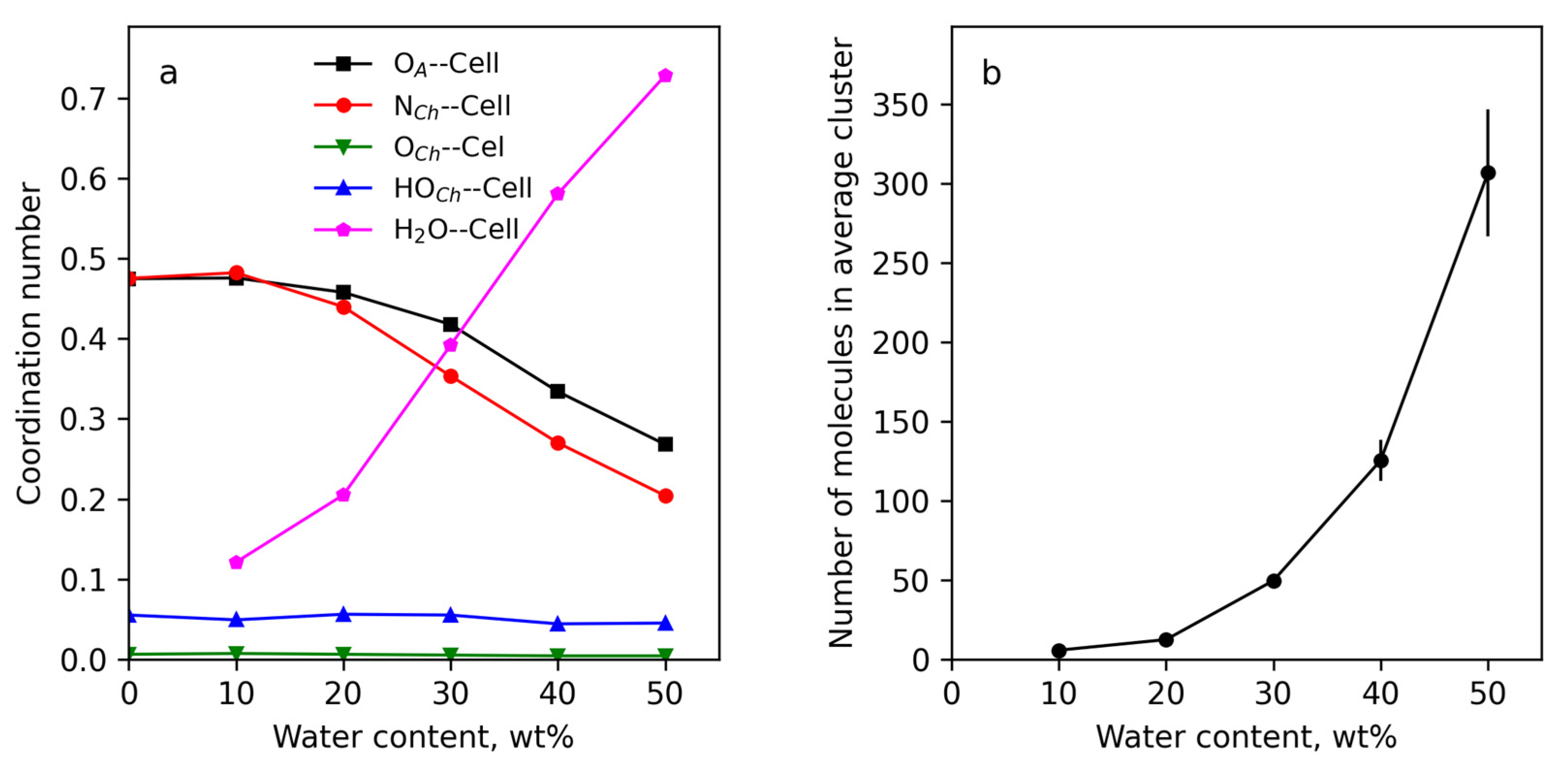
3.4.2. Structure of Cellulose Near-Surface Layer and Water Influence
3.5. Mechanical Properties and 3D Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Su, M.; Song, Y. Printable Smart Materials and Devices: Strategies and Applications. Chem. Rev. 2022, 122, 5144–5164. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Berto, F. 3D Printing of polymer composites: A short review. Mater. Des. Process. Commun. 2020, 2, e97. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin, R.; In Het Panhuis, M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Yoshida, K.; Takishima, Y.; Hara, Y.; Kawakami, M.; Furukawa, H. 3D printing for gel robotics. In Nano-, Bio-, Info-Tech Sensors, and 3D Systems II; Varadan, V.K., Ed.; SPIE: San Francisco, CA, USA, 2018; p. 46. [Google Scholar]
- Rahman, M.S.; Shiblee, M.N.I.; Ahmed, K.; Khosla, A.; Ogawa, J.; Kawakami, M.; Furukawa, H. Flexible and Conductive 3D Printable Polyvinylidene Fluoride and Poly(N,N-dimethylacrylamide) Based Gel Polymer Electrolytes. Macromol. Mater. Eng. 2020, 305, 2000262. [Google Scholar] [CrossRef]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Murphy, C.A.; Collins, M.N. Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym. Compos. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Li, V.C.F.; Kuang, X.; Mulyadi, A.; Hamel, C.M.; Deng, Y.; Qi, H.J. 3D printed cellulose nanocrystal composites through digital light processing. Cellulose 2019, 26, 3973–3985. [Google Scholar] [CrossRef]
- Nada, A.M.; Hassan, M.L. Thermal behavior of cellulose and some cellulose derivatives. Polym. Degrad. Stab. 2000, 67, 111–115. [Google Scholar] [CrossRef]
- Krumm, C.; Pfaendtner, J.; Dauenhauer, P.J. Millisecond Pulsed Films Unify the Mechanisms of Cellulose Fragmentation. Chem. Mater. 2016, 28, 3108–3114. [Google Scholar] [CrossRef]
- He, X.; Lu, Q. Design and fabrication strategies of cellulose nanocrystal-based hydrogel and its highlighted application using 3D printing: A review. Carbohydr. Polym. 2023, 301, 120351. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Håkansson, K.M.O.; Henriksson, I.C.; de la Peña Vázquez, C.; Kuzmenko, V.; Markstedt, K.; Enoksson, P.; Gatenholm, P. Solidification of 3D Printed Nanofibril Hydrogels into Functional 3D Cellulose Structures. Adv. Mater. Technol. 2016, 1, 1600096. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Rheological properties of cellulose nanofiber hydrogel for high-fidelity 3D printing. Carbohydr. Polym. 2021, 263, 117976. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sale, K.L.; Holmes, B.M.; Simmons, B.A.; Singh, S. Understanding the interactions of cellulose with ionic liquids: A molecular dynamics study. J. Phys. Chem. B 2010, 114, 4293–4301. [Google Scholar] [CrossRef]
- Markstedt, K.; Sundberg, J.; Gatenholm, P. 3D bioprinting of cellulose structures from an ionic liquid. 3D Print. Addit. Manuf. 2014, 1, 115–121. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Govinda, V.; Attri, P.; Venkatesu, P.; Venkateswarlu, P. Thermophysical properties of dimethylsulfoxide with ionic liquids at various temperatures. Fluid Phase Equilib. 2011, 304, 35–43. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Regueira, T.; Santos, L.M.N.B.F.; Fernandez, J.; Coutinho, J.A.P. Effect of water on the viscosities and densities of 1-butyl-3- methylimidazolium dicyanamide and 1-butyl-3-methylimidazolium tricyanomethane at atmospheric pressure. J. Chem. Eng. Data 2010, 55, 645–652. [Google Scholar] [CrossRef]
- Gunasekera, D.H.A.T.; Kuek, S.; Hasanaj, D.; He, Y.; Tuck, C.; Croft, A.K.; Wildman, R.D. Three dimensional ink-jet printing of biomaterials using ionic liquids and co-solvents. Faraday Discuss. 2016, 190, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, J.; Zhang, J.; Niu, Y.; Liu, C.Y.; He, J.; Zhang, J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012, 53, 2524–2531. [Google Scholar] [CrossRef]
- Wang, L.; Gao, L.; Cheng, B.; Ji, X.; Song, J.; Lu, F. Rheological behaviors of cellulose in 1-ethyl-3-methylimidazolium chloride/dimethylsulfoxide. Carbohydr. Polym. 2014, 110, 292–297. [Google Scholar] [CrossRef]
- Nazari, B.; Utomo, N.W.; Colby, R.H. The Effect of Water on Rheology of Native Cellulose/Ionic Liquids Solutions. Biomacromolecules 2017, 18, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Rudaz, C.; Budtova, T. Phase diagram, solubility limit and hydrodynamic properties of cellulose in binary solvents with ionic liquid. Carbohydr. Polym. 2014, 105, 237–243. [Google Scholar] [CrossRef]
- Liu, H.; Sale, K.L.; Simmons, B.A.; Singh, S. Molecular dynamics study of polysaccharides in binary solvent mixtures of an ionic liquid and water. J. Phys. Chem. B 2011, 115, 10251–10258. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.; Liu, Z.; Wang, W. Cosolvent or antisolvent? A molecular view of the interface between ionic liquids and cellulose upon addition of another molecular solvent. J. Phys. Chem. B 2013, 117, 11780–11792. [Google Scholar] [CrossRef]
- Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Ribeiro, M.C.C.; Smirnov, M.A. Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers 2022, 14, 5148. [Google Scholar] [CrossRef]
- De Silva, R.; Byrne, N. Utilization of cotton waste for regenerated cellulose fibres: Influence of degree of polymerization on mechanical properties. Carbohydr. Polym. 2017, 174, 89–94. [Google Scholar] [CrossRef]
- Michud, A.; Hummel, M.; Haward, S.; Sixta, H. Monitoring of cellulose depolymerization in 1-ethyl-3-methylimidazolium acetate by shear and elongational rheology. Carbohydr. Polym. 2015, 117, 355–363. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Elokhovsky, V.Y.; Karttunen, M. Polymerizable choline-and imidazolium-based ionic liquids reinforced with bacterial cellulose for 3d-printing. Polymers 2021, 13, 3044. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Sprenger, K.G.; Jaeger, V.W.; Pfaendtner, J. The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids. J. Phys. Chem. B 2015, 119, 5882–5895. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, D.; Nazarychev, V.; Fedotova, V.; Vorobiov, V.; Lukasheva, N.; Smirnov, M.; Karttunen, M. Investigation of structure and properties of polymerizable deep eutectic solvent based on choline chloride and acrylic acid. J. Mol. Liq. 2023, 370, 121030. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Wallingford CT. Gaussian 2016, 9. [Google Scholar]
- Woods, R.J.; Chappelle, R. Restrained electrostatic potential atomic partial charges for condensed-phase simulations of carbohydrates. J. Mol. Struct. THEOCHEM 2000, 527, 149–156. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L.; Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An Nlog (N) method for Ewald sums in large systems Particle mesh Ewald: An N -log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; et al. A smooth particle mesh Ewald method A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Mackerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins †. J. Phys. Chem. B 1998, 5647, 3586–3616. [Google Scholar] [CrossRef]
- Tolmachev, D.; Lukasheva, N.; Ramazanov, R.; Nazarychev, V.; Borzdun, N.; Volgin, I.; Andreeva, M.; Glova, A.; Melnikova, S.; Dobrovskiy, A.; et al. Computer Simulations of Deep Eutectic Solvents: Challenges, Solutions, and Perspectives. Int. J. Mol. Sci. 2022, 23, 645. [Google Scholar] [CrossRef] [PubMed]
- Bedrov, D.; Piquemal, J.; Borodin, O.; Mackerell, A.D.; Der, C.S. Molecular Dynamics Simulations of Ionic Liquids and Electrolytes Using Polarizable Force Fields. Chem. Rev. 2019, 119, 7940–7995. [Google Scholar] [CrossRef]
- Tolmachev, D.A.; Boyko, O.S.; Lukasheva, N.V.; Martinez-Seara, H.; Karttunen, M. Overbinding and Qualitative and Quantitative Changes Caused by Simple Na+ and K+ Ions in Polyelectrolyte Simulations: Comparison of Force Fields with and without NBFIX and ECC Corrections. J. Chem. Theory Comput. 2020, 16, 677–687. [Google Scholar] [CrossRef]
- Corrections, E.C.C. Changes in the Local Conformational States Caused by Simple Na + and K + Ions in Polyelectrolyte Simulations: Comparison of Seven Force Fields with and without NBFIX and without NBFIX and ECC Corrections. Polymers 2022, 14, 252. [Google Scholar]
- García, G.; Atilhan, M.; Aparicio, S. The impact of charges in force fi eld parameterization for molecular dynamics simulations of deep eutectic solvents. J. Mol. Liq. 2015, 211, 506–514. [Google Scholar] [CrossRef]
- Maglia de Souza, R.; Karttunen, M.; Ribeiro, M.C.C. Fine-Tuning the Polarizable CL&Pol Force Field for the Deep Eutectic Solvent Ethaline. J. Chem. Inf. Model. 2021, 61, 5938–5947. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, Z.; Zheng, L.; Kalhor, P.; Huai, Z.; Liu, Z. Molecular modelling of ionic liquids: General guidelines on fixed-charge force fields for balanced descriptions. J. Ion. Liq. 2022, 2, 100043. [Google Scholar] [CrossRef]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 04, 150. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Persson, J.; Chanzy, H.; Sugiyama, J. Combined Infrared and Electron Diffraction Study of the Polymorphism of Native Celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Multivariate statistical analysis of X-ray data from cellulose: A new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour. Technol. 2010, 101, 4461–4471. [Google Scholar] [CrossRef]
- Ottesen, V.; Syverud, K. Swelling of individual cellulose nanofibrils in water, role of crystallinity: An AFM study. Cellulose 2021, 28, 19–29. [Google Scholar] [CrossRef]
- Horkay, F.; Douglas, J.F. Polymer Gels: Basics, Challenges, and Perspectives. ACS Symp. Ser. 2018, 1296, 1–13. [Google Scholar] [CrossRef]
- Elhamarnah, Y.A.; Nasser, M.; Qiblawey, H.; Benamor, A.; Atilhan, M.; Aparicio, S. A comprehensive review on the rheological behavior of imidazolium based ionic liquids and natural deep eutectic solvents. J. Mol. Liq. 2019, 277, 932–958. [Google Scholar] [CrossRef]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of water at various temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Chen, P.; Sun, L.; Liu, Y.; Gao, C. A double network conductive gel with robust mechanical properties based on polymerizable deep eutectic solvent. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130349. [Google Scholar] [CrossRef]
- He, M.; Hou, Y.; Zhu, C.; He, M.; Jiang, Y.; Feng, G.; Liu, L.; Li, Y.; Chen, C.; Zhang, L. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjug. Chem. 2021, 32, 1915–1925. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Y.; Xu, M.; Qin, M.; Lan, W.; Huang, D.; Wei, Y.; Chen, W. High strength polyvinyl alcohol/polyacrylic acid (PVA/PAA) hydrogel fabricated by Cold-Drawn method for cartilage tissue substitutes. J. Biomater. Sci. Polym. Ed. 2020, 31, 1836–1851. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Oyen, M.L. Mechanical characterization of hydrogels. In The Mechanics of Hydrogels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. [Google Scholar]
- Shi, Y.; Xiong, D.; Liu, Y.; Wang, N.; Zhao, X. Swelling, mechanical and friction properties of PVA/PVP hydrogels after swelling in osmotic pressure solution. Mater. Sci. Eng. C 2016, 65, 172–180. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Lukasheva, N.V.; Smirnov, M.A. Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks. Polymers 2023, 15, 2156. https://doi.org/10.3390/polym15092156
Fedotova VS, Sokolova MP, Vorobiov VK, Sivtsov EV, Lukasheva NV, Smirnov MA. Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks. Polymers. 2023; 15(9):2156. https://doi.org/10.3390/polym15092156
Chicago/Turabian StyleFedotova, Veronika S., Maria P. Sokolova, Vitaly K. Vorobiov, Eugene V. Sivtsov, Natalia V. Lukasheva, and Michael A. Smirnov. 2023. "Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks" Polymers 15, no. 9: 2156. https://doi.org/10.3390/polym15092156
APA StyleFedotova, V. S., Sokolova, M. P., Vorobiov, V. K., Sivtsov, E. V., Lukasheva, N. V., & Smirnov, M. A. (2023). Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks. Polymers, 15(9), 2156. https://doi.org/10.3390/polym15092156







