New Physico-Chemical Analysis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites
2.3. Characterization Methods
2.3.1. Scanning Electron Microscopy
2.3.2. X-ray Diffraction
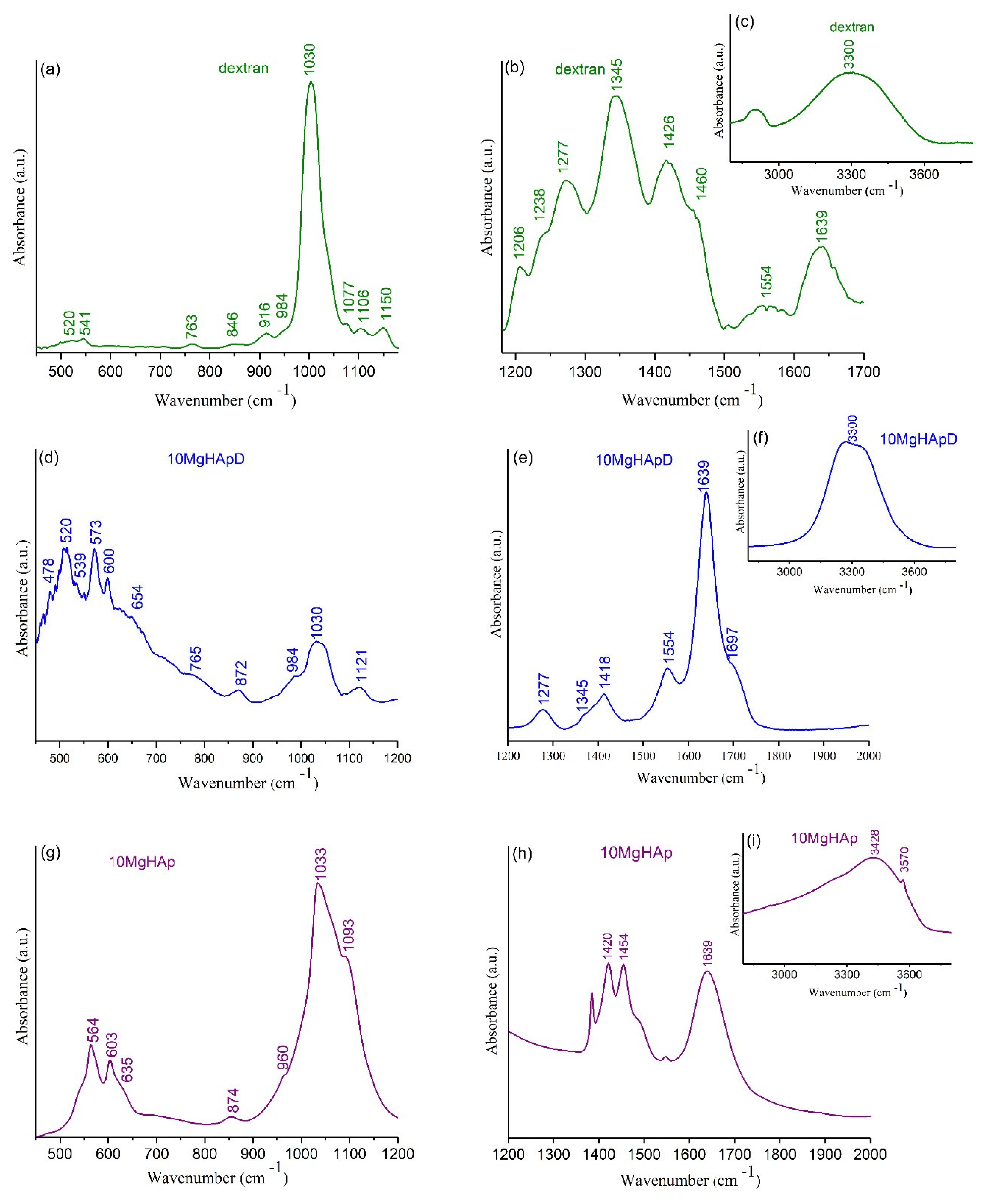
2.3.3. Fourier Transform Infrared Spectroscopy
2.3.4. Atomic Force Microscopy (AFM)
2.3.5. Monofractal and Multifractal Analysis
2.3.6. In Vitro Antimicrobial Assays
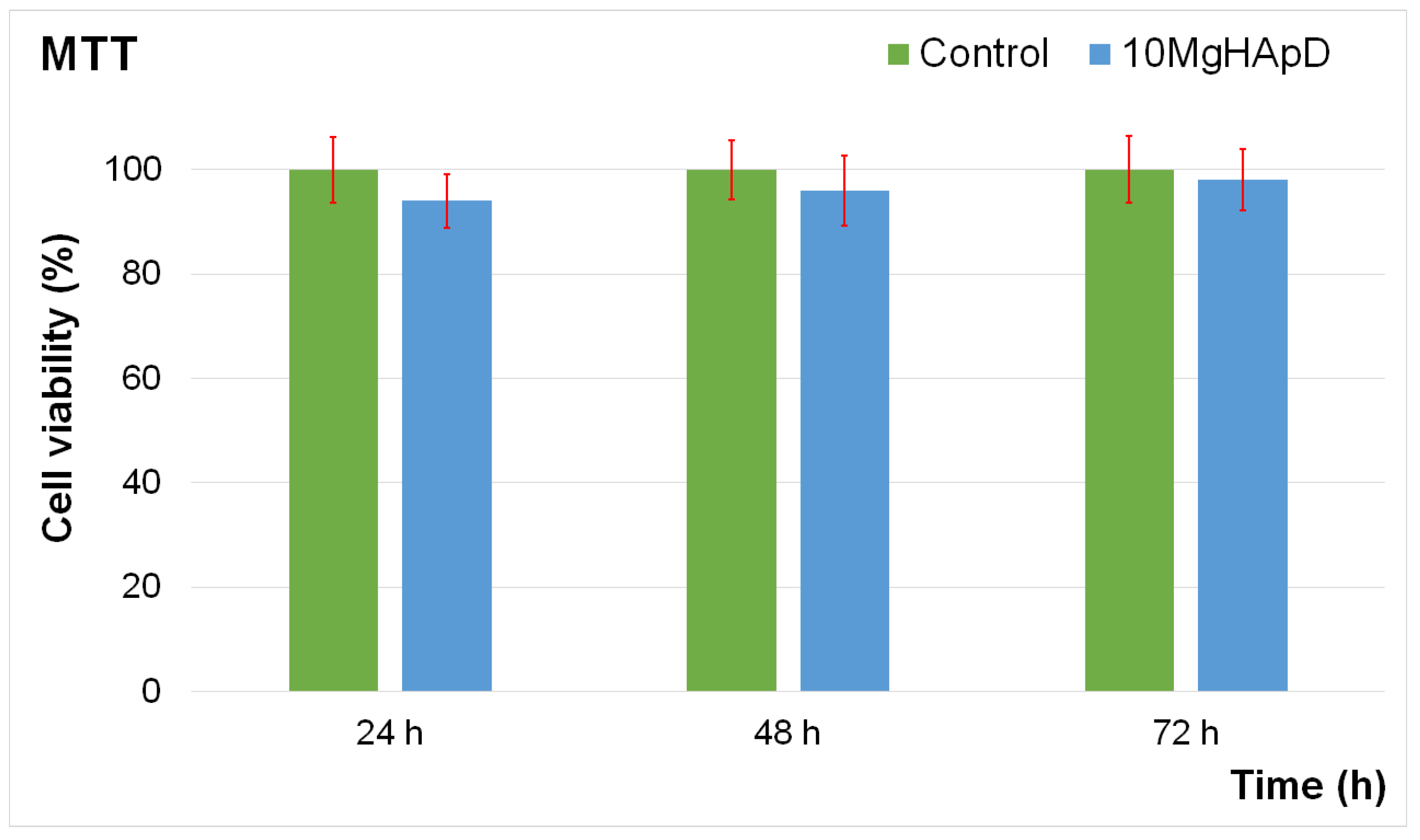
2.3.7. In Vitro Biocompatibility Assay
3. Results and Discussion
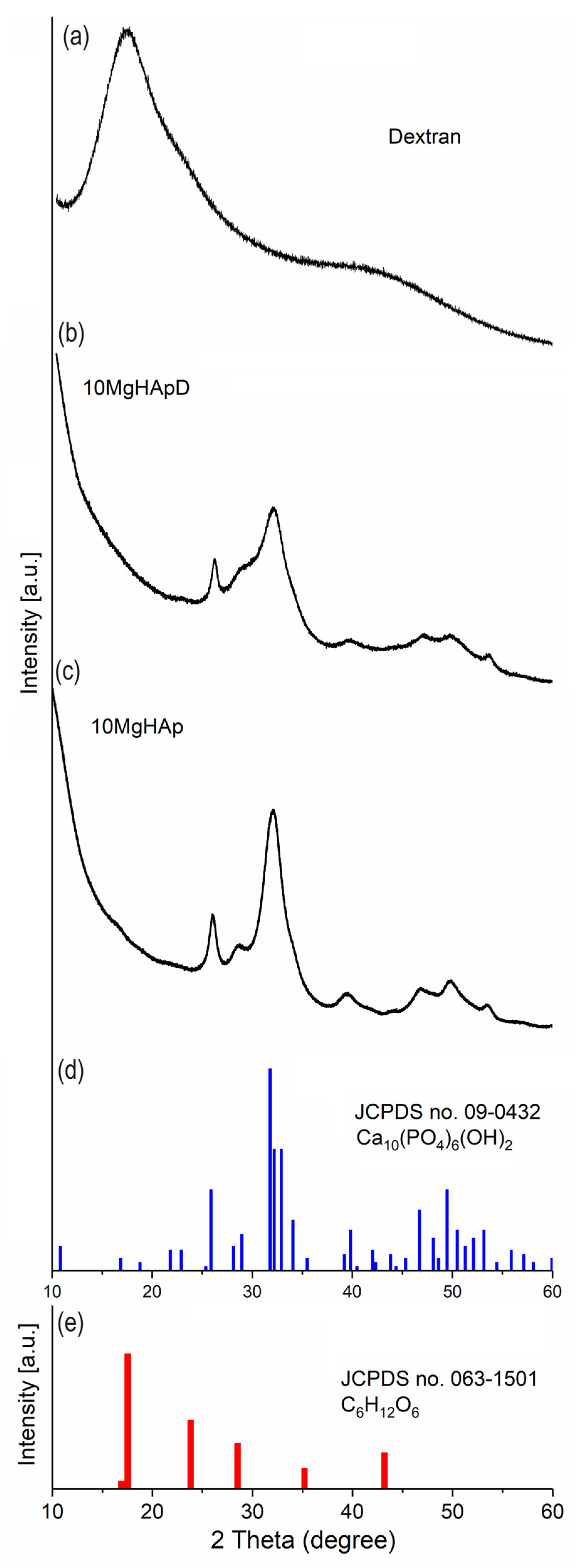
3.1. X-ray Diffraction
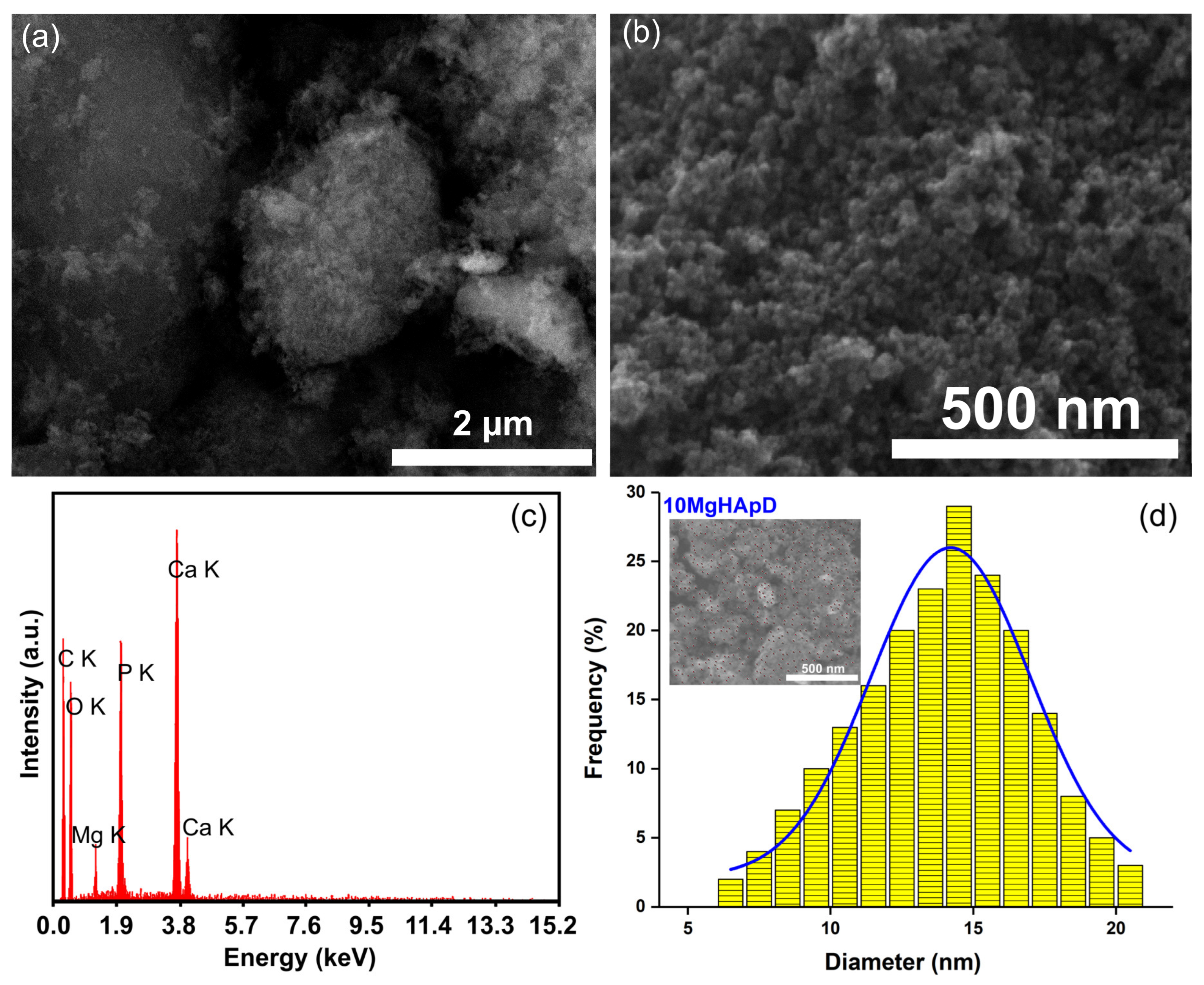
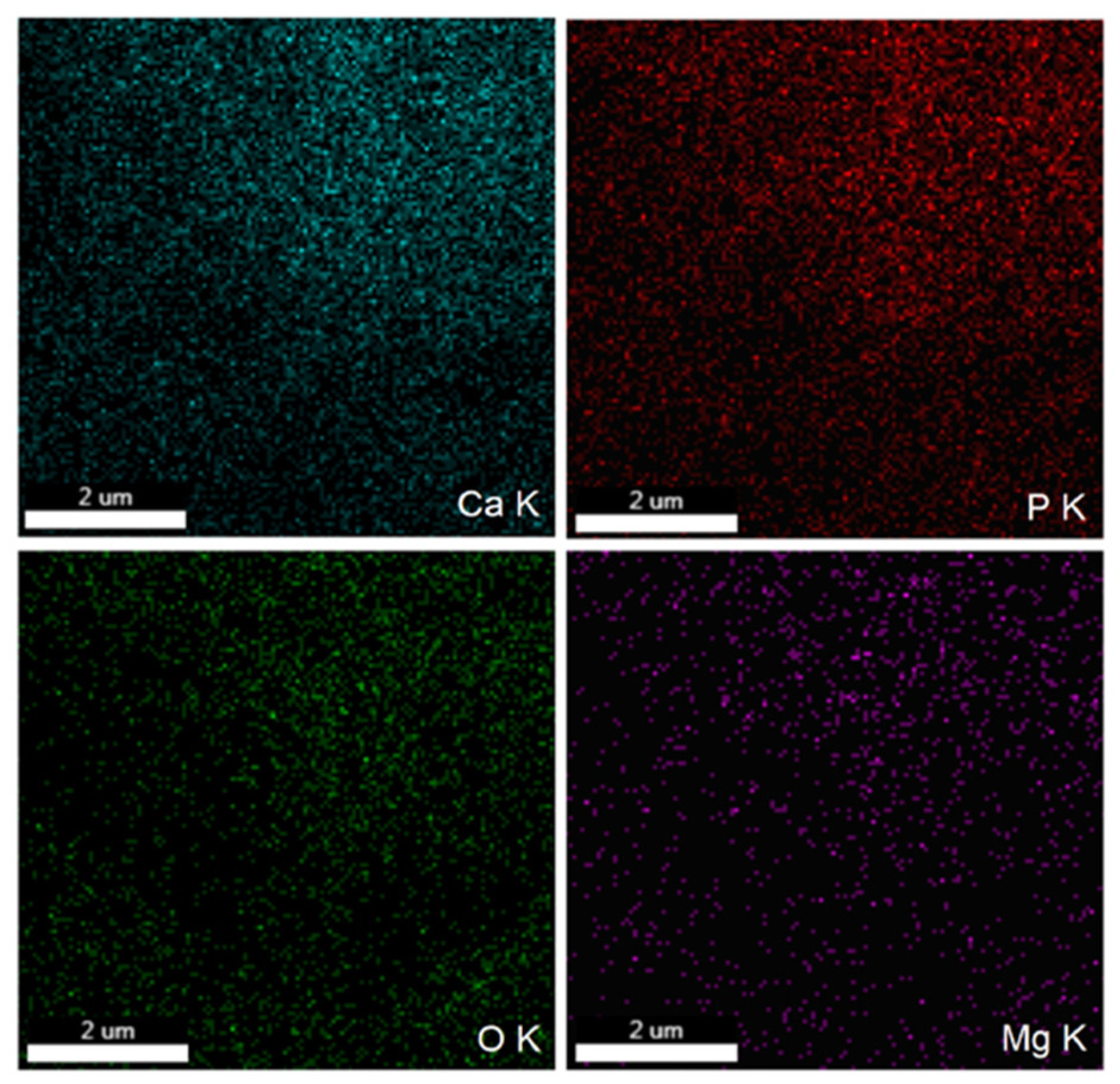
3.2. Scanning Electron Microscopy
3.3. Atomic Force Microscopy
3.4. Monofractal Analysis
3.5. Multifractal Analysis
3.6. Fourier Transform Infrared Spectroscopy
3.7. Antimicrobial Assay
3.8. In Vitro Biocompatibility Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef]
- Bengtsson, U.G.; Hylander, L.D. Increased mercury emissions from modern dental amalgams. Biometals 2017, 30, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. Nanotechnology and its Role in Caries Therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts Promote Streptococcus mutans Biofilm Matrix Degradation and Enhance Bacterial killing to Suppress Dental Caries In Vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in Preventive Dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Banghart, S.B. Synthesis of Extracellular Dextran by Cariogenic Bacteria and its Presence in Human Dental Plaque. Arch. Oral Biol. 1967, 12, 11–23. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Arcís, R.W.; López-Macipe, A.; Toledano, M.; Osorio, E.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Pascual, C.D. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent. Mater. 2002, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, Q.; Wang, Y.; Li, H.A.O. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dent. Mater. 2011, 27, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium incorporation into hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Sergi, R.; Bellucci, D.; Candidato Jr, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstić, A.; Jovanović, D.; Raičević, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowders for bone tissue engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Laisney, J.; Chevallet, M.; Fauquant, C.; Sageot, C.; Moreau, Y.; Predoi, D.; Herlin-Boime, N.; Lebrun, C.; Michaud-Soret, I. Ligand-Promoted Surface Solubilization of TiO2 Nanoparticles by the Enterobactin Siderophore in Biological Medium. Biomolecules 2022, 12, 1516. [Google Scholar] [CrossRef]
- Laisney, J.; Rosset, A.; Bartolomei, V.; Predoi, D.; Truffier-Boutry, D.; Artous, S.; Bergé, V.; Brochard, G.; Michaud-Soret, I. TiO2 nanoparticles coated with bio-inspired ligands for the safer-by-design development of photocatalytic paints. Environ. Sci. Nano 2021, 8, 297–310. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Lim, G.K.; Wang, J.; Ng, S.C.; Gan, L.M. Formation of nanocrystalline hydroxyapatite in nonionic surfactant emulsions. Langmuir 1999, 15, 7472–7477. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.C.; Landi, E.; Sandri, M. Magnesium doped hydroxyapatite: Synthesis and characterization. Key Eng. Mater. 2004, 264, 2051–2054. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhatt, H.A. Nanocrystalline hydroxyapatite doped with magnesium and zinc: Synthesis and characterization. Mater. Sci. Eng. C 2007, 27, 837–848. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef] [PubMed]
- Arul, K.T.; Kolanthai, E.; Manikandan, E.; Bhalerao, G.M.; Chandra, V.S.; Ramya, J.R.; Mudali, U.K.; Nair, K.G.M.; Kalkura, S.N. Green synthesis of magnesium ion incorporated nanocrystalline hydroxyapatite and their mechanical, dielectric and photoluminescence properties. Mater. Res. Bull. 2015, 67, 55–62. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Panneer, D.S.; Vivekananthan, S.; Sagadevan, S.; Lett, J.A. Microwave synthesis of hydroxyapatite encumbered with ascorbic acid intended for drug leaching studies. Mater. Res. Innov. 2020, 24, 171–178. [Google Scholar] [CrossRef]
- Dhanalakshmi, C.P.; Vijayalakshmi, L.; Narayanan, V. Synthesis and characterization of poly(4-vinyl pyridine-co-styrene)/FHAP nanocomposite, and its biomedical application. Appl. Nanosci. 2013, 3, 373–382. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles: Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccines Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Abu-Elsaad, N.; El-Kady, A.M.; Ibrahim, M.A. Improvement of physico-chemical properties of dextran-chitosan composite scaffolds by addition of nano-hydroxyapatite. Sci. Rep. 2018, 8, 12180. [Google Scholar] [CrossRef]
- Kaushik, S. Polymeric and Ceramic Nanoparticles: Possible Role in Biomedical Applications. In Handbook of Polymer and Ceramic Nanotechnology; Hussain, C.M., Thomas, S., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. 3D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2020, 109, 103822. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sutha, S.; Dhineshbabu, N.R.; Prabhu, M.; Rajendran, V. Mg-doped hydroxyapatite/chitosan composite coated 316l stainless steel implants for biomedical applications. J. Nanosci. Nanotechnol. 2015, 15, 4178–4187. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015, 849216. [Google Scholar] [CrossRef]
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 20 November 2023).
- Ţălu, Ș. Micro and Nanoscale Characterization of Three Dimensional Surfaces. Basics and Applications; Napoca Star Publishing House: Cluj-Napoca, Romania, 2015. [Google Scholar]
- Ţălu, Ş.; Abdolghaderi, S.; Pinto, E.P.; Matos, R.S.; Salerno, M. Advanced fractal analysis of nanoscale topography of Ag/DLC composite synthesized by RF-PECVD. Surf. Eng. 2020, 36, 713–719. [Google Scholar] [CrossRef]
- Bulinski, A.; Dimitrov, D. Statistical Estimation of the Shannon Entropy. Acta Math. Sin. Engl. Ser. 2019, 35, 17–46. [Google Scholar] [CrossRef]
- Matos, R.S.; Lopes, G.A.C.; Ferreira, N.S.; Pinto, E.P.; Carvalho, J.C.T.; Figueiredo, S.S.; Oliveira, A.F.; Zamora, R.R.M. Superficial Characterization of Kefir Biofilms Associated with Açaí and Cupuaçu Extracts. Arab. J. Sci. Eng. 2018, 43, 3371–3379. [Google Scholar] [CrossRef]
- Brown, C.; Liebovitch, L. Fractal Analysis; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Turculet, C.S.; Beuran, M.; Ghita, R.V.; Costescu, A.; Groza, A.; Chifiriuc, M.C.; Chapon, P.; Gaiaschi, S.; et al. Enamel Based Composite Layers Deposited on Titanium Substrate with Antifungal Activity. J. Spectrosc. 2016, 2016, 4361051. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Unterweger, H.; Tietze, R.; Janko, C.; Zaloga, J.; Lyer, S.; Dürr, S.; Taccardi, N.; Goudouri, O.M.; Hoppe, A.; Eberbeck, D.; et al. Development and characterization of magnetic iron oxide nanoparticles with a cisplatin-bearing polymer coating for targeted drug delivery. Int. J. Nanomed. 2014, 9, 3659–3676. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Mangalaraj, D.; Ponpandian, N. Controlled growth and investigations on the morphology and mechanical properties of hydroxyapatite/titania nanocomposite thin films. Compos. Sci. Technol. 2010, 70, 1645–1651. [Google Scholar] [CrossRef]
- Ahmadi, R.; Asadpourchallou, N.; Kaleji, B.K. In vitro study: Evaluation of mechanical behavior, corrosion resistance, antibacterial properties and biocompatibility of HAp/TiO2/Ag coating on Ti6Al4V/TiO2 substrate. Surf. Interfaces 2021, 24, 101072. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Schmähling, J.; Hamprecht, F.A. Generalizing the Abbott–Firestone curve by two new surface descriptors. Wear 2007, 262, 1360–1371. [Google Scholar] [CrossRef]
- Matos, R.S.; Pinto, E.P.; Pires, M.A.; Ramos, G.Q.; Ţălu, Ş.; Lima, L.S.; da Fonseca Filho, H.D. Evaluating the roughness dynamics of kefir biofilms grown on Amazon cupuaçu juice: A monofractal and multifractal approach. Microscopy 2023, dfad040. [Google Scholar] [CrossRef]
- Ramos, G.Q.; Matos, R.S.; da Fonseca Filho, H.D. Advanced Microtexture Study of Anacardium occidentale L. Leaf Surface from the Amazon by Fractal Theory. Microsc. Microanal. 2020, 26, 989–996. [Google Scholar] [CrossRef]
- Korpi, A.G.; Ţălu, Ş.; Bramowicz, M.; Arman, A.; Kulesza, S.; Pszczolkowski, B.; Jurečka, S.; Mardani, M.; Luna, C.; Balashabadi, P.; et al. Minkowski functional characterization and fractal analysis of surfaces of titanium nitride films. Mater. Res. Express 2019, 6, 086463. [Google Scholar] [CrossRef]
- Zelati, A.; Mardani, M.; Rezaee, S.; Matos, R.S.; Pires, M.A.; Da Fonseca Filho, H.D.; Das, A.; Hafezi, F.; Rad, G.A.; Kumar, S.; et al. Morphological and multifractal properties of Cr thin films deposited onto different substrates. Microsc. Res. Tech. 2023, 86, 157–168. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Patra, N.; Salerno, M. Micromorphological characterization of polymer-oxide nanocomposite thin films by atomic force microscopy and fractal geometry analysis. Prog. Org. Coat. 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Salcedo, M.O.C.; Zamora, R.R.M.; Carvalho, J.C.T. Study fractal leaf surface of the plant species Copaifera sp. using the Microscope Atomic-Force-AFM. Rev. ECIPerú 2016, 13, 10–16. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Solaymani, S.; Bramowicz, M.; Naseri, N.; Kulesza, S.; Ghaderi, A. Surface micromorphology and fractal geometry of Co/CP/X (X = Cu, Ti, SM and Ni) nanoflake electrocatalysts. RSC Adv. 2016, 6, 27228–27234. [Google Scholar] [CrossRef]
- Bullmore, E.; Barnes, A.; Bassett, D.S.; Fornito, A.; Kitzbichler, M.; Meunier, D.; Suckling, J. Generic aspects of complexity in brain imaging data and other biological systems. Neuroimage 2009, 47, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.P.; Matos, R.S.; Pinto, E.P.; Santos, S.B.; Monteiro, M.D.S.; da Fonseca Filho, H.D.; Almeida, L.E. Advanced Microtexture Evaluation of Dextran Biofilms Obtained from Low Cost Substrate Loaded with Maytenus rigida Extract. Mater. Res. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Shakoury, R.; Arman, A.; Ţălu, Ş.; Ghosh, K.; Rezaee, S.; Luna, C.; Mwema, F.; Sherafat, K.; Salehi, M.; Mardani, M. Optical properties, microstructure, and multifractal analyses of ZnS thin films obtained by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2020, 31, 5262–5273. [Google Scholar] [CrossRef]
- Pinto, E.P.; Matos, R.S.; Pires, M.A.; Lima, L.d.S.; Ţălu, Ş.; da Fonseca Filho, H.D.; Ramazanov, S.; Solaymani, S.; Larosa, C. Nanoscale 3D Spatial Analysis of Zirconia Disc Surfaces Subjected to Different Laser Treatments. Fractal Fract. 2023, 7, 160. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, B.; Dey, A.; Mukhopadhyay, S.S. Studies on processing and characterization of hydroxyapatite biomaterials from different bio wastes. J. Miner. Mater. Charact. Eng. 2012, 11, 55–67. [Google Scholar] [CrossRef]
- Varma, H.K.; Babu, S. Synthesis of Calcium Phosphate Bioceramics by Citrate Gel Pyrolysis Method. Ceram. Int. 2005, 31, 109–114. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Obada, D.O.; Dauda, E.T.; Dodoo-Arhin, D. Experimental data on the characterization of hydroxyapatite synthesized from biowastes. Data Brief 2019, 26, 104485. [Google Scholar] [CrossRef]
- Rocha, J.H.G.; Lemos, A.F.; Kannan, S.; Agathopoulos, S.; Ferreira, J.M.F. Hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. J. Mater. Chem. 2005, 15, 5007–5011. [Google Scholar] [CrossRef]
- Can, H.K.; Kavlak, S.; ParviziKhosroshahi, S.; Güner, A. Preparation, characterization and dynamical mechanical properties of dextran-coated iron oxide nanoparticles (DIONPs). Artif. Cells Nanomed. Biotechnol. 2018, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Hradil, J.; Pisarev, A.; Babič, M.; Horák, D. Dextran-modified iron oxide nanoparticles. China Particuology 2007, 5, 162–168. [Google Scholar] [CrossRef]
- Salem, R.M.; Zhang, C.; Chou, L. Effect of Magnesium on Dentinogenesis of Human Dental Pulp Cells. Int. J. Biomater. 2021, 2021, 6567455. [Google Scholar] [CrossRef] [PubMed]
- NICDR. Dental Caries (Tooth Decay) in Adults (Age 20 to 64). 2014. Available online: http://www.nidcr.nih.gov.ezproxy.bu.edu/DataStatistics/FindDataByTopic/DentalCaries/DentalCariesAdults20to64.htm (accessed on 10 November 2023).
- Flaxman, D.A.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Memish, Z.A. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the global burden of disease study. Lancet 2012, 380, 2163–2196. [Google Scholar]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine; Karger: Basel, Switzerland, 1991. [Google Scholar]
- Joo, L.; Ong, D.; Chanm, C.N. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 1999, 28, 667–707. [Google Scholar]
- Marques, C.F.; Olhero, S.; Abrantes, J.C.C.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M.F. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Chung, R.-J.; Hsieh, M.-F.; Huang, C.-W.; Perng, L.-H.; Wen, H.-W.; Chin, T.-S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. B 2006, 76, 169–178. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Predoi, D.; Buton, N.; Megier, C.; Stan, G.E. Dextran-Thyme Magnesium-Doped Hydroxyapatite Composite Antimicrobial Coatings. Coatings 2020, 10, 57. [Google Scholar] [CrossRef]
- Monzavi, A.; Eshraghi, S.; Hashemian, R.; Momen-Heravi, F. In vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogens. Clin. Oral Investig. 2015, 19, 349–356. [Google Scholar] [CrossRef]
- Delbet, P. Politique Préventive du Cancer: Cytophylaxie; Denoël: Paris, France, 1944. [Google Scholar]
- Houlihan, A.J.; Russell, J.B. The effect of calcium and magnesium on the activity of bovicin HC5 and nisin. Curr. Microbiol. 2006, 53, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Patoare, Y.; Karim, P.; Rayhan, I.; Quadir, M.A.; Hasnat, A. Effect of magnesium and zinc on antimicrobial activities of some antibiotics. Pak. J. Pharm. Sci. 2005, 18, 57–61. [Google Scholar] [PubMed]
- Som, A.; Yang, L.; Wong, G.C.; Tew, G.N. Divalent metal ion triggered activity of a synthetic antimicrobial in cardiolipin membranes. J. Am. Chem. Soc. 2009, 131, 1510215103. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, L. Calcium and Magnesium Ions Are Membrane-Active against Stationary-Phase Staphylococcus aureus with High Specificity. Sci. Rep. 2016, 6, 20628. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mukherjee, S.; Matthews, P.M.; Hammad, L.A.; Kearns, D.B.; Dann, C.E. Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. J. Bacteriol. 2013, 195, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chai, Y.; Guo, J.H.; Losick, R. Evidence for cyclic Di-GMP-Mediated signaling in Bacillus subtilis. J. Bacteriol. 2012, 194, 5080–5090. [Google Scholar] [CrossRef]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6, 907. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.-Q.; Lin, Y.-J.; Wei, M.; Evans, D.G.; Duan, X. Controllable preparation of nano-MgO and investigation of its bactericidal properties. J. Inorg. Biochem. 2005, 99, 986–993. [Google Scholar] [CrossRef]
- Amiri, S.; Ramezani, R.; Aminlari, M. Antibacterial Activity of Dextran-Conjugated Lysozyme against Escherichia coli and Staphylococcus aureus in Cheese Curd. J. Food Prot. 2008, 71, 411–415. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Arnold, L.W.; Babcock, G.F. Dextran sulphate: An adjuvant for cell-mediated immune responses. Immunology 1977, 32, 963–974. [Google Scholar]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V. Influence of the Biological Medium on the Properties of Magnesium Doped Hydroxyapatite Composite Coatings. Coatings 2023, 13, 409. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gregorini, R.; Ripamonti, A.; Roveri, N.; Shah, J.S. The role of magnesium on the structure of biological apatites. Calcif. Tissue Int. 1992, 50, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Perez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B 2020, 191, 110996. [Google Scholar] [CrossRef]
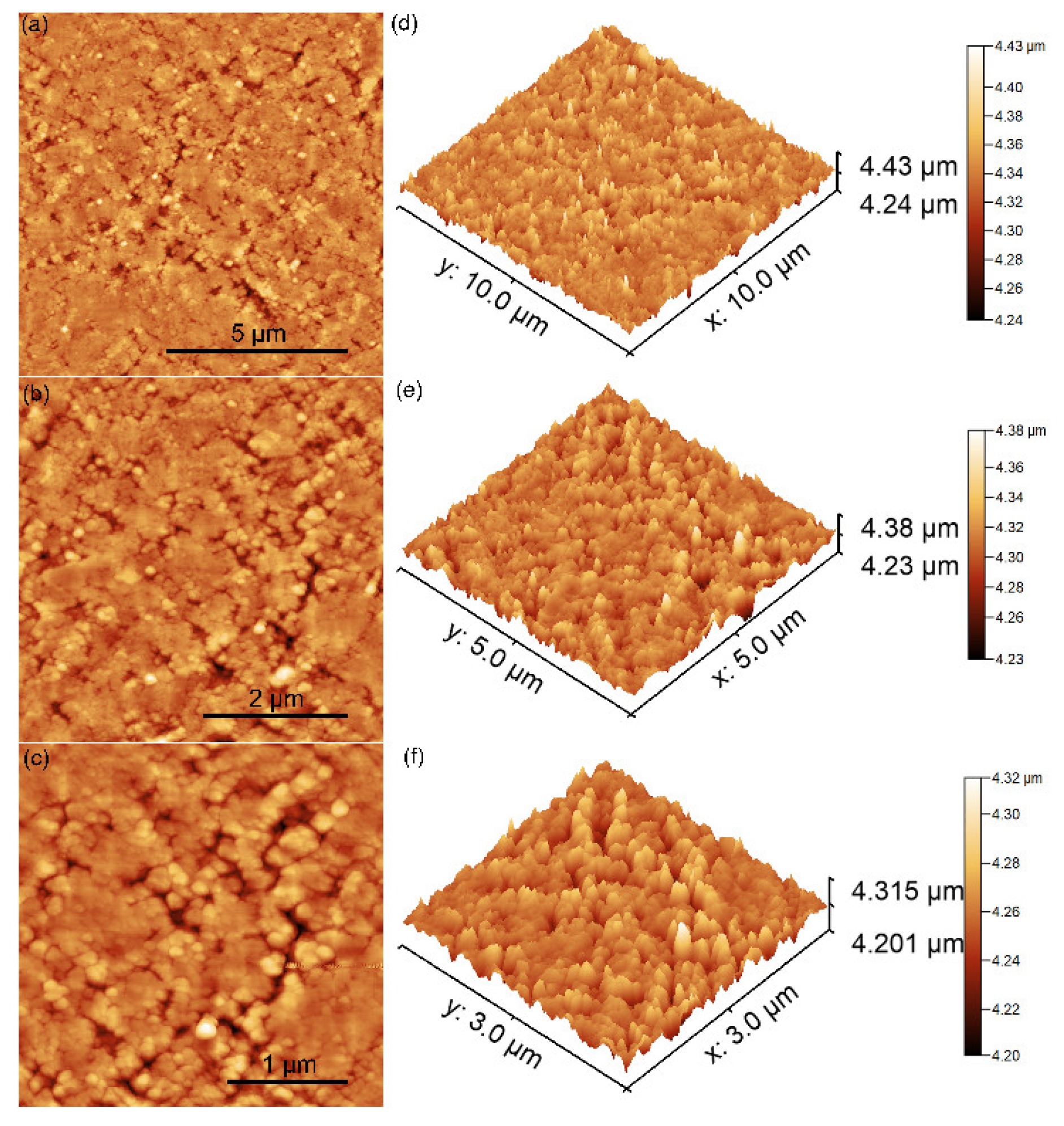
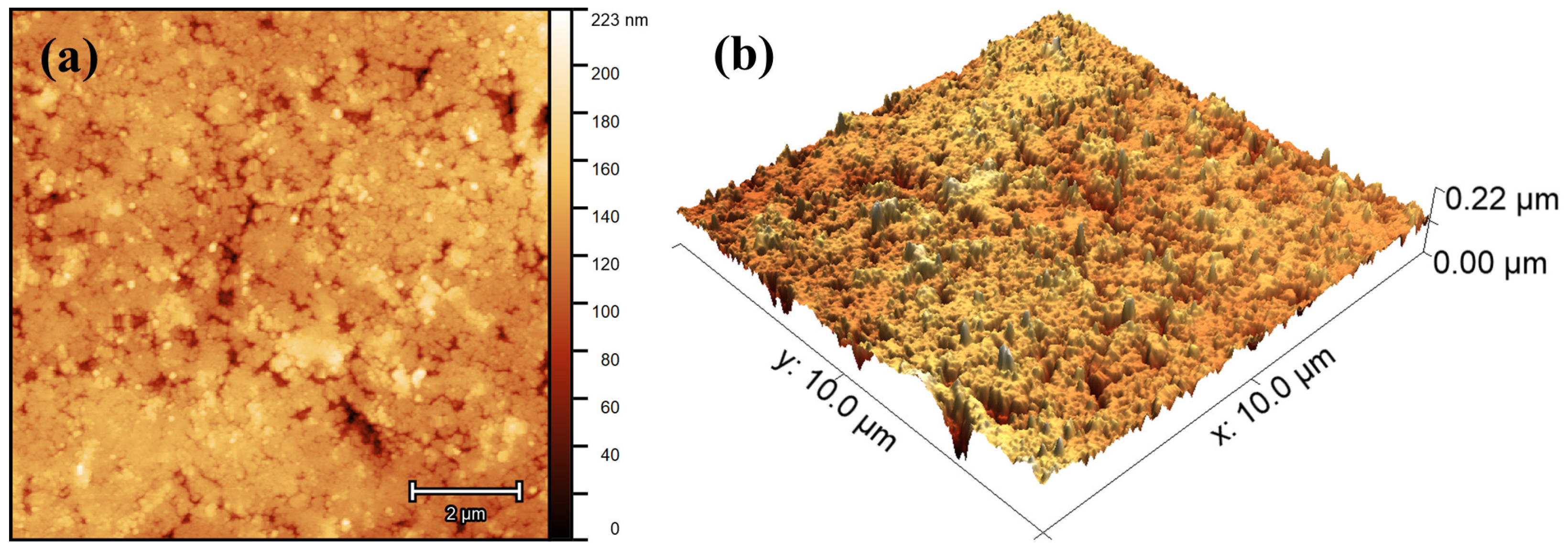
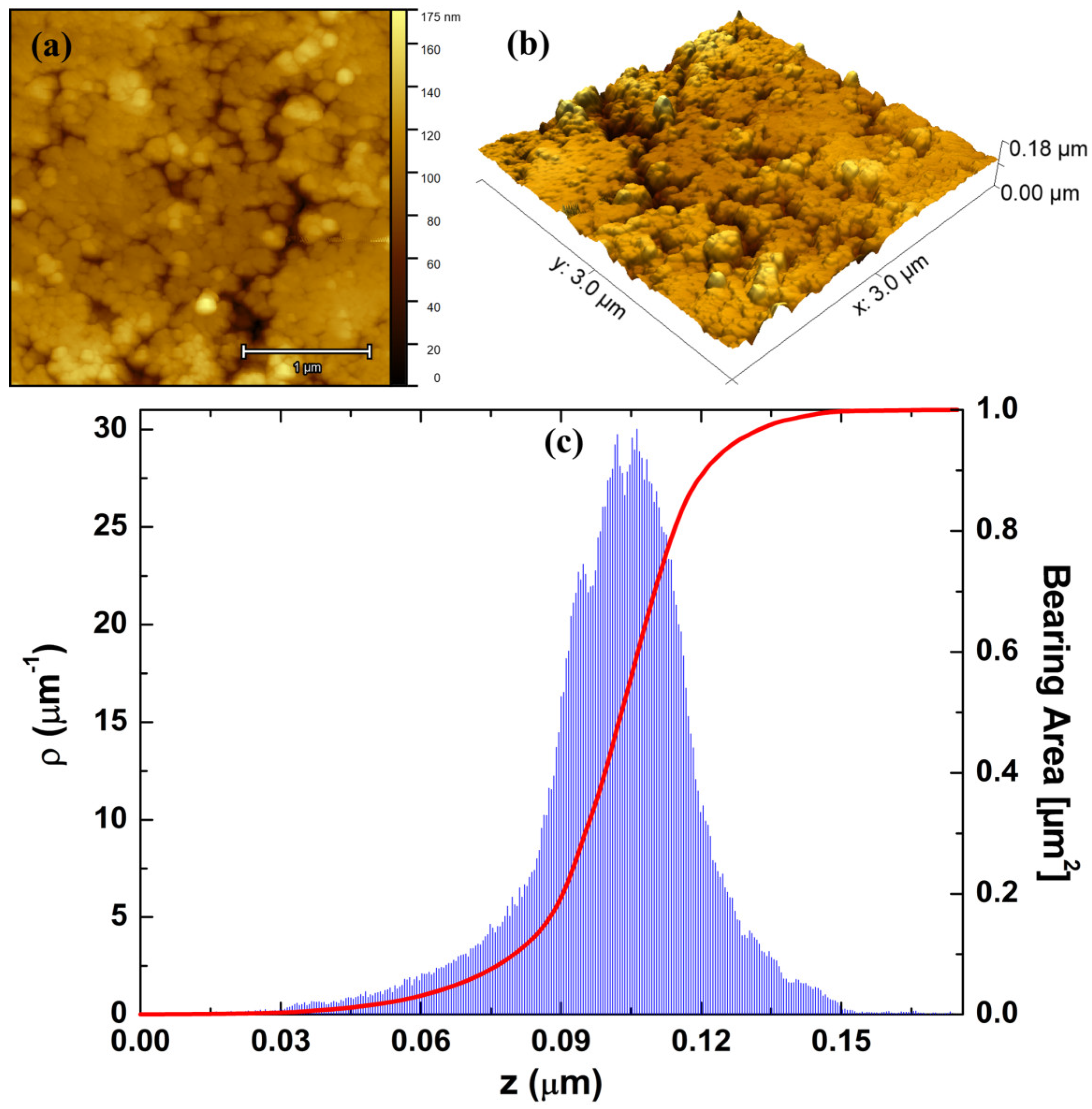
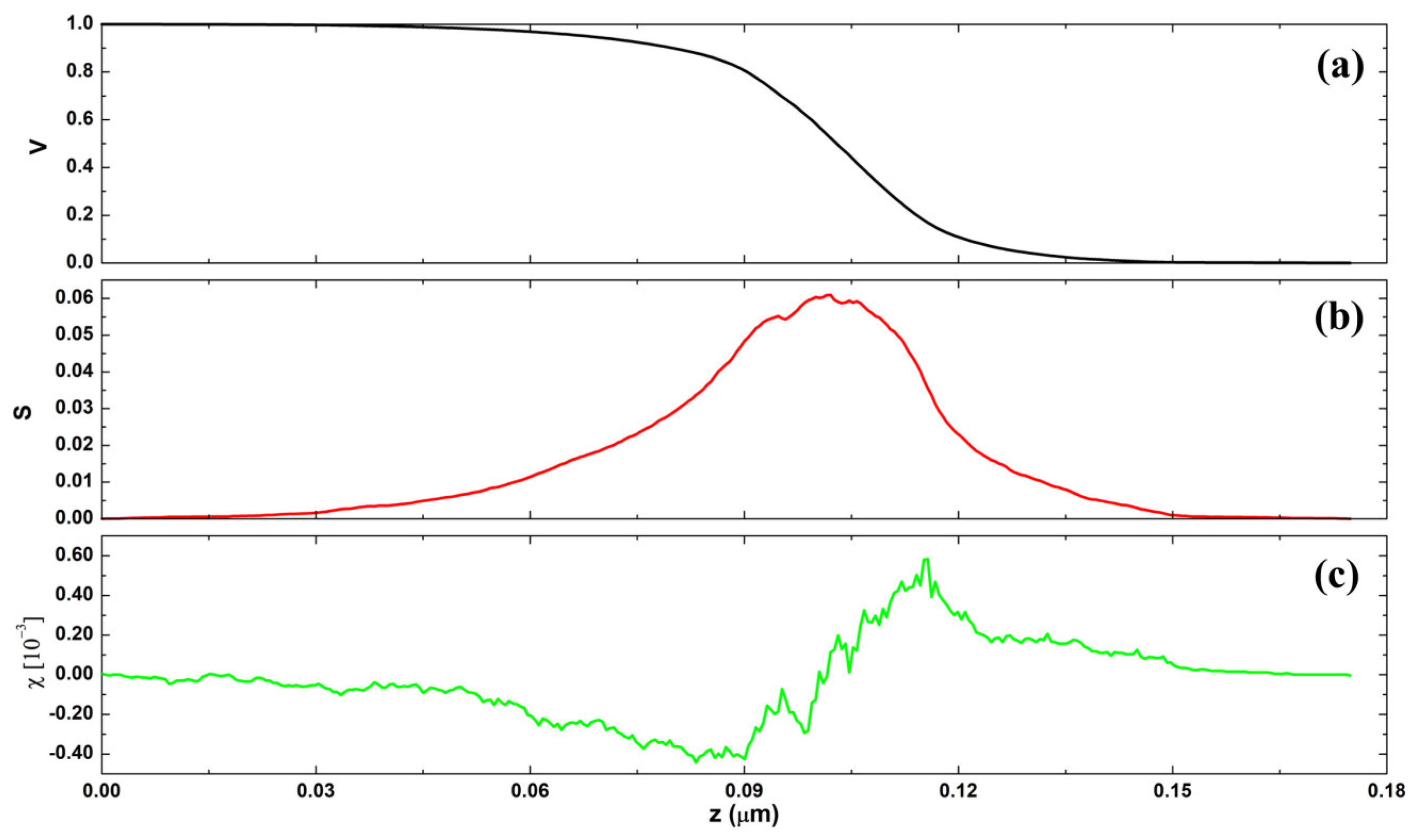

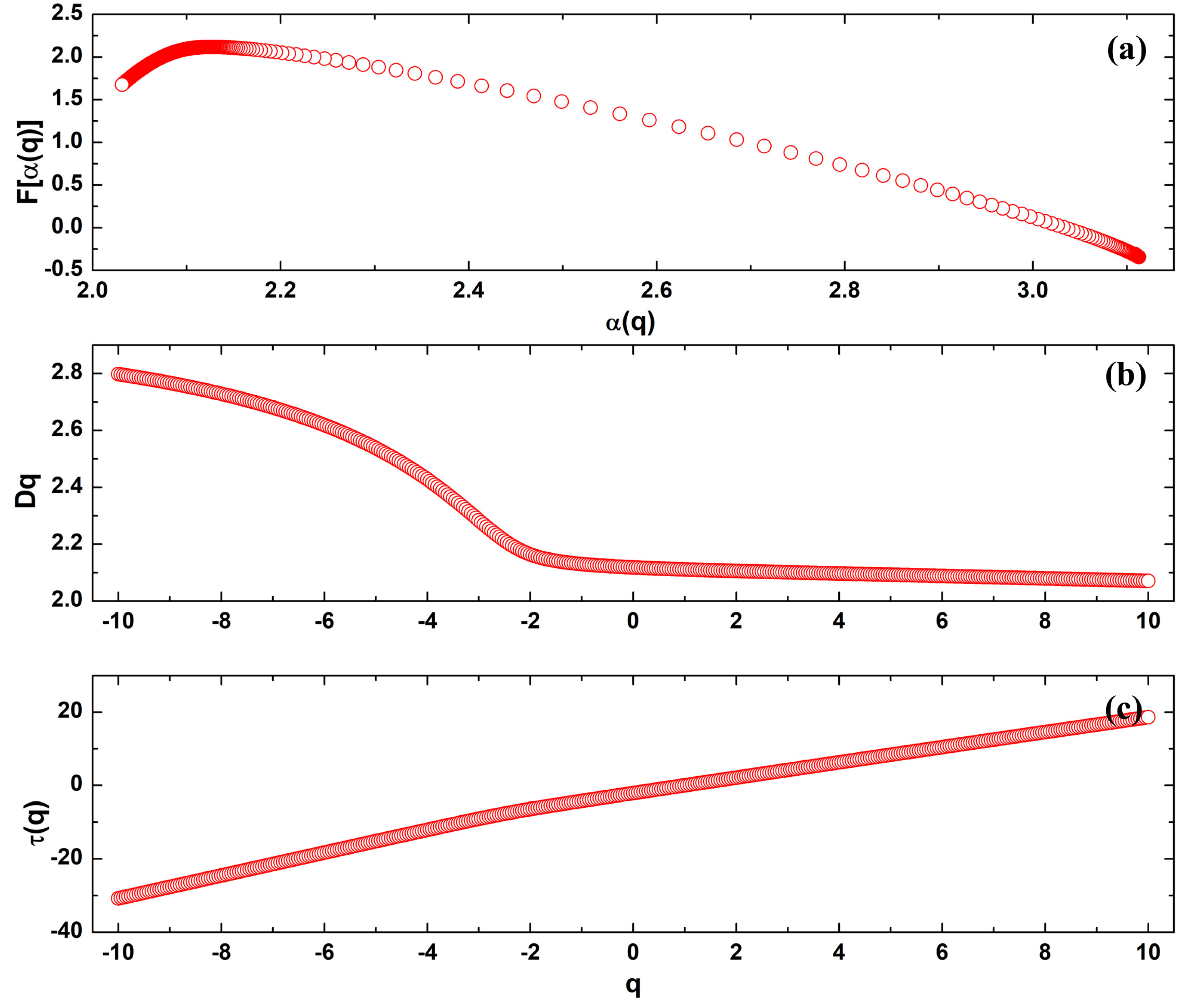

| Sample | Parameters | |||||
|---|---|---|---|---|---|---|
| Sa (nm) | Ssk | Sku | Sp (nm) | Sv (nm) | Sz (nm) | |
| 10MgHApD | 13.0 ± 0.2 | −0.7 ± 0.1 | 3.1 ± 0.3 | 68.2 ± 4.7 | 102.8 ± 0.8 | 180.9 ± 15.0 |
| Sample | Parameters | |||
|---|---|---|---|---|
| 10MgHApD | FD | H | FS | E |
| 2.243 ± 0.007 | 0.757 ± 0.007 | 0.361 ± 0.160 | 0.897 ± 0.006 | |
| Sample | Parameters | |||||
|---|---|---|---|---|---|---|
| αmax | αmin | Δa | f(αmax) | f(αmin) | Δf | |
| 10MgHApD | 3.109 | 2.031 | 1.078 | −0.310 | 1.675 | 1.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Ţălu, Ş.; Ghegoiu, L.; Matos, R.S.; da Fonseca Filho, H.D.; Trusca, R. New Physico-Chemical Analysis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites. Polymers 2024, 16, 125. https://doi.org/10.3390/polym16010125
Predoi D, Ciobanu SC, Iconaru SL, Ţălu Ş, Ghegoiu L, Matos RS, da Fonseca Filho HD, Trusca R. New Physico-Chemical Analysis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites. Polymers. 2024; 16(1):125. https://doi.org/10.3390/polym16010125
Chicago/Turabian StylePredoi, Daniela, Steluta Carmen Ciobanu, Simona Liliana Iconaru, Ştefan Ţălu, Liliana Ghegoiu, Robert Saraiva Matos, Henrique Duarte da Fonseca Filho, and Roxana Trusca. 2024. "New Physico-Chemical Analysis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites" Polymers 16, no. 1: 125. https://doi.org/10.3390/polym16010125
APA StylePredoi, D., Ciobanu, S. C., Iconaru, S. L., Ţălu, Ş., Ghegoiu, L., Matos, R. S., da Fonseca Filho, H. D., & Trusca, R. (2024). New Physico-Chemical Analysis of Magnesium-Doped Hydroxyapatite in Dextran Matrix Nanocomposites. Polymers, 16(1), 125. https://doi.org/10.3390/polym16010125













