Poly(ethylene glycol) Methyl Ether Acrylate-Grafted Chitosan-Based Micro- and Nanoparticles as a Drug Delivery System for Antibiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods and Instrumentation
2.2.1. CS-PEGA Synthesis
2.2.2. Preparation of MPs/NPs Based on Chitosan Derivative
2.2.3. Determination of Structural and Morphological Characteristics of MPs/NPs
2.2.4. MP/NP Water Sorption Capacity
2.2.5. MP/NP Drug-Loading Capacity
2.2.6. In Vitro Drug Release from MPs/NPs
2.2.7. MPs/NPs Hemocompatibility
2.2.8. MPs/NPs Cytotoxicity
Cell Culture
3. Results and Discussion
3.1. Preparation of MPs/NPs Based on Chitosan Derivative
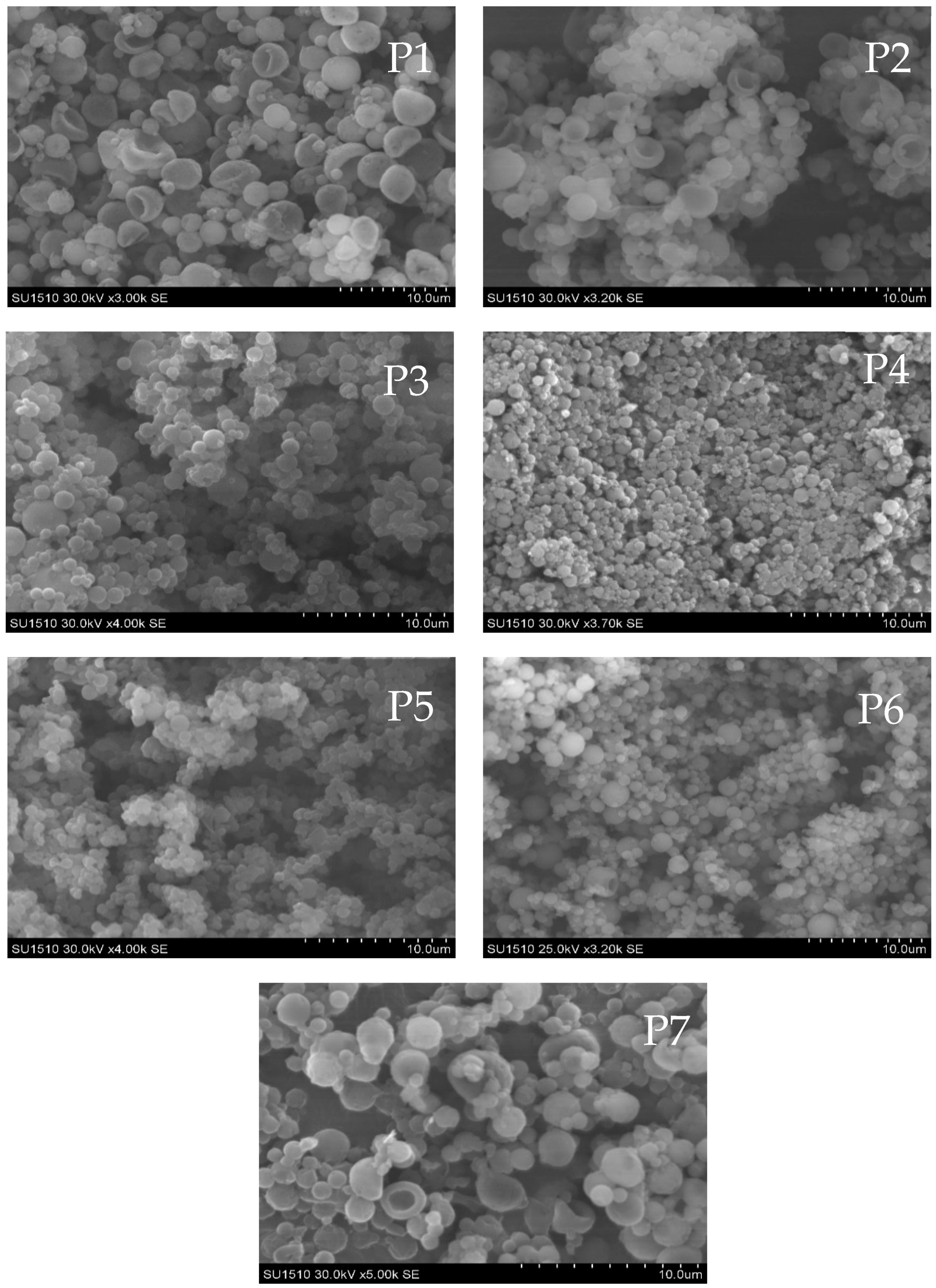
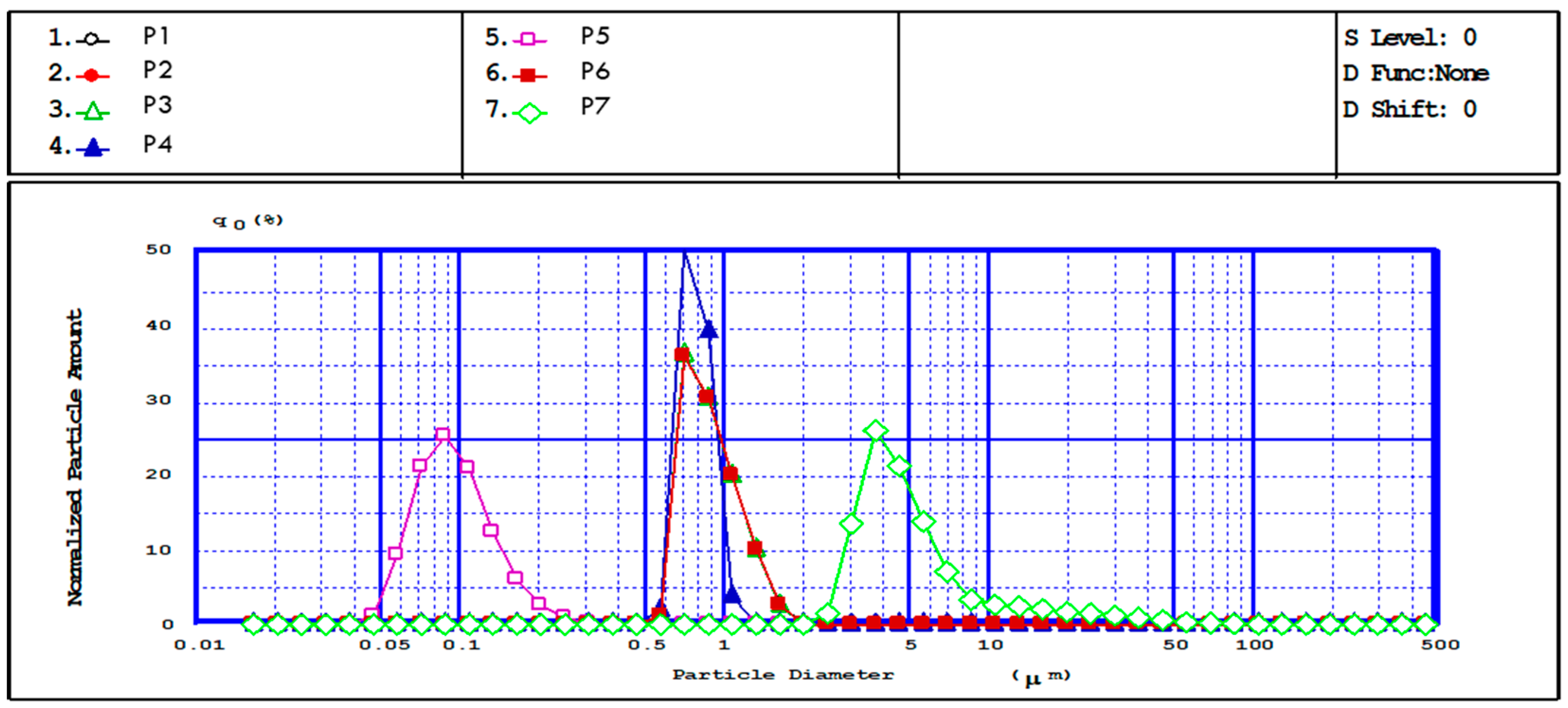
3.2. Structural and Morphological Characteristics of MPs/NPs
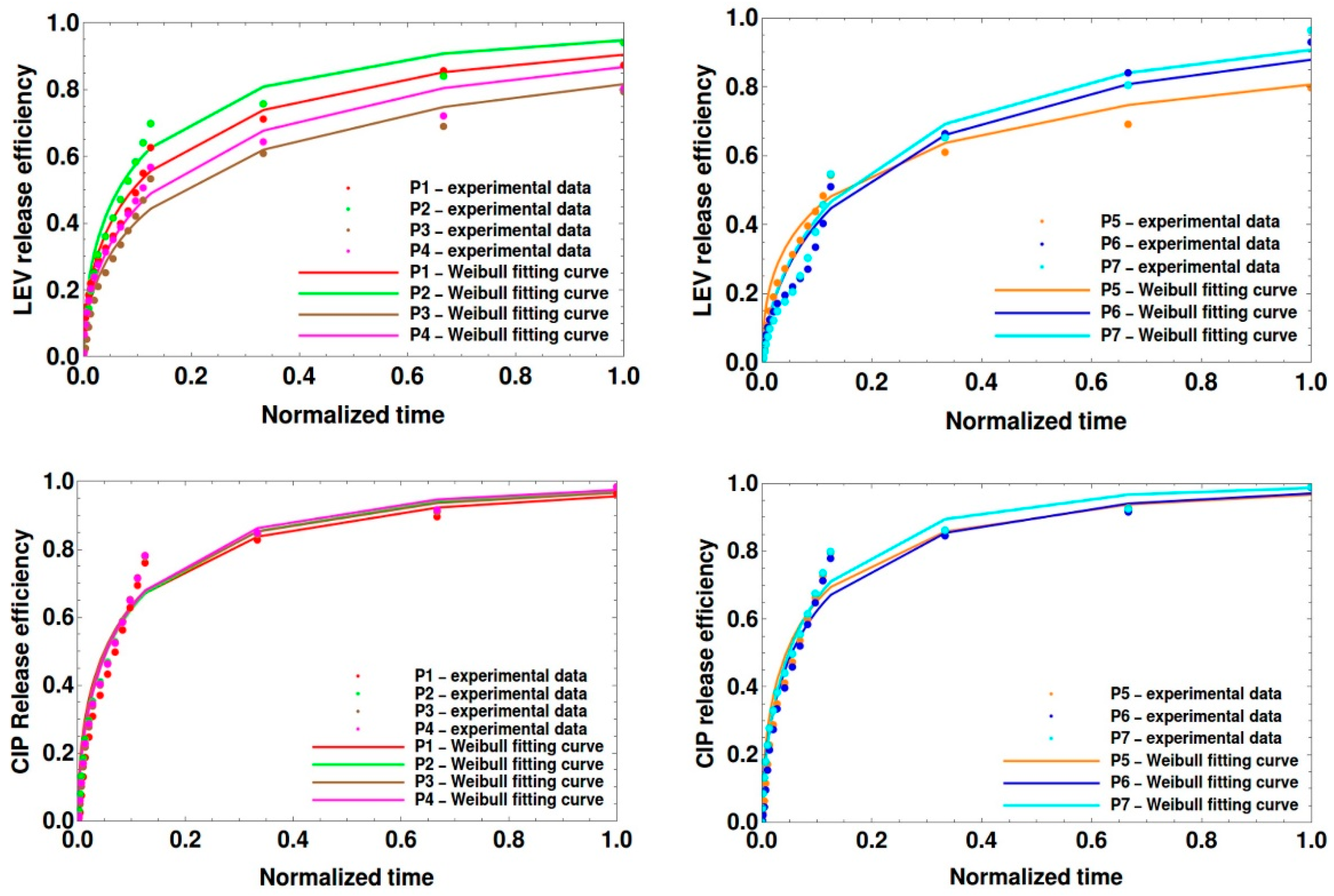
Theoretical Modeling of Drug Release
3.3. Biological Properties of MPs/NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Humelnicu, A.C.; Rotaru, R.; Fortuna, M.E.; Patras, X.; Teodorescu, M.; Tamba, B.I.; Harabagiu, V. Chitosan-Based Drug Delivery Systems. In Chitin and Chitosan; Broek, L.A.M., Boeriu, C.G., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2019; Chapter 11; pp. 260–289. [Google Scholar]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm. 1995, 116, 1–9. [Google Scholar] [CrossRef]
- Narguess, M.; Ahmed, H.M.; Elwahy, T.A.S.; Youssef, E.S.; Emtithal, A.E.S. Enhanced antibacterial activity of Egyptian local insects’ chitosan-based nanoparticles loaded with ciprofloxacin-HC. Int. J. Biol. Macromol. 2019, 126, 262–272. [Google Scholar]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based Nanoparticles as Drug Delivery Systems: A Review on Two Decades of Research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Barbieri, S.; Buttini, F.; Rossi, A.; Bettini, R.; Colombo, P.; Ponchel, G.; Sonvico, F. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015, 491, 99–104. [Google Scholar] [CrossRef]
- Islam, N.; Ferro, V. Recent Advances in Chitosan-Based Nanoparticulate Pulmonary Drug Delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2017, 13, 72–819. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H. Chitosan nanoparticles prepared by ionotropic gelation: An overview of recent advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Mazancová, P.; Némethová, V.; Treľová, D.; Kleščíková, L.; Lacík, I.; Rázga, F. Dissociation of chitosan/tripolyphosphate complexes into separate components upon pH elevation. Carbohydr. Polym. 2018, 192, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jătariu Cadinoiu, A.N.; Holban, M.N.; Peptu, C.A.; Sava, A.; Costuleanu, M.; Popa, M. Double crosslinked interpenetrated network in nanoparticle form for drug targeting--preparation, characterization and biodistribution studies. Int. J. Pharm. 2012, 436, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.A.; Buhus, G.; Popa, M.; Perichaud, A.; Costin, D. Double cross-linked chitosan–gelatin particulate systems for ophthalmic applications. J. Bioact. Compat. Polym. 2010, 25, 98–116. [Google Scholar] [CrossRef]
- Geng, Z.; Ji, Y.; Yu, S.; Liu, Q.; Zhou, Z.; Guo, C.; Lu, D.; Pei, D. Preparation and characterization of a dual cross-linking injectable hydrogel based on sodium alginate and chitosan quaternary ammonium salt. Carbohydr Res. 2021, 507, 108389. [Google Scholar] [CrossRef]
- Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N. About mechanism of Chitosan cross-linking with glutaraldehyde. Russ. J. Bioorg. Chem. 2009, 35, 360–369. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Hukkeri, V.I.; Sung, H.W.; Liang, H.F. A novel method for the synthesis of the PEG-crosslinked Chitosan with a pH-independent swelling behavior. Macromol. Biosci. 2005, 5, 925–928. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Popa, M. New folic acid-chitosan derivative based nanoparticles—Potential applications in cancer therapy. Cellul. Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Wulandari, I.O.; Santjojo, D.J.D.H.; Shobirin, R.A.; Sabarudin, A. Characteristics and magnetic properties of chitosan-coated fe3o4 nanoparticles prepared by ex-situ co-precipitation method. Rasayan J. Chem. 2017, 10, 1348–1358. [Google Scholar]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2021, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Logigan, C.L.; Delaite, C.; Tiron, C.E.; Peptu, C.; Popa, M.; Peptu, C.A. Chitosan Grafted Poly (Ethylene Glycol) Methyl Ether Acrylate Particulate Hydrogels for Drug Delivery Applications. Gels 2022, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemist 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41, 113–119. [Google Scholar] [CrossRef]
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A.; Zhao, X. Quinolones: Action and resistance updated. Curr. Top. Med. Chem. 2009, 9, 981–998. [Google Scholar] [CrossRef]
- Dalhoff, A. Resistance surveillance studies: A multifaceted problem—The fluoroquinolone example. Infection 2012, 40, 239–262. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–995. [Google Scholar] [CrossRef]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef] [PubMed]
- López-López, M.; Fernández-Delgado, A.; Moyá, M.L.; Blanco-Arévalo, D.; Carrera, C.; de la Haba, R.R.; Ventosa, A.; Bernal, E.; López-Cornejo, P. Optimized Preparation of Levofloxacin Loaded Polymeric Nanoparticles. Pharmaceutics 2019, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Mohammadi, S.S.; Montaseri, H.; Khezri, E. Nanoparticles of Chitosan Loaded Ciprofloxacin: Fabrication and Antimicrobial Activity. Adv. Pharm. Bull. 2017, 7, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Yin, R.; Ma, G.; Yang, D.; Nie, J. Electrospinning of methoxy poly(ethylene glycol)-grafted chitosan and poly(ethylene oxide) blend aqueous solution. Carbohydr. Polym. 2011, 83, 270–276. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Rajamanickam, V.M.; Yaspal, M.; Singh, S. Investigations on Agglomeration and Haemocompatibility of Vitamin E TPGS Surface Modified Berberine Chloride Nanoparticles. BioMed Res. Int. 2014, 2014, 951942. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Tiron, C.; Hoiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef]
- Shahriari, M.H.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Preparation and characterization of eugenol-loaded oligochitosan nanoparticles through sol–gel and emulsion/sol–gel methods. Polym. Bull. 2018, 75, 3035–3051. [Google Scholar] [CrossRef]
- Martins, A.F.; PiaiIvânia, J.F.; Schuquel, T.A.; Muniz, E.C. Polyelectrolyte complexes of chitosan/heparin and N,N,N-trimethyl chitosan/heparin obtained at different pH: I. Preparation, characterization, and controlled release of heparin. Colloid Polym. Sci. 2011, 289, 1133–1144. [Google Scholar] [CrossRef]
- Facchi, S.P.; Scariot, D.B.; Bueno, P.V.A.; Souza, P.R.; Figueiredo, L.C.; Follmann, H.D.M.; Martins, A.F. Preparation and cytotoxicity of N-modified chitosan nanoparticles applied in curcumin delivery. Int. J. Biol. Macromol. 2016, 87, 237–245. [Google Scholar] [CrossRef]
- Jafary, F.; Panjehpour, M.; Varshosaz, J.; Yaghmaei, P. Stability improvement of immobilized alkaline phosphatase using chitosan nanoparticles. Braz. J. Chem. Eng. 2016, 33, 243–250. [Google Scholar] [CrossRef]
- Contreras, C.A.; Sugita, S.; Ramos, E. Preparation of Sodium Aluminate From Basic Aluminium Sulfate. J. Mater. Process. Adv. Technol. 2006, 8, 122–129. [Google Scholar]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Likhitkar, S. Investigation of magnetically enhanced swelling behavior of superparamagnetic starch nanoparticles. Bull. Mater. Sci. 2013, 36, 15–24. [Google Scholar] [CrossRef]
- Hunter, R.J. Applications of the zeta potential. In Zeta Potential in Colloid Science: Principles and Applications; Hunter, R.J., Ed.; Academic Press: London, UK, 1988; pp. 219–257. [Google Scholar]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target. 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5—Mathematical Models of Drug Release; Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. ISBN 9780081000922. [Google Scholar]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, L.P.; Ghazali, M.; Ashrafi, H.; Azadi, A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon 2020, 6, 2. [Google Scholar]
- Kosmidis, K.; Macheras, P. On the dilemma of fractal or fractional kinetics in drug release studies: A comparison between Weibull and Mittag-Leffler functions. Int. J. Pharm. 2018, 543, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Jha, P.K. Studying Drug Release through Polymeric Controlled Release Formulations in United States Pharmacopoeia 2 Apparatus Using Multiphysics Simulation and Experiments. Mol. Pharm. 2021, 18, 2600–2611. [Google Scholar] [CrossRef]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical modelling of drug release from swellable polymeric nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Nottale, L. Scale Relativity and Fractal Space-Time—A New Approach to Unifying Relativity and Quantum Mechanics; Imperial College Press: London, UK, 2011. [Google Scholar]
- Băcăiță, E.S.; Peptu, C.A.; Savin (Logigan), C.L.; Luțcanu, M.; Agop, M. Manifest/non-manifest drug release patterns from polysaccharide based hydrogels—Case study on cyclodextrin—K carrageenan crosslinked hydrogels. Polymers 2021, 13, 4147. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Băcăiţă, S.; Radu, V.; Agop, M.; Ignat, L.; Uritu, C.M.; Maier, S.S.; Pinteala, M. Mathematical Modelling Of The Release Profile of Anthraquinone-Derived Drugs Encapsulated on Magnetite Nanoparticles. Rev. Roum. Chim. 2013, 58, 217–221. [Google Scholar]
- Amin, K.; Dannenfelser, R. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
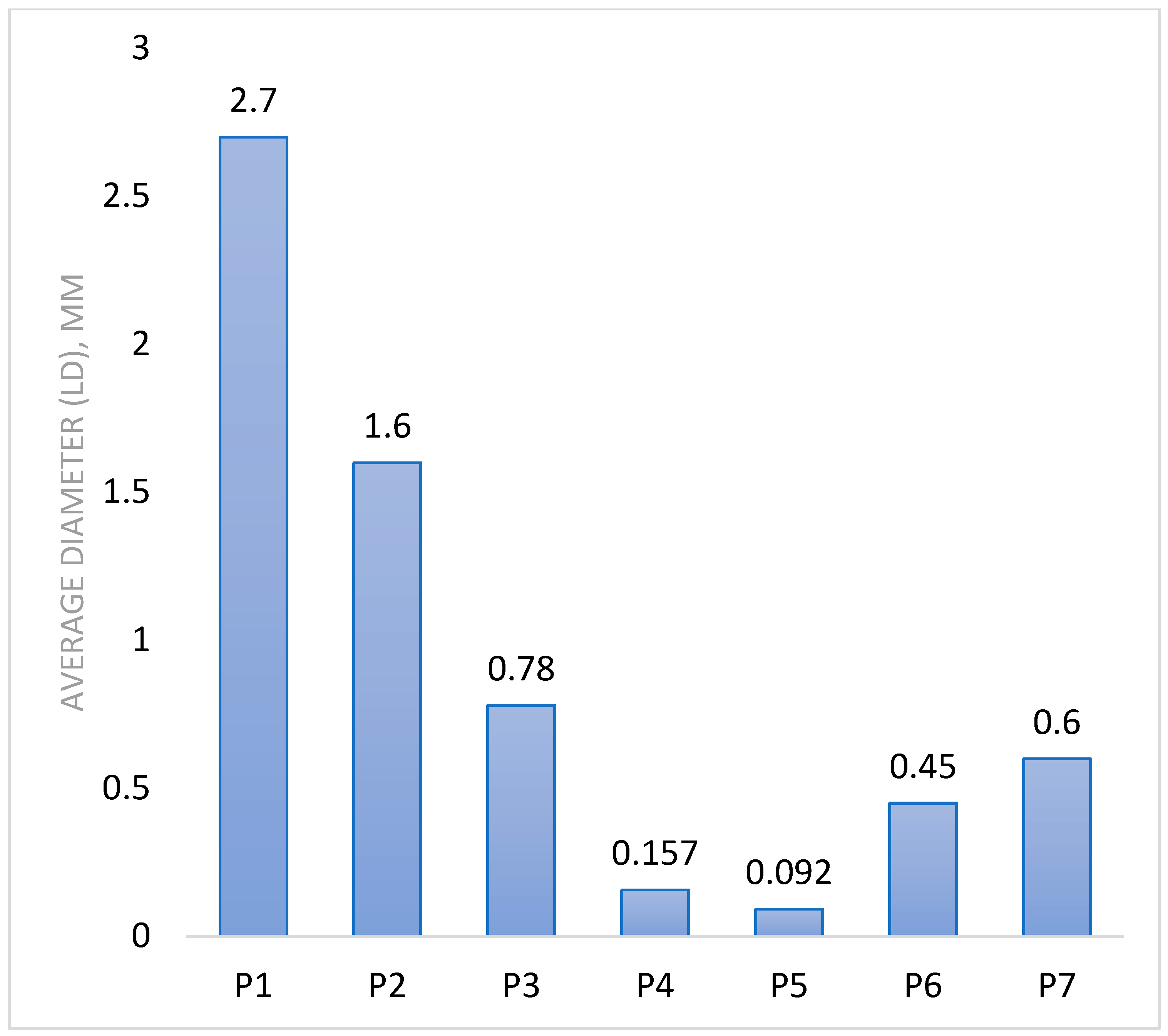
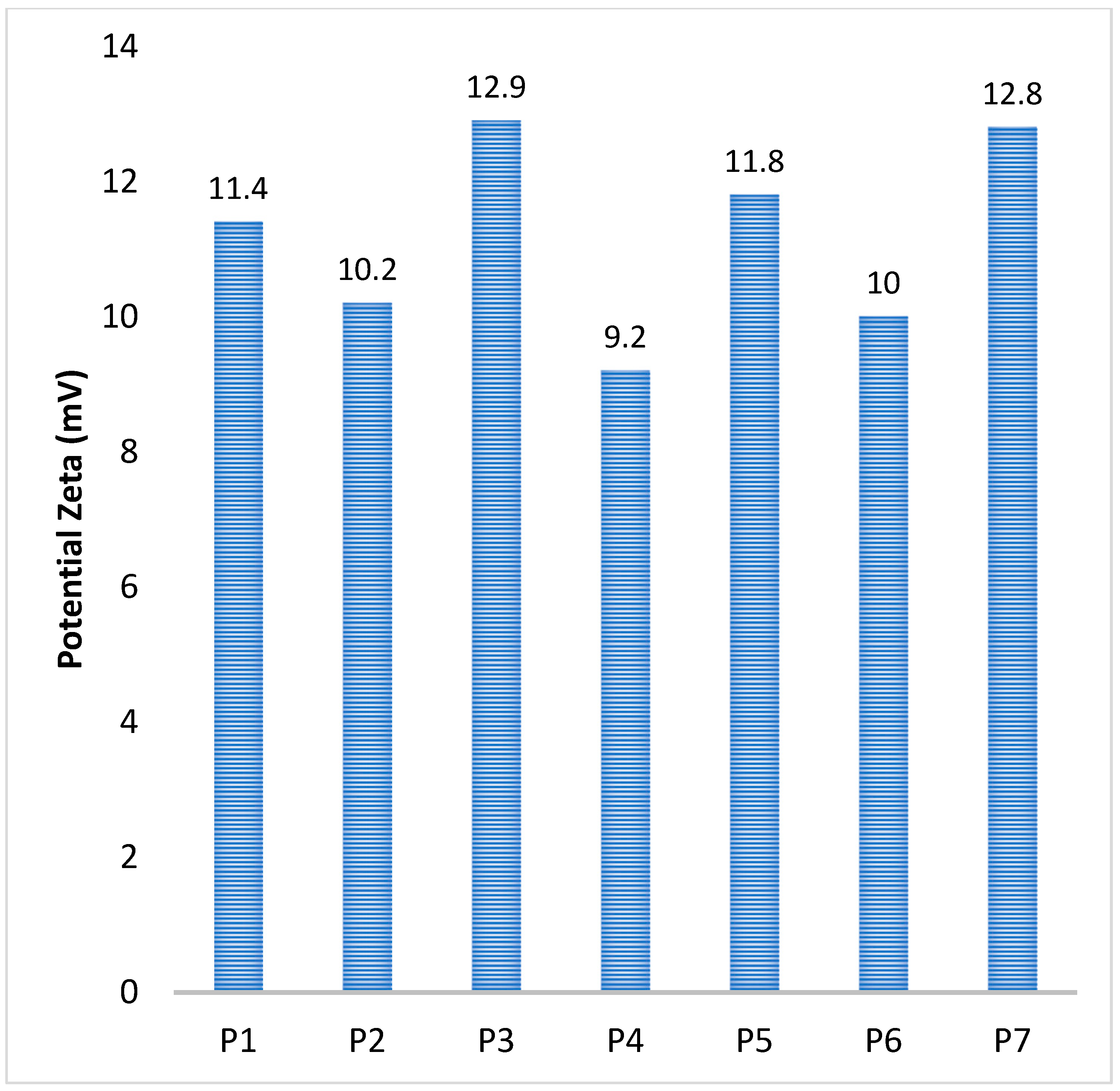



| Sample Code | Polymer Solution Concentration, % | Molar Ratio NH2/Na2SO4 | Speed, rpm | Water Phase, mL | The Organic Phase, mL | Surfactants Concentration, % | Ionic/Covalent Crosslinking Time, h | Average Diameter (LD), µm | Potential Zeta (mV) |
|---|---|---|---|---|---|---|---|---|---|
| P0 | 0.5 | 1:3 | 5000 | 50 | 200 | 2 | 1 | - | - |
| P1 | 0.5 | 1:4 | 5000 | 50 | 200 | 2 | 1 | 2.7 | 11.4 ± 0.2 |
| P2 | 0.5 | 1:4 | 9000 | 50 | 200 | 2 | 1 | 1.6 | 10.2 ± 0.3 |
| P3 | 0.5 | 1:4 | 12,000 | 50 | 200 | 2 | 1 | 0.78 | 12.9 ± 0.6 |
| P4 | 0.5 | 1:4 | 15,000 | 50 | 200 | 2 | 1 | 0.157 | 9.2 ± 0.3 |
| P5 | 0.35 | 1:4 | 15,000 | 50 | 200 | 2 | 1 | 0.092 | 11.8 ± 0.6 |
| P6 | 0.5 | 1:5 | 15,000 | 50 | 200 | 2 | 1 | 0.45 | 10 ± 0.4 |
| P7 | 0.75 | 1:4 | 15,000 | 50 | 200 | 2 | 1 | 0.6 | 12.8 ± 0.3 |
| P8 | 0.5 | 1:6 | 15,000 | 50 | 200 | 2 | 1 | 0.45 | 15 ± 0.4 |
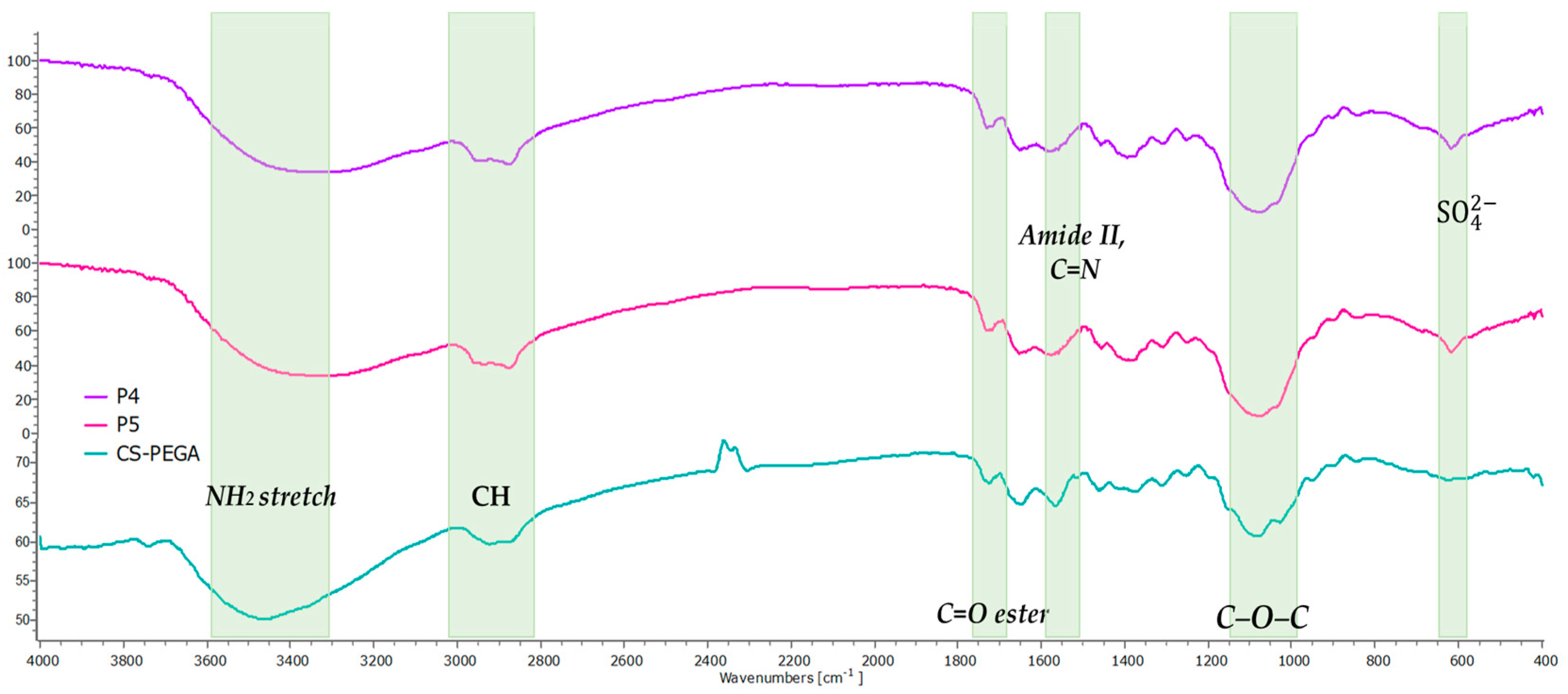
| Sample | Wavelength (cm−1) | Absorption Band |
|---|---|---|
| P4 and P5 | 617 | The new bond formed between the SO42− anions and NH3+ cations (ionic crosslinking process) |
| 1086 | Stretching vibrations -C-O-C | |
| 1575 | Imine linkages -C=N- |
| Sample Code | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
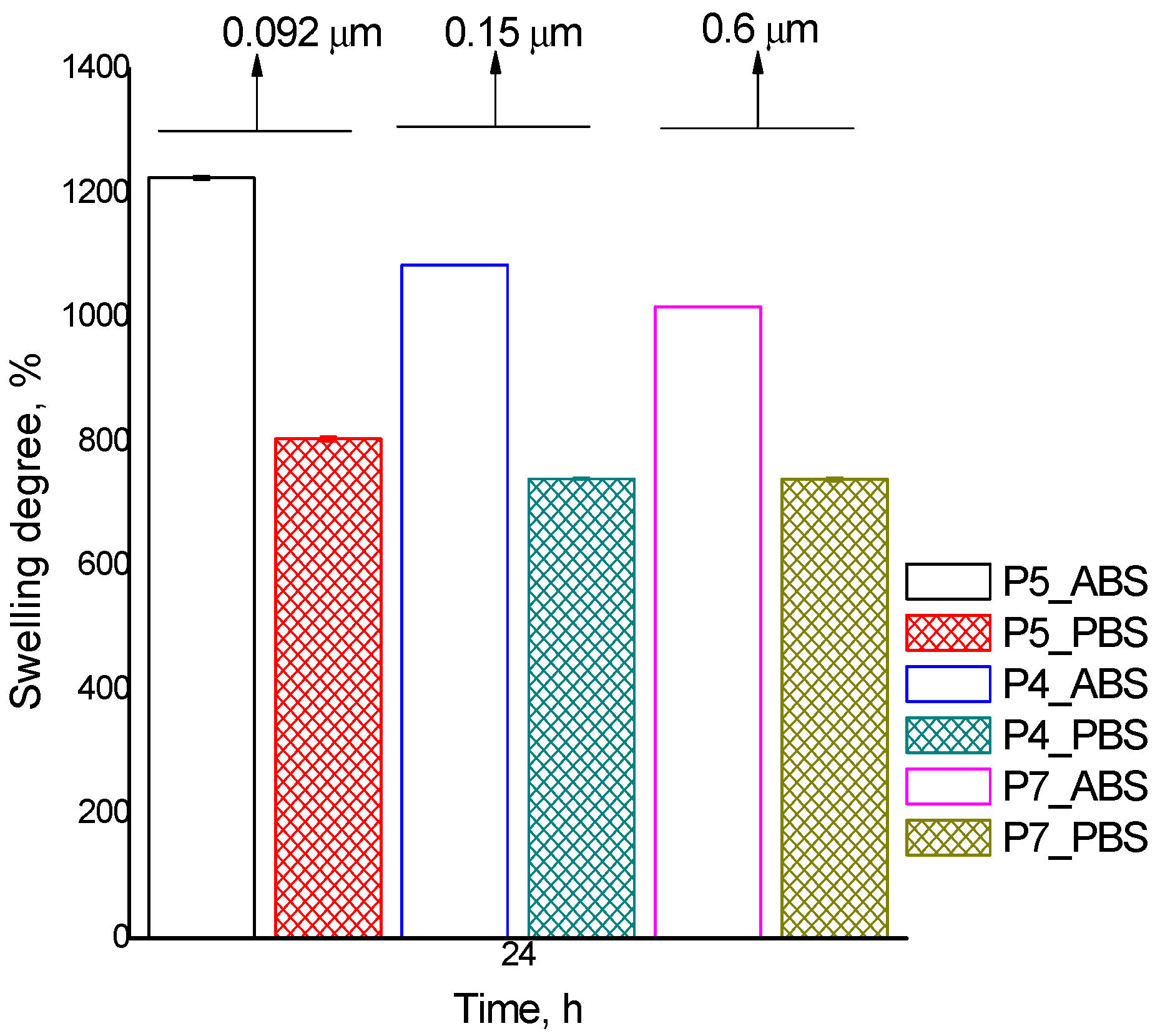
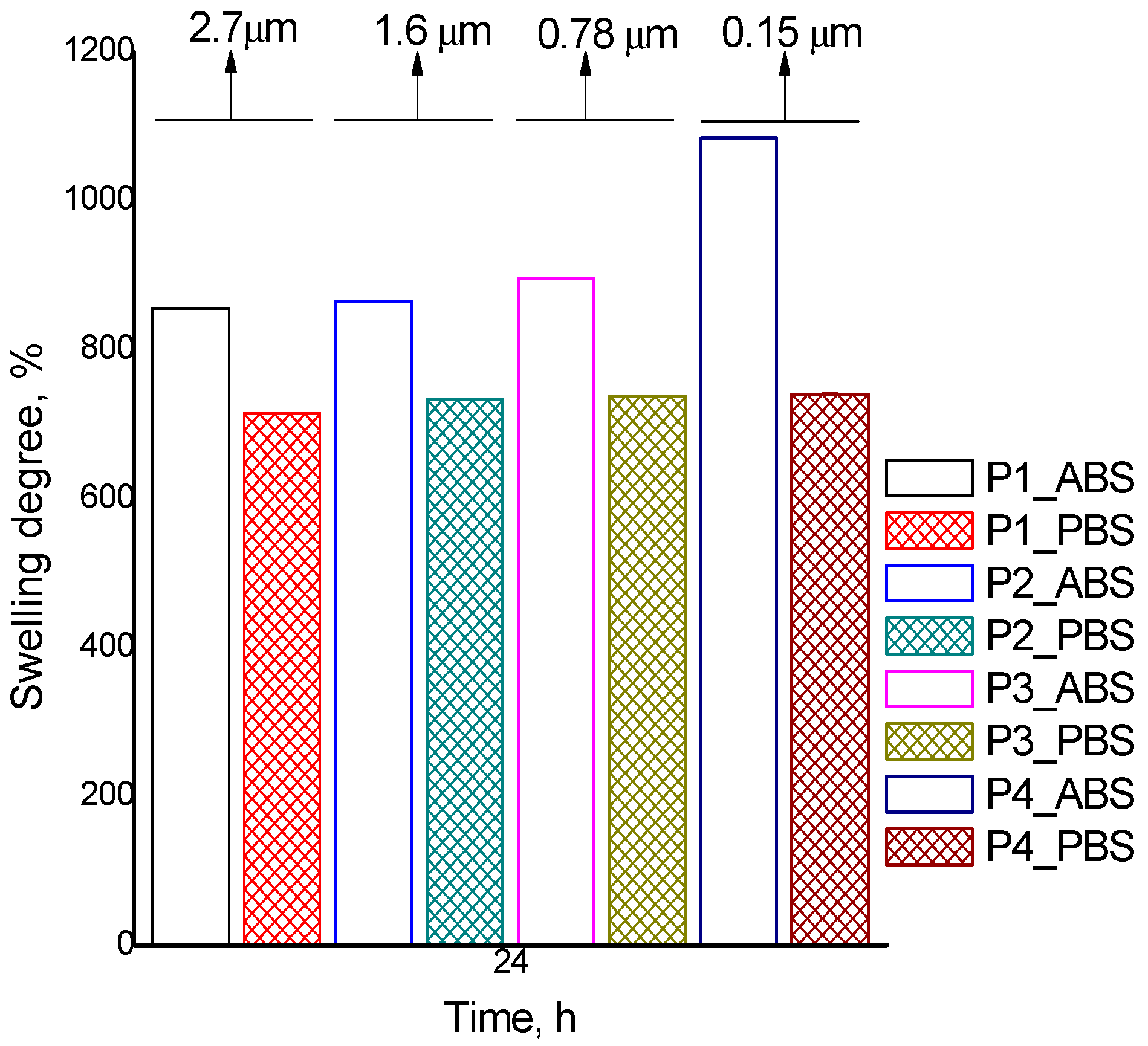
| Q ABS, % | 855 | 864 | 894 | 1084 | 1225 | 1046 | 1017 |
| Q PBS, % | 714 | 732 | 737 | 740 | 804 | 749 | 739 |
| Sample Code | P1 | P2 | P3 | P4 | P5 | P6 | P7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP |
| mg LEV/CIP/mg MPs/NPs | 1.663 | 1.617 | 1.609 | 1.627 | 1.956 | 1.633 | 2.010 | 1.665 | 2.054 | 1.666 | 1.698 | 1.666 | 1.624 | 1.638 |
| Encapsulation efficiency, % | 82.18 | 99.96 | 84.68 | 99.92 | 84.52 | 99.92 | 87.5 | 99.90 | 87.9 | 99.98 | 85.94 | 99.96 | 83.15 | 99.94 |
| Sample Code | P1 | P2 | P3 | P4 | P5 | P6 | P7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP | LEV | CIP |
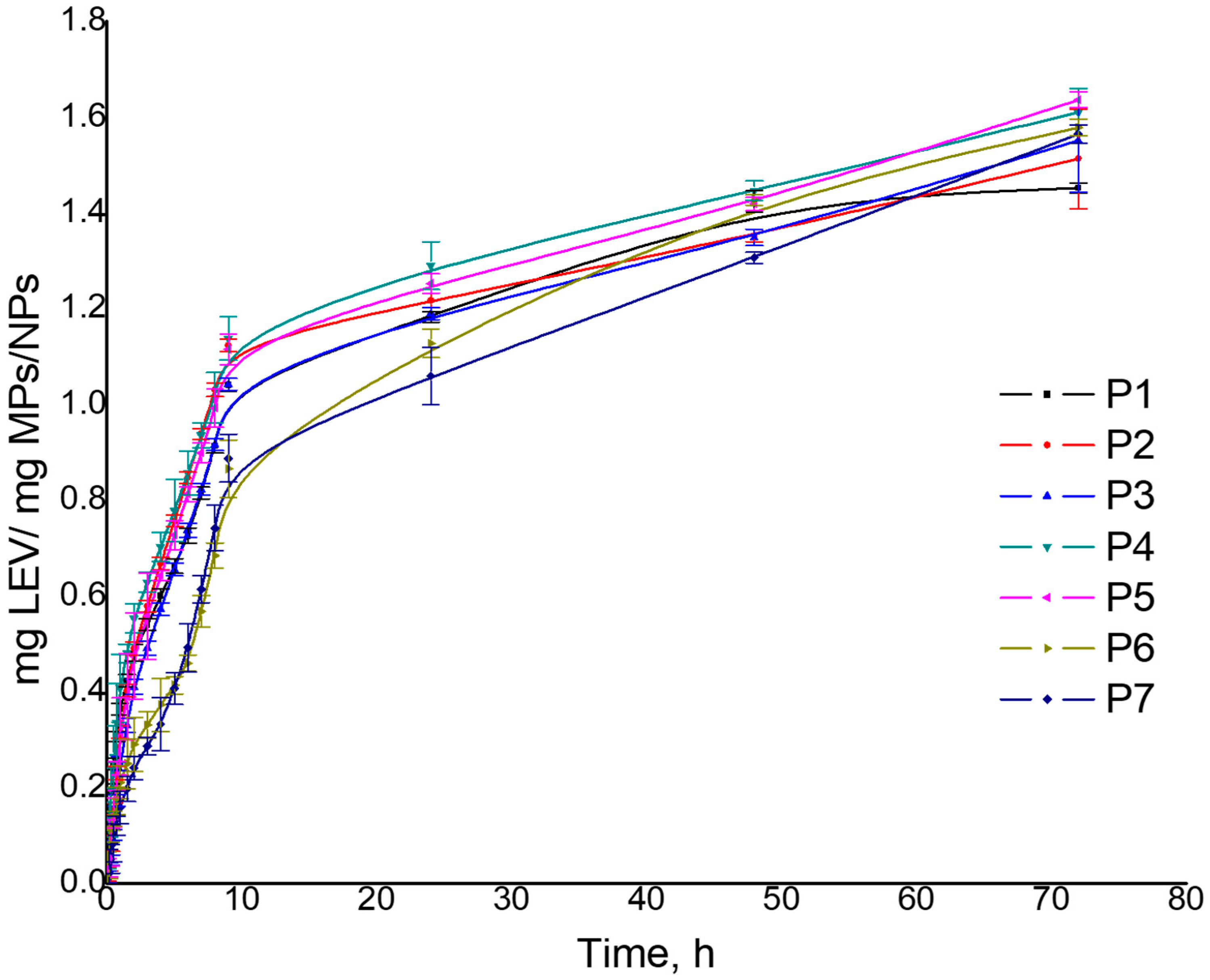
| mg LEV/CIP/mg MPs/NPs | 1.452 | 1.560 | 1.513 | 1.602 | 1.552 | 1.605 | 1.610 | 1.639 | 1.637 | 1.663 | 1.579 | 1.646 | 1.565 | 1.621 |
| Release efficiency, % | 87.29 | 96.43 | 93.98 | 98.43 | 79.30 | 98.33 | 80.25 | 98.42 | 79.68 | 99.81 | 92.95 | 98.80 | 96.35 | 98.94 |
| Sample Code | Polymer Solution Concentration (%) | Molar Ratio NH2/Na2SO4 | Speed (rpm) | Average Diameter (µm) | LEV | CIP | ||
|---|---|---|---|---|---|---|---|---|
| a | b | a | b | |||||
| P1 | 0.5 | 1:4 | 5000 | 2.7 | 2.36 | 0.51 | 3.16 | 0.5 |
| P2 | 0.5 | 1:4 | 9000 | 1.6 | 2.97 | 0.53 | 3.58 | 0.56 |
| P3 | 0.5 | 1:4 | 12,000 | 0.78 | 1.7 | 0.51 | 3.44 | 0.53 |
| P4 | 0.5 | 1:4 | 15,000 | 0.157 | 2.03 | 0.53 | 3.73 | 0.57 |
| P5 | 0.35 | 1:4 | 15,000 | 0.092 | 1.65 | 0.44 | 3.44 | 0.51 |
| P6 | 0.5 | 1:5 | 15,000 | 0.45 | 2.12 | 0.61 | 3.58 | 0.56 |
| P7 | 0.75 | 1:4 | 15,000 | 0.6 | 2.39 | 0.64 | 4.43 | 0.61 |
| Sample Code | Average Diameter (µm) | LEV | CIP | ||
|---|---|---|---|---|---|
| DF | DF | ||||
| P1 | 2.7 | 1.547 | 3.92 | 2.07 | 4.00 |
| P2 | 1.6 | 0.684 | 3.77 | 0.826 | 3.57 |
| P3 | 0.78 | 0.093 | 3.92 | 0.188 | 3.77 |
| P4 | 0.157 | 0.004 | 3.77 | 0.008 | 3.51 |
| P5 | 0.092 | 0.001 | 4.54 | 0.002 | 3.92 |
| P6 | 0.45 | 0.039 | 3.28 | 0.065 | 3.57 |
| P7 | 0.6 | 0.078 | 3.13 | 0.144 | 3.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logigan, C.-L.; Delaite, C.; Popa, M.; Băcăiță, E.S.; Tiron, C.E.; Peptu, C.; Peptu, C.A. Poly(ethylene glycol) Methyl Ether Acrylate-Grafted Chitosan-Based Micro- and Nanoparticles as a Drug Delivery System for Antibiotics. Polymers 2024, 16, 144. https://doi.org/10.3390/polym16010144
Logigan C-L, Delaite C, Popa M, Băcăiță ES, Tiron CE, Peptu C, Peptu CA. Poly(ethylene glycol) Methyl Ether Acrylate-Grafted Chitosan-Based Micro- and Nanoparticles as a Drug Delivery System for Antibiotics. Polymers. 2024; 16(1):144. https://doi.org/10.3390/polym16010144
Chicago/Turabian StyleLogigan, Corina-Lenuța, Christelle Delaite, Marcel Popa, Elena Simona Băcăiță, Crina Elena Tiron, Cristian Peptu, and Cătălina Anișoara Peptu. 2024. "Poly(ethylene glycol) Methyl Ether Acrylate-Grafted Chitosan-Based Micro- and Nanoparticles as a Drug Delivery System for Antibiotics" Polymers 16, no. 1: 144. https://doi.org/10.3390/polym16010144
APA StyleLogigan, C.-L., Delaite, C., Popa, M., Băcăiță, E. S., Tiron, C. E., Peptu, C., & Peptu, C. A. (2024). Poly(ethylene glycol) Methyl Ether Acrylate-Grafted Chitosan-Based Micro- and Nanoparticles as a Drug Delivery System for Antibiotics. Polymers, 16(1), 144. https://doi.org/10.3390/polym16010144









