Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
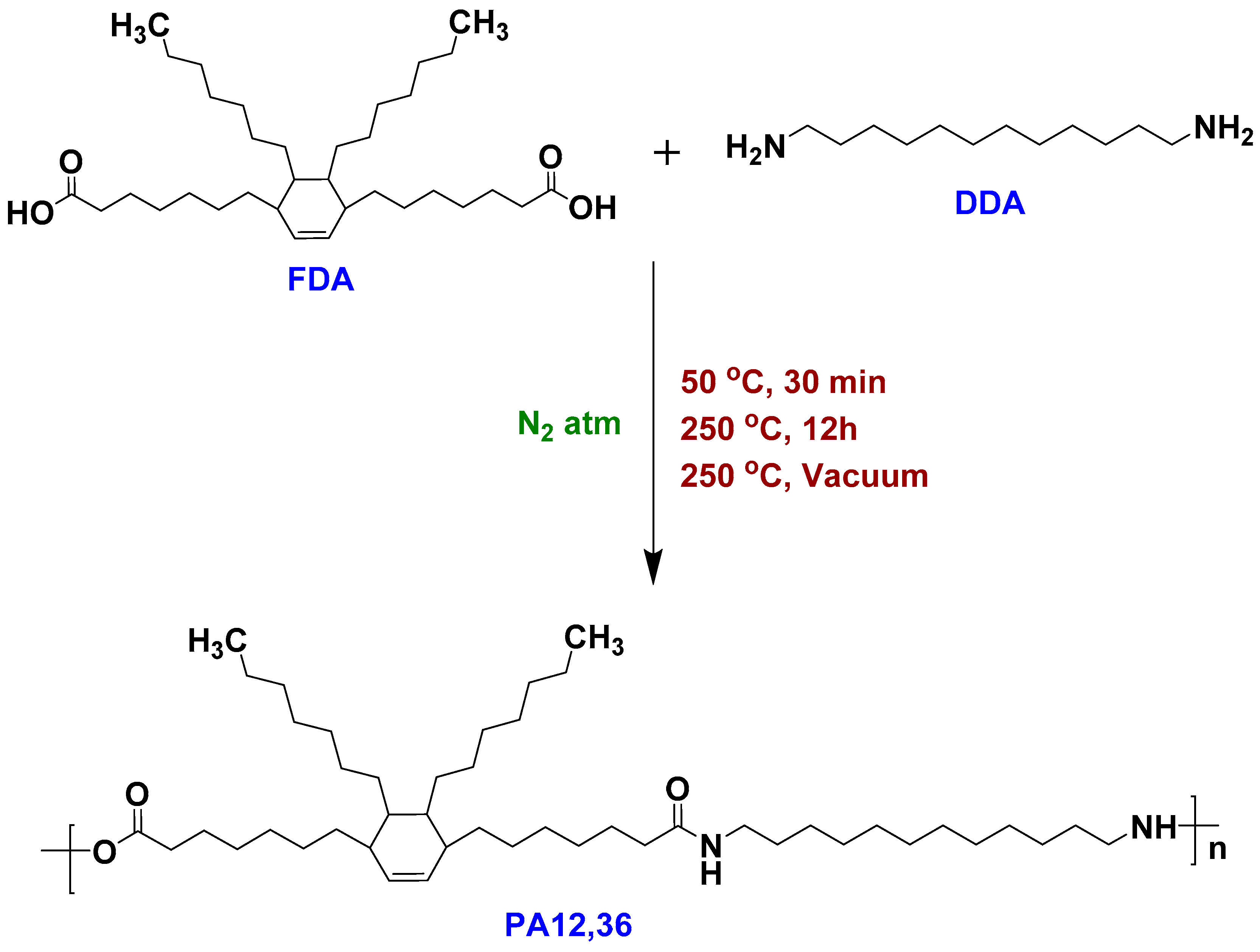
2.2. Synthesis of PA12,36
2.3. Foaming Process
2.4. Characterization
3. Results and Discussion
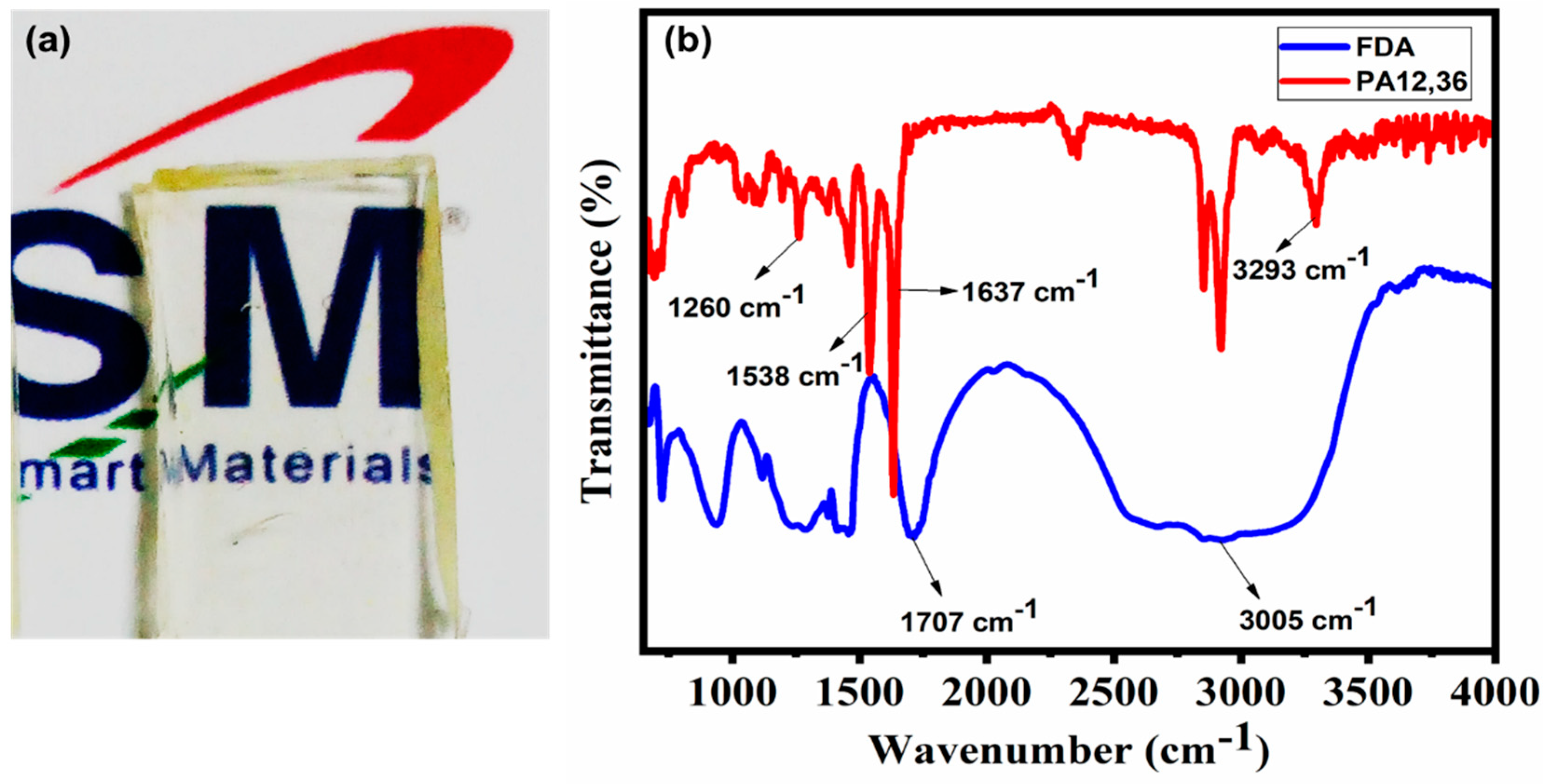
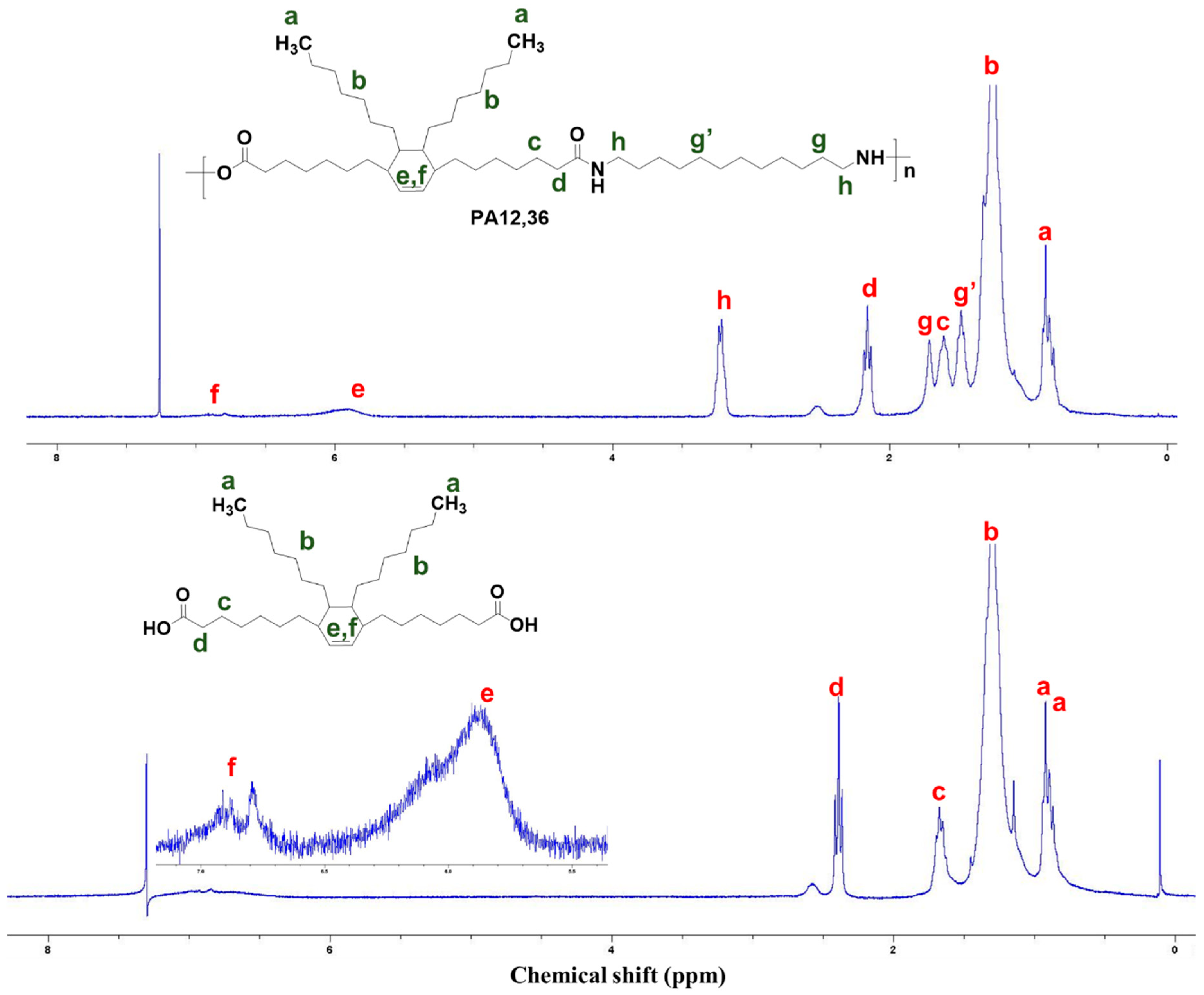
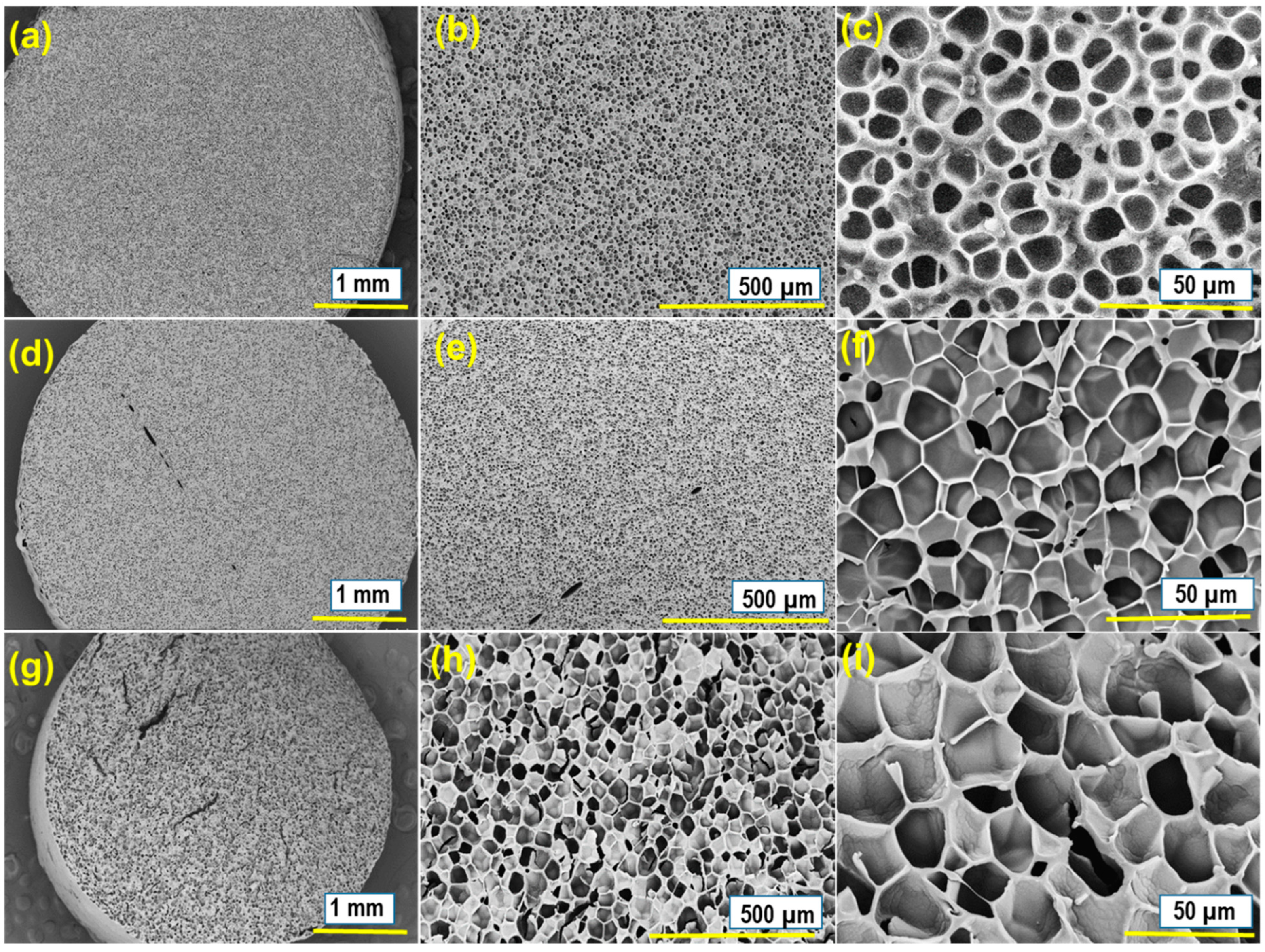
3.1. Structure Characterization
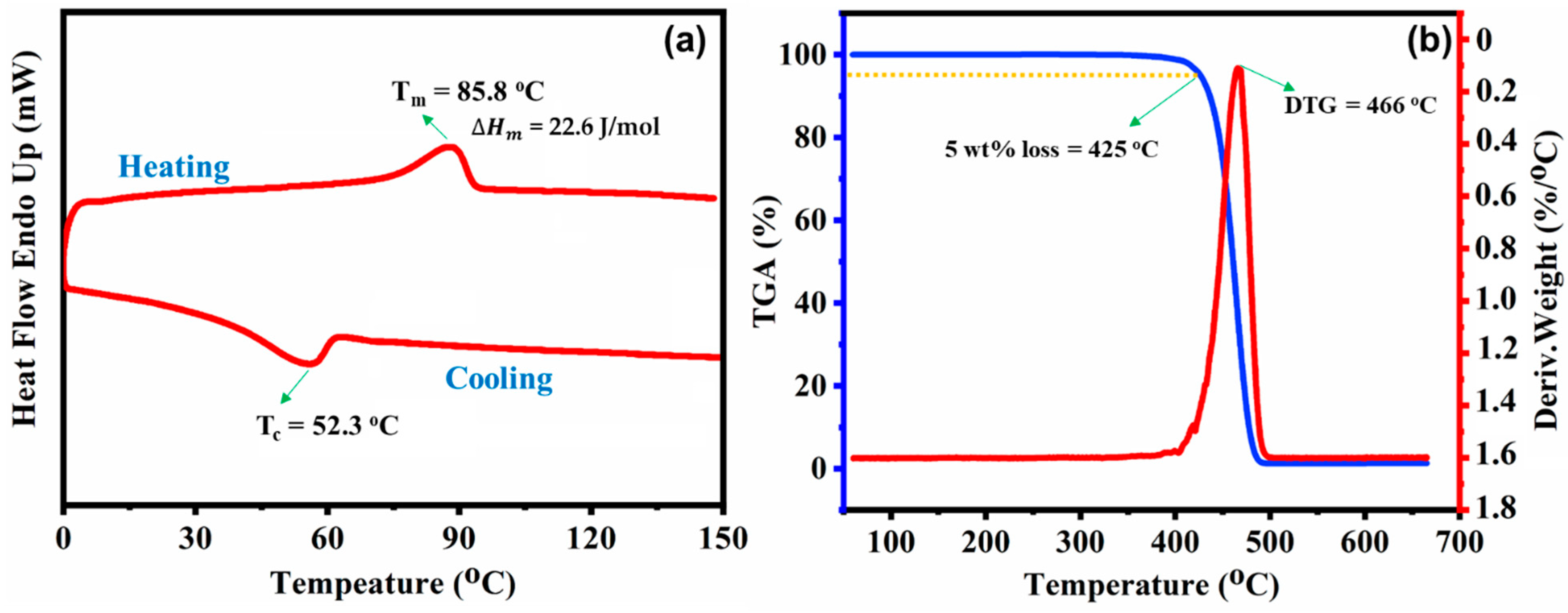
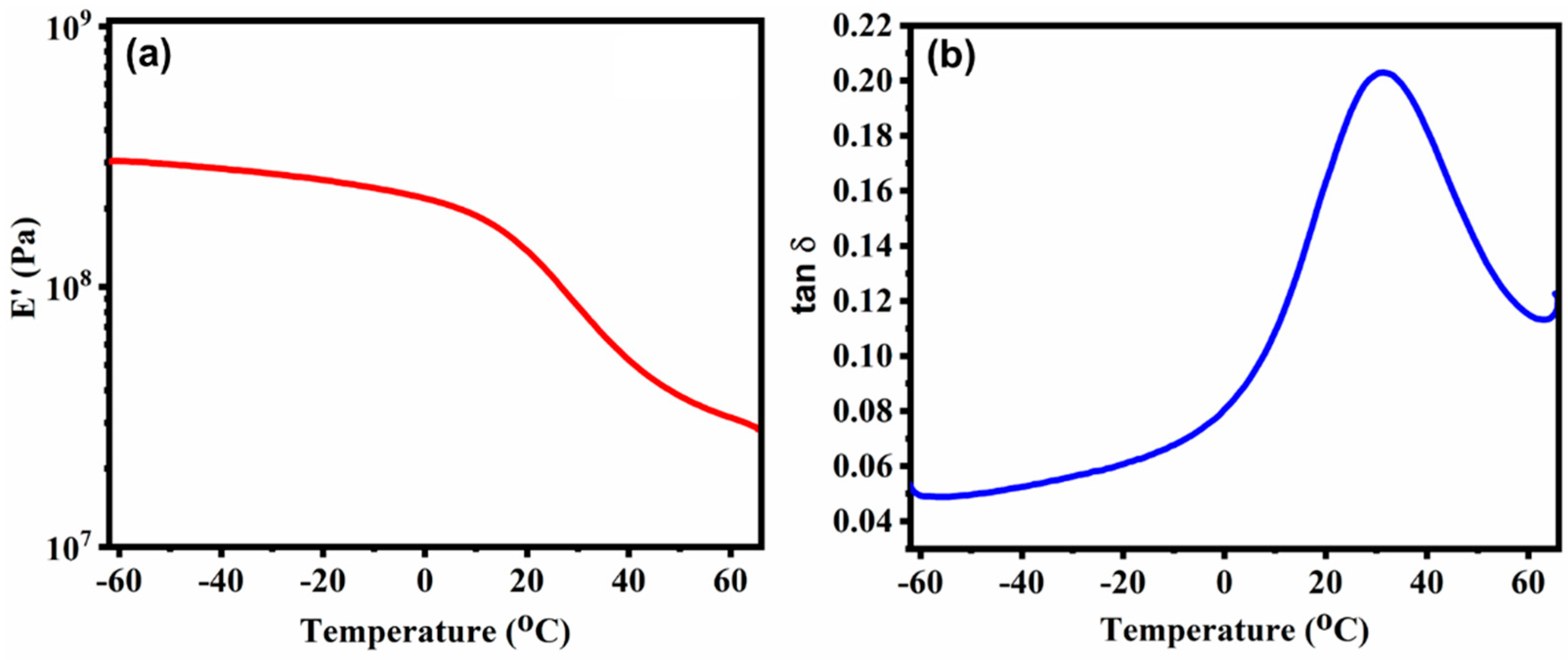
3.2. Thermal Properties
3.3. Tensile Properties
3.4. Foaming Performance
3.5. Expansion Ratio (ER), Average Cell Diameter (D), and Cell Density
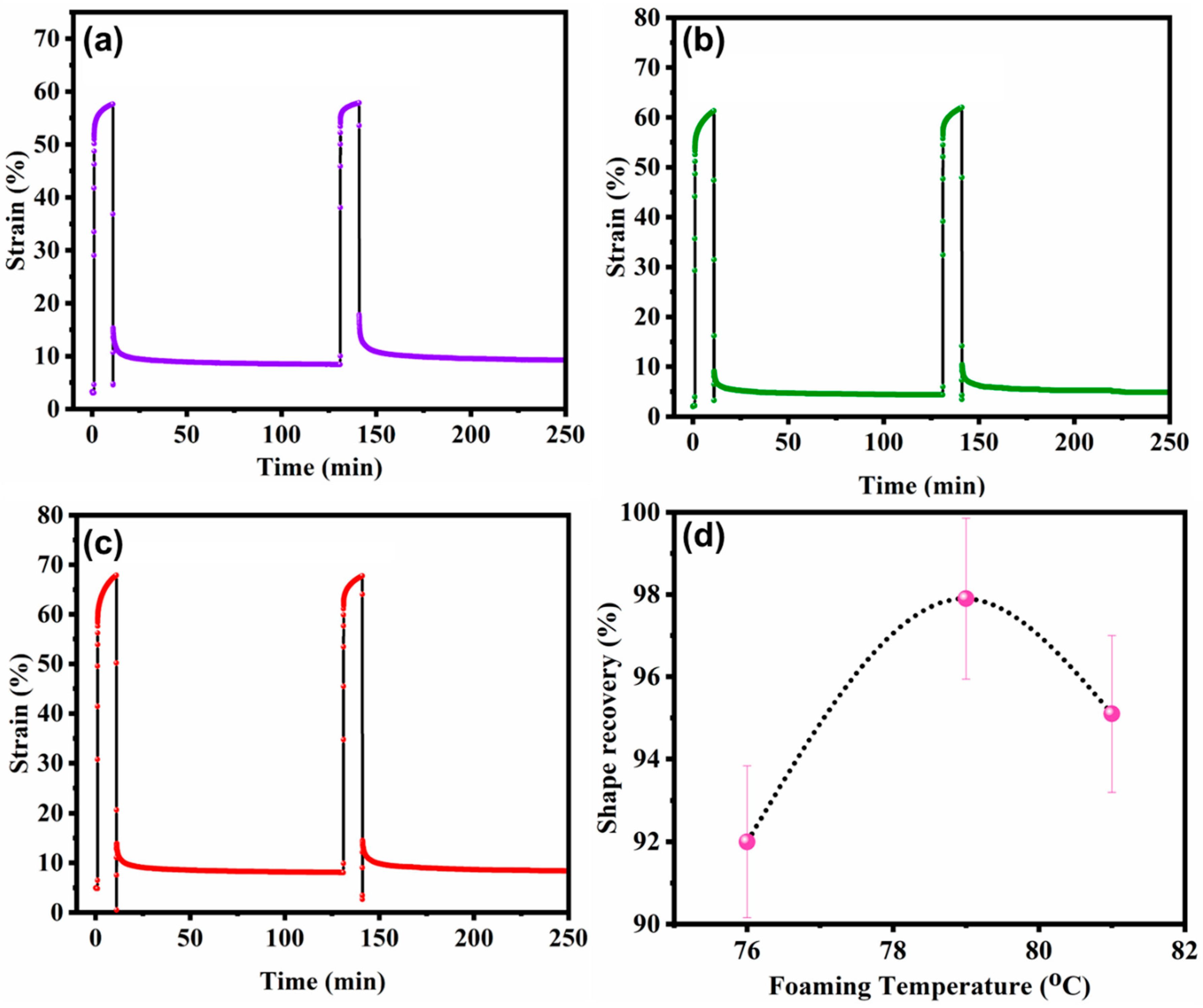
3.6. Cyclic Creep Recovery and Dimensional Stability
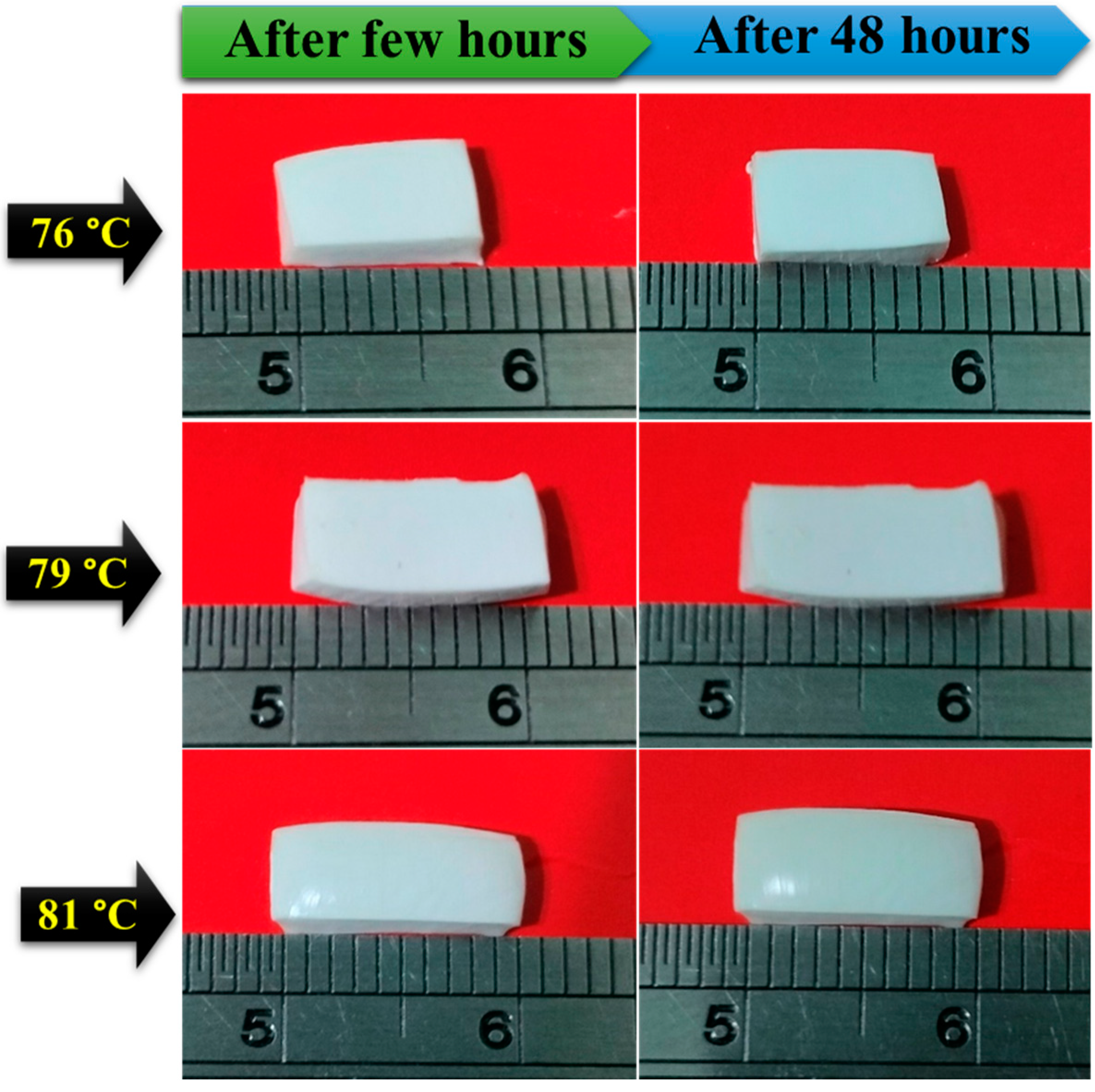
3.7. Dimensional Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer Nano-Foams for Insulating Applications Prepared from CO2 Foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Wang, C.; Park, C.B. Ultra-Lightweight, Super Thermal-Insulation and Strong Pp/CNT Microcellular Foams. Compos. Sci. Technol. 2020, 191, 108084. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Wang, G.; Qiao, Y.; Chen, Z.; Zhang, A.; Park, C.B. Fabrication of Outstanding Thermal-Insulating, Mechanical Robust and Superhydrophobic PP/CNT/Sorbitol Derivative Nanocomposite Foams for Efficient Oil/Water Separation. J. Hazard. Mater. 2021, 418, 126295. [Google Scholar] [CrossRef] [PubMed]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A Review and Evaluation of Thermal Insulation Materials and Methods for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Dickson, T.; Pavía, S. Energy Performance, Environmental Impact and Cost of A Range Of Insulation Materials. Renew. Sustain. Energy Rev. 2021, 140, 110752. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Muraza, O.; Aditiya, H.B. A Review on Insulation Materials for Energy Conservation in Buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, S.; Chen, X.; Liu, H.; Deng, J.; Li, S.; Feng, X.; Li, Y.; Wu, X.; Pan, K. Thermoplastic Polyamide Elastomers: Synthesis, Structures/Properties, and Applications. Macromol. Mater. Eng. 2021, 306, 100568. [Google Scholar] [CrossRef]
- Marchildon, K. Polyamides–still strong after seventy years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Pervaiz, M.; Faruq, M.; Jawaid, M.; Sain, M. Polyamides: Developments and Applications towards Next-Generation Engineered Plastics. Curr. Org. Synth. 2017, 14, 146–155. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Ge, Y.; Zhao, C.; Wei, L.; Hu, D.; Liu, T.; Zhang, L.; Zhao, L.; Park, C.B. Microcellular Extrusion Foaming of Long-Chain Branched Polyamide 6 Composites. J. Supercrit. Fluids 2023, 199, 105953. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.; Gong, S.; Ma, S.; Yang, H.; Pan, K.; Deng, J. Preparation Methods, Performance Improvement Strategies, and Typical Applications of Polyamide Foams. Ind. Eng. Chem. Res. 2021, 60, 17365–17378. [Google Scholar] [CrossRef]
- Lima, G.M.; Bose, R.K. Production and Application of Polymer Foams Employing Supercritical Carbon Dioxide. Adv. Polym. Technol. 2022, 2022, 8905115. [Google Scholar] [CrossRef]
- Li, Y.; Gong, P.; Liu, Y.; Niu, Y.; Park, C.B.; Li, G. Environmentally Friendly and Zero-Formamide EVA/LDPE Microcellular Foams Via Supercritical Carbon Dioxide Solid Foaming. ACS Appl. Polym. Mater. 2021, 3, 4213–4222. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Peng, X. Applications and Challenges of Supercritical Foaming Technology. Polymers 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, J.; Ge, C.; Zhao, G.; Park, C.B. Nanocellular Poly (Ether-Block-Amide)/MWCNT Nanocomposite Films Fabricated by Stretching-Assisted Microcellular Foaming for High-Performance EMI Shielding Applications. J. Mater. Chem. C 2021, 9, 1245–1258. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Azdast, T.; Doniavi, A.; Lee, R.E. Multi-Objective Optimization of Heat Transfer Mechanisms of Microcellular Polymeric Foams from Thermal-Insulation Point of View. Therm. Sci. Eng. Prog. 2019, 9, 21–29. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, G.; Zhao, J.; Zhang, A.; Zhao, G. Super-Elastic and Structure-Tunable Poly (Ether-Block-Amide) Foams Achieved by Microcellular Foaming. J. CO2 Util. 2022, 55, 101807. [Google Scholar] [CrossRef]
- Li, X.; Lai, J.; Wei, J.; Peng, X.; Wu, J.; Geng, L. Structure and Morphology of Thermoplastic Polyamide 6 Elastomers with Different Soft Segment Content and Their Foaming Behavior Using Supercritical CO2. Polym Eng Sci. 2022, 62, 103–115. [Google Scholar] [CrossRef]
- Ito, S.; Matsunaga, K.; Tajima, M.; Yoshida, Y. Generation of Microcellular Polyurethane with Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2007, 106, 3581–3586. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Kemmere, M.F.; Keurentjes, J.T. Foam processing of Poly (Ethylene-Co-Vinyl Acetate) Rubber Using Supercritical Carbon Dioxide. Polymer 2004, 45, 7539–7547. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Hossieny, N.; Jahani, D.; Park, C.B. Characterization of Hard-Segment Crystalline Phase of Poly (Ether-Block-Amide) (PEBAX®) Thermoplastic Elastomers in The Presence of Supercritical CO2 and Its Impact on Foams. Polymer 2017, 114, 15–27. [Google Scholar] [CrossRef]
- Yeh, S.K.; Liu, W.H.; Huang, Y.M. Carbon Dioxide-Blown Expanded Polyamide Bead Foams with Bimodal Cell Structure. Ind. Eng. Chem. Res. 2019, 58, 2958–2969. [Google Scholar] [CrossRef]
- Xu, M.; Lu, J.; Zhao, J.; Wei, L.; Liu, T.; Zhao, L.; Park, C.B. Rheological and Foaming Behaviors of Long-Chain Branched Polyamide 6 With Controlled Branch Length. Polymer 2021, 224, 123730. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Y.; Liu, T.; Zhao, L.; Park, C.B. Determination of Modified Polyamide 6’s Foaming Windows by Bubble Growth Simulations Based on Rheological Measurements. J. Appl. Polym. Sci. 2019, 136, 48138. [Google Scholar] [CrossRef]
- Faridirad, F.; Ahmadi, S.; Barmar, M. Polyamide/Carbon Nanoparticles Nanocomposites: A Review. Polym Eng Sci. 2017, 57, 475–494. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, C.; Liu, T. 2D Nanosheet-Constructed Hybrid Nanofillers for Polymer Nanocomposites with Synergistic Dispersion and Function. APL Mater. 2019, 7, 080904. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, C.W.; Tasi, M.C.; Rwei, S.P.; Lee, Y.H. Biomass Thermoplastic (Co) polyamide Elastomers Synthesized from a Fatty Dimer Acid: A Sustainable Route toward a New Era of Uniform and Bimodal Foams. Ind. Eng. Chem. Res. 2021, 60, 12139–12154. [Google Scholar] [CrossRef]
- Munny, A.A.; Ali, S.M.; Kabir, G.; Moktadir, M.A.; Rahman, T.; Mahtab, Z. Enablers of Social Sustainability in The Supply Chain: An Example of Footwear Industry from An Emerging Economy. Sustain. Prod. Consum. 2019, 20, 230–242. [Google Scholar] [CrossRef]
- Lunchev, A.V.; Tham, S.C.; Lipik, V.; Tok, A.L.Y. Carbon Nanomaterials as Additives to Ethylene Vinyl Acetate Copolymer Foams for Sport Footwear Applications. Polym. Adv. Technol. 2022, 33, 863–869. [Google Scholar] [CrossRef]
- Ikram, M. Transition Toward Green Economy: Technological Innovation’s Role in the Fashion Industry. Curr. Opin. Green Sustain. Chem. 2022, 37, 100657. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Zhou, B.; Zhang, X. Upcycling of textile and footwear wastes for synergistical reinforcement of cement mortar. Compos. Commun. 2023, 41, 101646. [Google Scholar] [CrossRef]
- Júnior, C.P.; Mendonca, A.V.; Fim, F.C.; Silva, L.B. Recycling EVA Waste: An Opportunity for the Footwear Industry-Rheological Properties of EVA Waste Composites Using Torque Rheometry. J. Polym. Environ. 2022, 30, 2155–2164. [Google Scholar] [CrossRef]
- Meier, M.A. Plant-Oil-Based Polyamides and Polyurethanes: Toward Sustainable Nitrogen-Containing Thermoplastic Materials. Macromol. Rapid Commun. 2019, 40, 1800524. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, W.; Cai, L.; Jiang, T.; Wang, Z. Castor Oil-Based Multi-Functional Monomers and their Application in Polyamide Design. Ind. Crops Prod. 2023, 203, 117188. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, C.W.; Rwei, S.P. Highly Stretchable Fully Biomass Autonomic Self-Healing Polyamide Elastomers and Their Foam for Selective Oil Absorption. Polymers 2021, 13, 3089. [Google Scholar] [CrossRef] [PubMed]
- Nurhamiyah, Y.; Amir, A.; Finnegan, M.; Themistou, E.; Edirisinghe, M.; Chen, B. Wholly Biobased, Highly Stretchable, Hydrophobic, and Self-Healing Thermoplastic Elastomer. ACS Appl. Mater. Interfaces 2021, 13, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Pagacz, J.; Raftopoulos, K.N.; Leszczyńska, A.; Pielichowski, K. Bio-Polyamides Based on Renewable Raw Materials: Glass Transition and Crystallinity Studies. J. Therm. Anal. Calorim. 2016, 123, 1225–1237. [Google Scholar] [CrossRef]
- Lu, H.; Xu, X.; Li, X.; Zhang, Z. Morphology, Crystallization and Dynamic Mechanical Properties of PA66/Nano-SiO2 Composites. Bull. Mater. Sci. 2006, 29, 485–490. [Google Scholar] [CrossRef]
- Hablot, E.; Donnio, B.; Bouquey, M.; Avérous, L. Dimer Acid-Based Thermoplastic Bio-Polyamides: Reaction Kinetics, Properties and Structure. Polymer 2010, 51, 5895–5902. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Spoljaric, S.; Seppälä, J. Redefining Polyamide Property Profiles Via Renewable Long-Chain Aliphatic Segments: Towards Impact Resistance and Low Water Absorption. Eur. Polym. J. 2018, 109, 16–25. [Google Scholar] [CrossRef]
- Nurhamiyah, Y.; Chen, B. Biobased Polyamide 4, 36 Thermoplastic Elastomer and its Cellulose Nanocomposites. Macromol. Chem. Phys. 2023, 224, 2300013. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, C.W.; Chou, Y.L.; Rwei, S.P.; Ramaraj, S.K. Biomass Chemical Upcycling of Waste rPET for the Fabrication of Formamide-Free TPEE Microcellular Foams Via ScCO2 Foaming. J. CO2 Util. 2022, 65, 102199. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, Y.H.; Rwei, S.P.; Lee, Y.H. Biomass Upcycling of Waste rPET to Higher-Value New-Easy-Recyclable Microcellular Thermoplastic (Co) Polyamide Foams and Hot-Melt Adhesives. Mater. Today Chem. 2022, 26, 101101. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Zhao, J.; Xu, Z.; Li, S.; Zhao, G. Corrugated Thermoplastic Polyurethane Foams with High Mechanical Strength Fabricated by Integrating Fused Filament Fabrication and Microcellular Foaming Using Supercritical CO2. J. CO2 Util. 2022, 66, 102293. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Fabrication of High-Expansion Microcellular PLA Foams Based on Pre-Isothermal Cold Crystallization and Supercritical CO2 Foaming. Polym. Degrad. Stab. 2018, 156, 75–88. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, G.; Huang, P.; Xu, D.; Zhai, W. Fundamental Influences of Crosslinking Structure on The Cell Morphology, Creep Property, Thermal Property, and Recycling Behavior of Microcellular EPDM Foams Blown with Compressed CO2. Ind. Eng. Chem. Res. 2020, 59, 1534–1548. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhu, Q.S.; Dong, B.B.; Wu, H.H.; Liu, C.T.; Shen, C.Y.; Turng, L.S.; Geng, T. The Effects of Nanoclay and Deformation Conditions on the Inelastic Behavior of Thermoplastic Polyurethane Foams. Polym. Test. 2019, 79, 106043. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, C.W.; Chou, C.H.; Lin, C.H.; Chen, Y.H.; Chen, C.W.; Way, T.F.; Rwei, S.P. Sustainable Polyamide Elastomers From a Bio-Based Dimer Diamine for Fabricating Highly Expanded and Facilely Recyclable Microcellular Foams via Supercritical CO2 Foaming. Eur. Polym. J. 2021, 160, 110765. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Ranganathan, P.; Mutharani, B.; Shiu, J.-W.; Rwei, S.-P.; Chang, Y.-H.; Chiu, F.-C. Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties. Polymers 2024, 16, 159. https://doi.org/10.3390/polym16010159
Chen C-W, Ranganathan P, Mutharani B, Shiu J-W, Rwei S-P, Chang Y-H, Chiu F-C. Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties. Polymers. 2024; 16(1):159. https://doi.org/10.3390/polym16010159
Chicago/Turabian StyleChen, Chin-Wen, Palraj Ranganathan, Bhuvanenthiran Mutharani, Jia-Wei Shiu, Syang-Peng Rwei, Yen-Hsiang Chang, and Fang-Chyou Chiu. 2024. "Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties" Polymers 16, no. 1: 159. https://doi.org/10.3390/polym16010159
APA StyleChen, C.-W., Ranganathan, P., Mutharani, B., Shiu, J.-W., Rwei, S.-P., Chang, Y.-H., & Chiu, F.-C. (2024). Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties. Polymers, 16(1), 159. https://doi.org/10.3390/polym16010159








