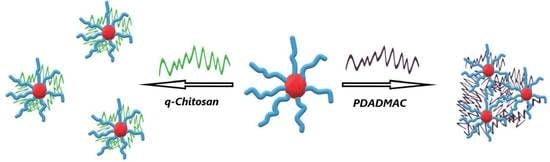
A Comparison of Interpolyelectrolyte Complexes (IPECs) Made from Anionic Block Copolymer Micelles and PDADMAC or q-Chitosan as Polycation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Complexes
2.3. Methods
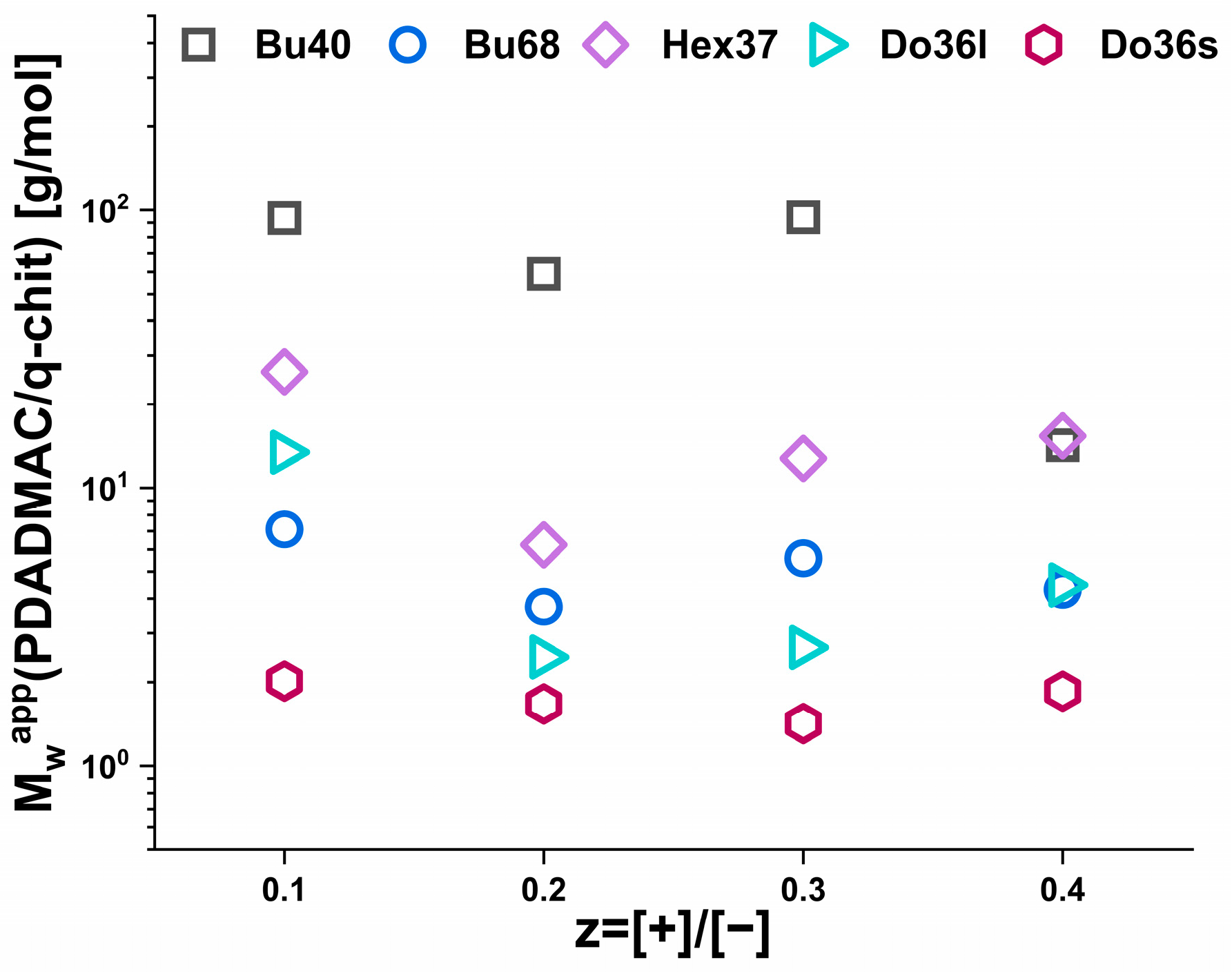
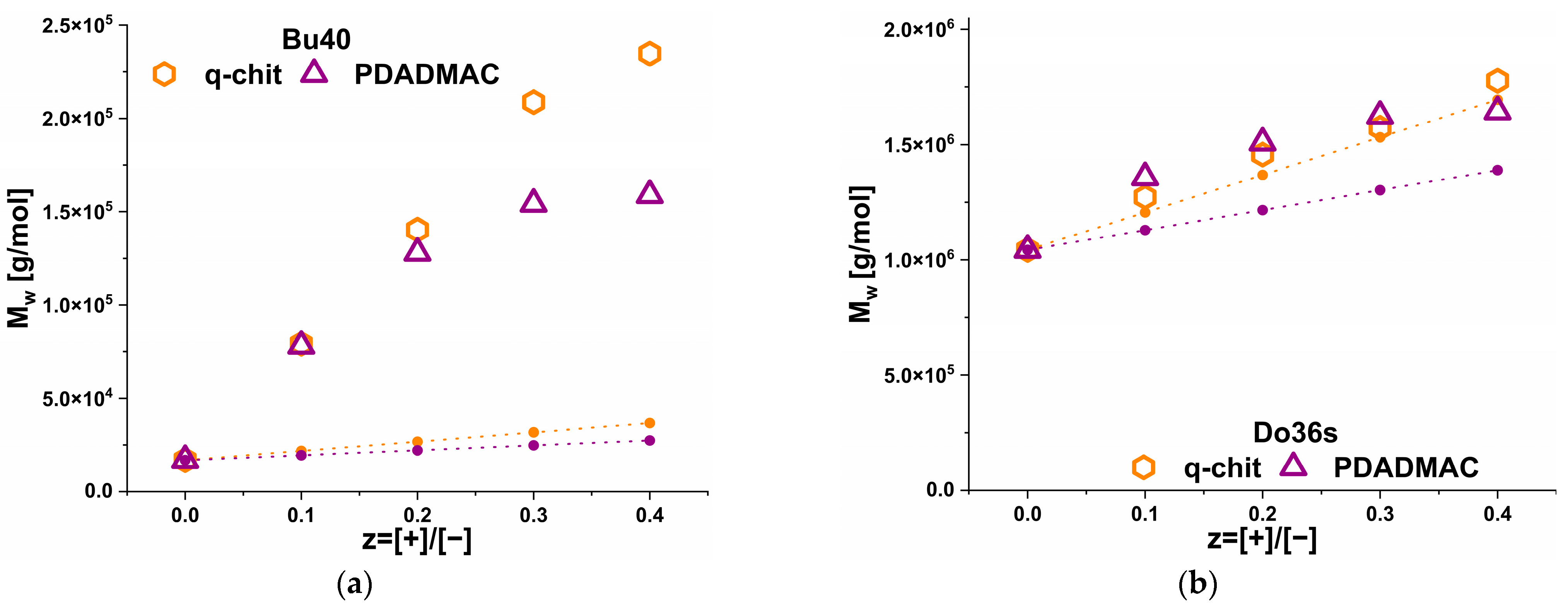
3. Results
3.1. Phase Behaviour
3.2. Turbidity Measurements
3.3. Static Light Scattering (SLS)
3.4. Small Angle Neutron Scattering (SANS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pergushov, D.V.; Müller, A.H.E.; Schacher, F.H. Micellar Interpolyelectrolyte Complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Machinskaya, A.E.; Vasilevskaya, V.V. The Formation of Interpolymer Complexes in Mixtures of Weak Polyelectrolytes. Polym. Sci.-Ser. A 2016, 58, 606–612. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Mussabayeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte Complexes: Advances and Prospects of Application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Kabanov, V.A. Basic Properties of Soluble Interpolyelectrolyte Complexes Applied to Bioengineering and Cell Transformations. In Macromolecular Complexes in Chemistry and Biology; Springer: Berlin/Heidelberg, Germany, 1994; pp. 151–174. [Google Scholar]
- Chelushkin, P.S.; Lysenko, E.A.; Bronich, T.K.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Polyion Complex Nanomaterials from Block Polyelectrolyte Micelles and Linear Polyelectrolytes of Opposite Charge. 2. Dynamic Properties. J. Phys. Chem. B 2008, 112, 7732–7738. [Google Scholar] [CrossRef]
- Burkhardt, M.; Ruppel, M.; Tea, S.; Drechsler, M.; Schweins, R.; Pergushov, D.V.; Gradzielski, M.; Zezin, A.B.; Müller, A.H.E. Water-Soluble Interpolyelectrolyte Complexes of Polyisobutylene-Block-Poly(Methacrylic Acid) Micelles: Formation and Properties. Langmuir 2008, 24, 1769–1777. [Google Scholar] [CrossRef]
- Laaser, J.E.; Jiang, Y.; Petersen, S.R.; Reineke, T.M.; Lodge, T.P. Interpolyelectrolyte Complexes of Polycationic Micelles and Linear Polyanions: Structural Stability and Temporal Evolution. J. Phys. Chem. B 2015, 119, 15919–15928. [Google Scholar] [CrossRef]
- Laaser, J.E.; Lohmann, E.; Jiang, Y.; Reineke, T.M.; Lodge, T.P. Architecture-Dependent Stabilization of Polyelectrolyte Complexes between Polyanions and Cationic Triblock Terpolymer Micelles. Macromolecules 2016, 49, 6644–6654. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Remizova, E.V.; Gradzielski, M.; Lindner, P.; Feldthusen, J.; Zezin, A.B.; Müller, A.H.E.; Kabanov, V.A. Micelles of Polyisobutylene-Block-Poly(Methacrylic Acid) Diblock Copolymers and Their Water-Soluble Interpolyelectrolyte Complexes Formed with Quaternized Poly (4-Vinylpyridine). Polymer 2004, 45, 367–378. [Google Scholar] [CrossRef]
- Chelushkin, P.S.; Lysenko, E.A.; Bronich, T.K.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Polyion Complex Nanomaterials from Block Polyelectrolyte Micelles and Linear Polyelectrolytes of Opposite Charge: 1. Solution Behavior. J. Phys. Chem. B 2007, 111, 8419–8425. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, H.; Tang, Y. Changes in Layer Properties and Physical Properties of Papers from Old Corrugated Container Fibres with PDADMAC/HEC Polyelectrolyte Multilayers. Adv. Mater. Res. 2012, 496, 67–70. [Google Scholar] [CrossRef]
- Wandrey, C.; Hernández-Barajas, J.; Hunkeler, D. Diallyldimethylammonium Chloride and Its Polymers. Adv. Polym. Sci. 1999, 145, 125–182. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y. Bacteria-Removing and Bactericidal Efficiencies of Pdadmac Composite Coagulants in Enhanced Coagulation Treatment. Clean-Soil Air Water 2013, 41, 37–42. [Google Scholar] [CrossRef]
- Jha, P.K.; Desai, P.S.; Li, J.; Larson, R.G. PH and Salt Effects on the Associative Phase Separation of Oppositely Charged Polyelectrolytes. Polymers 2014, 6, 1414–1436. [Google Scholar] [CrossRef]
- Queirós, M.V.A.; Loh, W. Preparation of Poly(Acrylate)/Poly(Diallyldimethylammonium) Coacervates without Small Counterions and Their Phase Behavior upon Salt Addition towards Poly-Ions Segregation. Polymers 2021, 13, 2259. [Google Scholar] [CrossRef]
- Herslöf-Björling, Å.; Björling, M.; Sundelöf, L.O. The Counter- and Colon Influence on the Interaction between Sodium Hyaluronate and Tetradecyltrimethylammonium Bromide. Langmuir 1999, 15, 353–357. [Google Scholar] [CrossRef]
- Jemili, N.; Fauquignon, M.; Grau, E.; Fatin-Rouge, N.; Dole, F.; Chapel, J.P.; Essafi, W.; Schatz, C. Complexation in Aqueous Solution of a Hydrophobic Polyanion (PSSNa) Bearing Different Charge Densities with a Hydrophilic Polycation (PDADMAC). Polymers 2022, 14, 2404. [Google Scholar] [CrossRef]
- Kuzminskaya, O.; Riemer, S.; Dalgliesh, R.; Almásy, L.; Hoffmann, I.; Gradzielski, M. Structure and Phase Behavior of Interpolyelectrolyte Complexes of PDADMAC and Hydrophobically Modified PAA (HM-PAA). Macromol. Chem. Phys. 2023, 224, 2200276. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Baldrick, P. The Safety of Chitosan as a Pharmaceutical Excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitosan: Manufacture, Properties, and Uses. In Chitosan: Manufacture, Properties and Usage; Davis, S.P., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 133–216. ISBN 978-1-61728-831-9. [Google Scholar]
- Krayukhina, M.A.; Samoilova, N.A.; Yamskov, I.A. Polyelectrolyte Complexes of Chitosan: Formation, Properties and Applications. Russ. Chem. Rev. 2008, 77, 799–813. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Jin, S.; Chen, Y. Structure and Properties of the Polyelectrolyte Complex of Chitosan with Poly(Methacrylic Acid). Polym. Int. 2007, 56, 1305–1312. [Google Scholar] [CrossRef]
- Carvalho, S.G.; dos Santos, A.M.; Silvestre, A.L.P.; Meneguin, A.B.; Ferreira, L.M.B.; Chorilli, M.; Gremião, M.P.D. New Insights into Physicochemical Aspects Involved in the Formation of Polyelectrolyte Complexes Based on Chitosan and Dextran Sulfate. Carbohydr. Polym. 2021, 271, 118436. [Google Scholar] [CrossRef]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte Complex Formation Using Alginate and Chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- de Lima, C.R.M.; de Souza, P.R.S.; Stopilha, R.T.; de Morais, W.A.; Silva, G.T.M.; Nunes, J.S.; Wanderley Neto, A.O.; Pereira, M.R.; Fonseca, J.L.C. Formation and Structure of Chitosan–Poly(Sodium Methacrylate) Complex Nanoparticles. J. Dispers. Sci. Technol. 2018, 39, 83–91. [Google Scholar] [CrossRef]
- Colombani, O.; Ruppel, M.; Schubert, F.; Zettl, H.; Pergushov, D.V.; Müller, A.H.E. Synthesis of Poly(n-Butyl Acrylate)-Block-Poly(Acrylic Acid) Diblock Copolymers by ATRP and Their Micellization in Water. Macromolecules 2007, 40, 4338–4350. [Google Scholar] [CrossRef]
- Azeri, Ö.; Schönfeld, D.; Noirez, L.; Gradzielski, M. Structural Control in Micelles of Alkyl Acrylate-Acrylate Copolymers via Alkyl Chain Length and Block Length. Colloid Polym. Sci. 2020, 298, 829–840. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Jamroży, K.; Batys, P.; Michna, A. Influence of Ionic Strength on Poly(Diallyldimethylammonium Chloride) Macromolecule Conformations in Electrolyte Solutions. J. Colloid Interface Sci. 2014, 435, 182–190. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial Activity of Methylated Chitosan and Chitooligomer Derivatives: Synthesis and Structure Activity Relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Jónsdóttir, S.; Steinsson, H.; Másson, M. N-Selective “One Pot” Synthesis of Highly N-Substituted Trimethyl Chitosan (TMC). Carbohydr. Polym. 2008, 74, 740–744. [Google Scholar] [CrossRef]
- Kerker, M. The Scatering of Light and Other Electromagnetic Radiation; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Itakura, M.; Shimada, K.; Matsuyama, S.; Saito, T.; Kinugasa, S. A Convenient Method to Determine the Rayleigh Ratio with Uniform Polystyrene Oligomers. J. Appl. Polym. Sci. 2006, 99, 1953–1959. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, T.-L. 16. Colloidal Solutions. Methods Exp. Phys. 1987, 23, 489–543. [Google Scholar]
- Keiderling, U. The New “BerSANS-PC” Software for Reduction and Treatment of Small Angle Neutron Scattering Data. Appl. Phys. A Mater. Sci. Process. 2002, 74, 1455–1457. [Google Scholar] [CrossRef]
- SasView Software. Available online: www.sasview.org (accessed on 10 January 2022).
- Chiappisi, L.; Prévost, S.; Grillo, I.; Gradzielski, M. Chitosan/Alkylethoxy Carboxylates: A Surprising Variety of Structures. Langmuir 2014, 30, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ritacco, H.; Ortega, F.; Svitova, T.; Radke, C.J.; Rubio, R.G. Adsorption Kinetics and Mechanical Properties of Ultrathin Polyelectrolyte Multilayers: Liquid-Supported versus Solid-Supported Films. J. Phys. Chem. B 2009, 113, 7128–7137. [Google Scholar] [CrossRef] [PubMed]






| Chemical Formula | Abbreviation | Mn [g/mol] | Đ |
|---|---|---|---|
| nBu40-b-AA167 | Bu40 | 17,180 | 1.15 |
| nBu68-b-AA167 | Bu68 | 20,680 | 1.19 |
| nHex37-b-AA169 | Hex37 | 17,960 | 1.12 |
| nDo36-b-AA127 | Do36l | 17,860 | 1.20 |
| nDo36-b-AA71 | Do36s | 13,950 | 1.28 |
| Bu40 Complexes | z = [+]/[−] | Rg [nm] | Nagg [−] | Nagg [+] | |
|---|---|---|---|---|---|
| 0 | 3.3 | 1 | 0 | 0.19 | |
| with q-chit | 0.1 | 5.4 | 3.6 | 0.1 | 0.20 |
| 0.2 | 6.6 | 5.1 | 0.3 | 0.19 | |
| 0.3 | 7.5 | 6.4 | 0.5 | 0.20 | |
| 0.4 | 8.9 | 6.3 | 0.7 | 0.13 | |
| with PDADMAC | 0.1 | 6.4 | 3.9 | 0.1 | 0.12 |
| 0.2 | 7.1 | 5.7 | 0.2 | 0.14 | |
| 0.3 | 7.7 | 6.1 | 0.3 | 0.13 | |
| 0.4 | 8.8 | 5.7 | 0.4 | 0.09 | |
| Do36s Complexes | z = [+]/[−] | Rg [nm] | Nagg [−] | Nagg [+] | |
| 0 | 8.0 | 74.6 | 0 | 0.81 | |
| with q-chit | 0.1 | 8.5 | 78.8 | 0.9 | 0.82 |
| 0.2 | 9.5 | 79.4 | 1.9 | 0.67 | |
| 0.3 | 10.1 | 76.6 | 2.8 | 0.61 | |
| 0.4 | 10.7 | 78.3 | 3.8 | 0.58 | |
| with PDADMAC | 0.1 | 8.8 | 89.8 | 0.7 | 0.79 |
| 0.2 | 9.4 | 92.6 | 1.4 | 0.72 | |
| 0.3 | 9.9 | 93.1 | 2.2 | 0.66 | |
| 0.4 | 10.3 | 88.3 | 2.7 | 0.60 |
| Bu40 Complexes | z = [+]/[−] | R [nm] | Mw (⸱105) [g/mol] | Nagg [−] |
|---|---|---|---|---|
| with q-chit | 0.1 | 5.82 | 5.46 | 24.5 |
| 0.2 | 6.18 | 6.54 | 23.9 | |
| 0.3 | 6.18 | 6.56 | 20.2 | |
| 0.4 | 5.77 | 5.34 | 14.2 | |
| with PDADMAC | 0.1 | 5.56 | 4.76 | 23.9 |
| 0.2 | 5.80 | 5.40 | 23.9 | |
| 0.3 | 5.65 | 5.00 | 19.8 | |
| 0.4 | 5.71 | 5.16 | 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeri, Ö.; Schönfeld, D.; Dai, B.; Keiderling, U.; Noirez, L.; Gradzielski, M. A Comparison of Interpolyelectrolyte Complexes (IPECs) Made from Anionic Block Copolymer Micelles and PDADMAC or q-Chitosan as Polycation. Polymers 2023, 15, 2204. https://doi.org/10.3390/polym15092204
Azeri Ö, Schönfeld D, Dai B, Keiderling U, Noirez L, Gradzielski M. A Comparison of Interpolyelectrolyte Complexes (IPECs) Made from Anionic Block Copolymer Micelles and PDADMAC or q-Chitosan as Polycation. Polymers. 2023; 15(9):2204. https://doi.org/10.3390/polym15092204
Chicago/Turabian StyleAzeri, Özge, Dennis Schönfeld, Bin Dai, Uwe Keiderling, Laurence Noirez, and Michael Gradzielski. 2023. "A Comparison of Interpolyelectrolyte Complexes (IPECs) Made from Anionic Block Copolymer Micelles and PDADMAC or q-Chitosan as Polycation" Polymers 15, no. 9: 2204. https://doi.org/10.3390/polym15092204
APA StyleAzeri, Ö., Schönfeld, D., Dai, B., Keiderling, U., Noirez, L., & Gradzielski, M. (2023). A Comparison of Interpolyelectrolyte Complexes (IPECs) Made from Anionic Block Copolymer Micelles and PDADMAC or q-Chitosan as Polycation. Polymers, 15(9), 2204. https://doi.org/10.3390/polym15092204