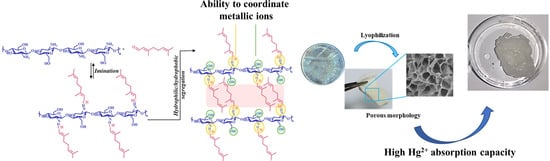
Citryl-Imino-Chitosan Xerogels as Promising Materials for Mercury Recovery from Waste Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
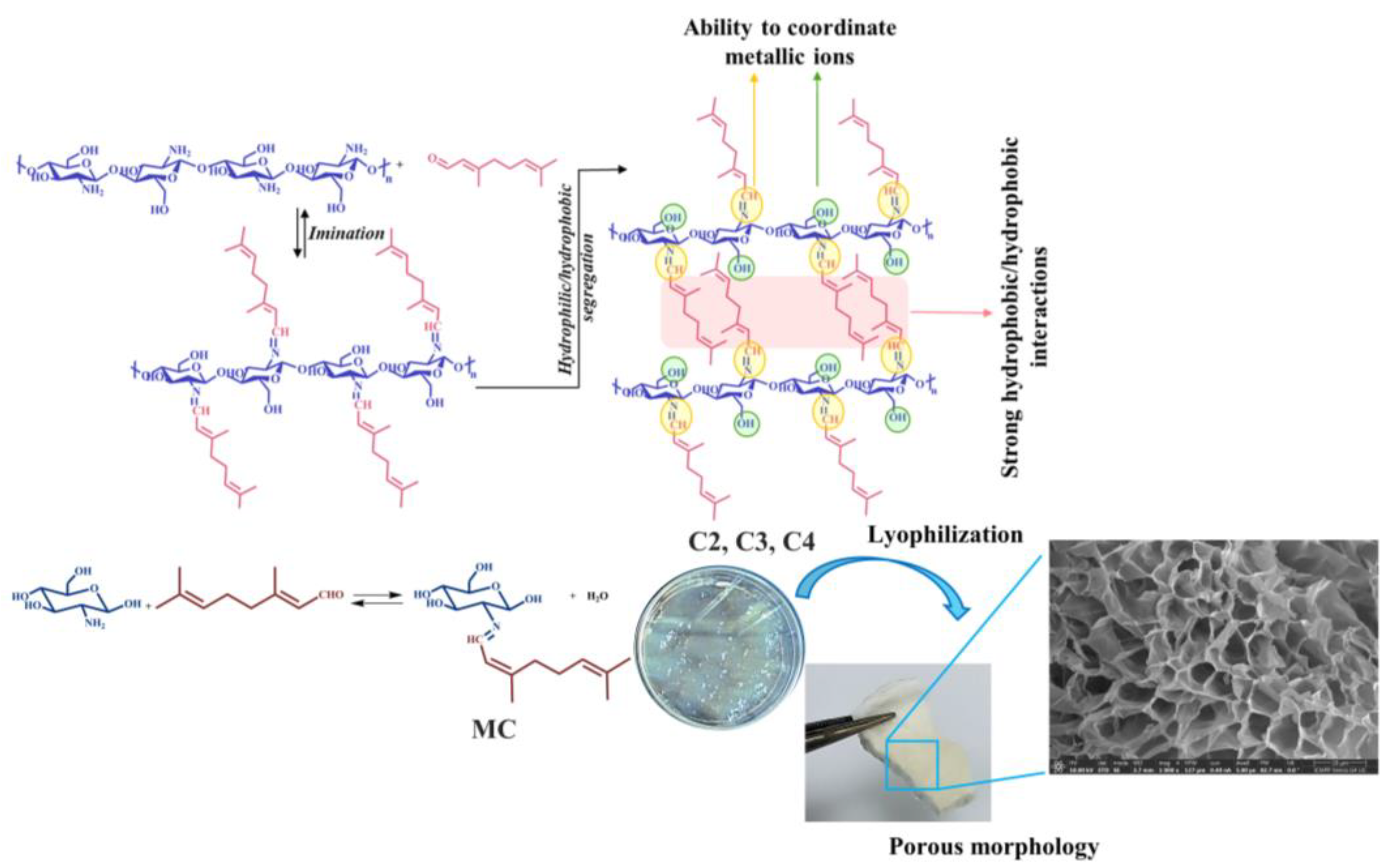
2.2. The Obtaining of the Hydrogels
2.3. The Obtaining of the Model Compound (MC)
2.4. Characterization Techniques
3. Results and Discussions
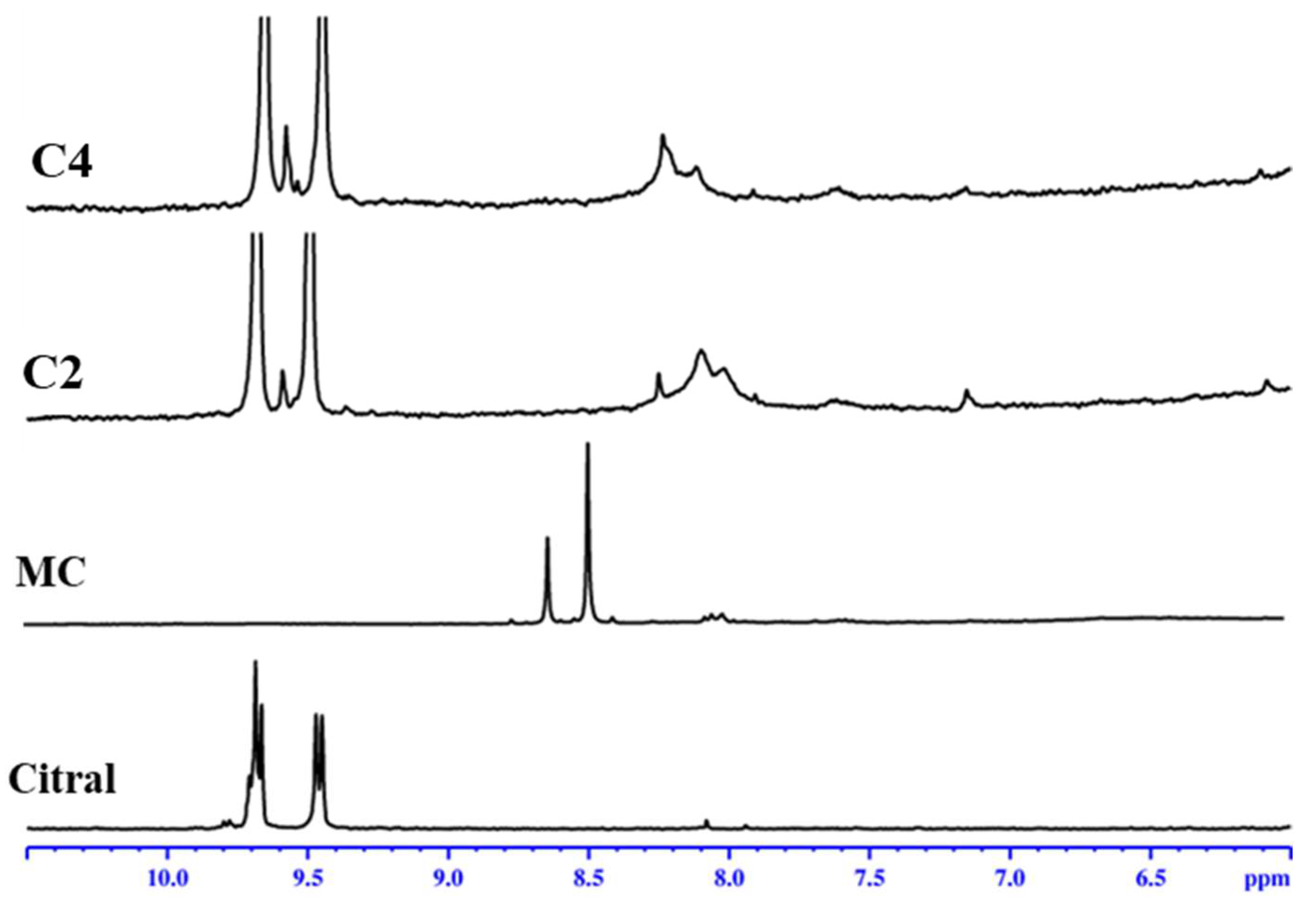
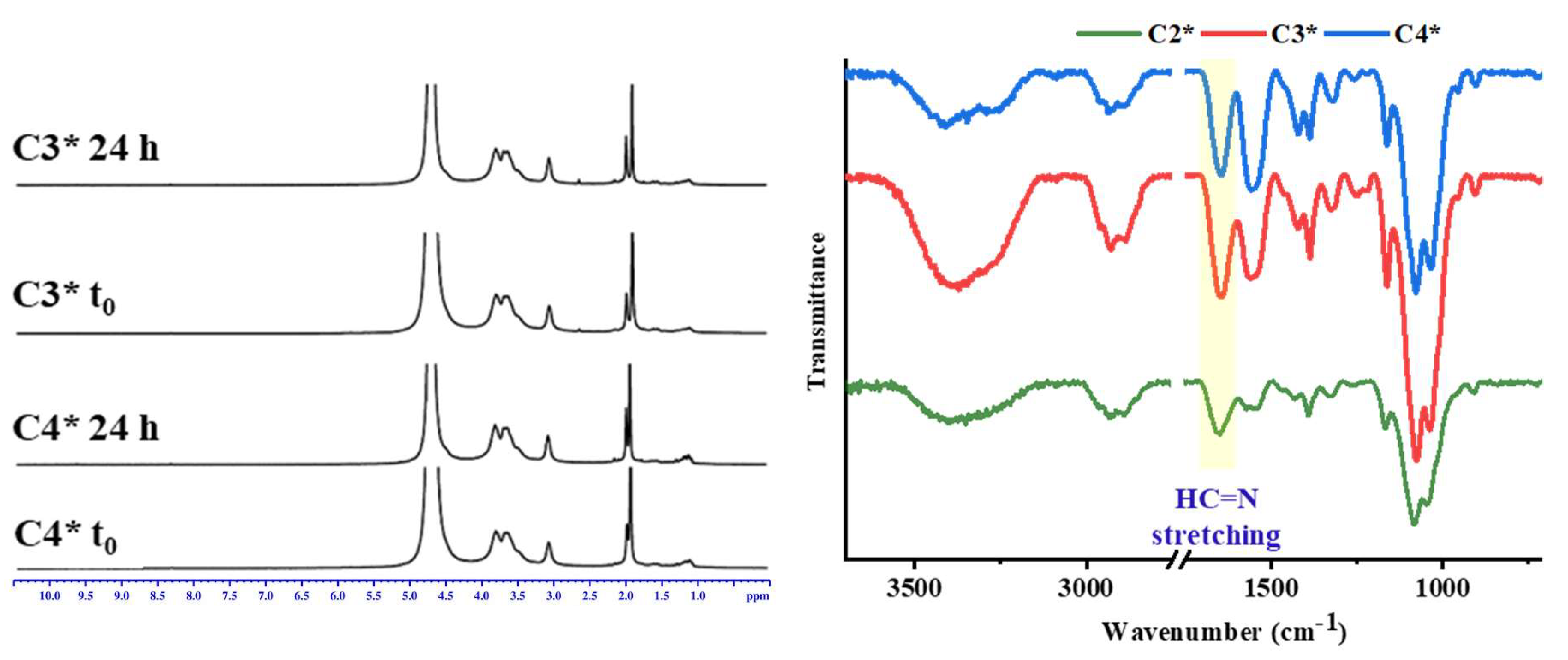
3.1. Structural Characterization by 1H-NMR
3.2. Structural Characterization by FTIR
3.3. Morphological Characterization by SEM Porosity and Gel Fraction
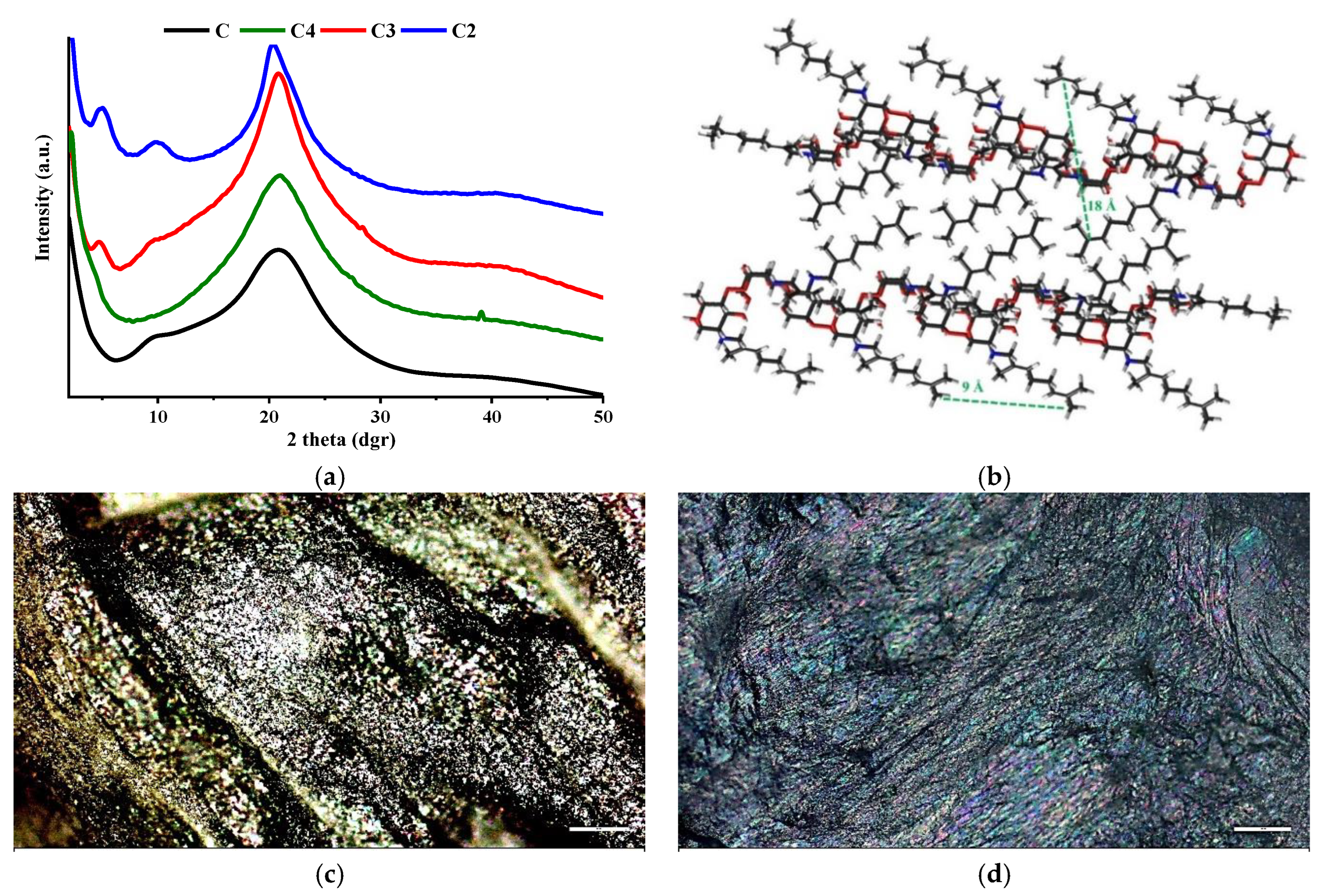
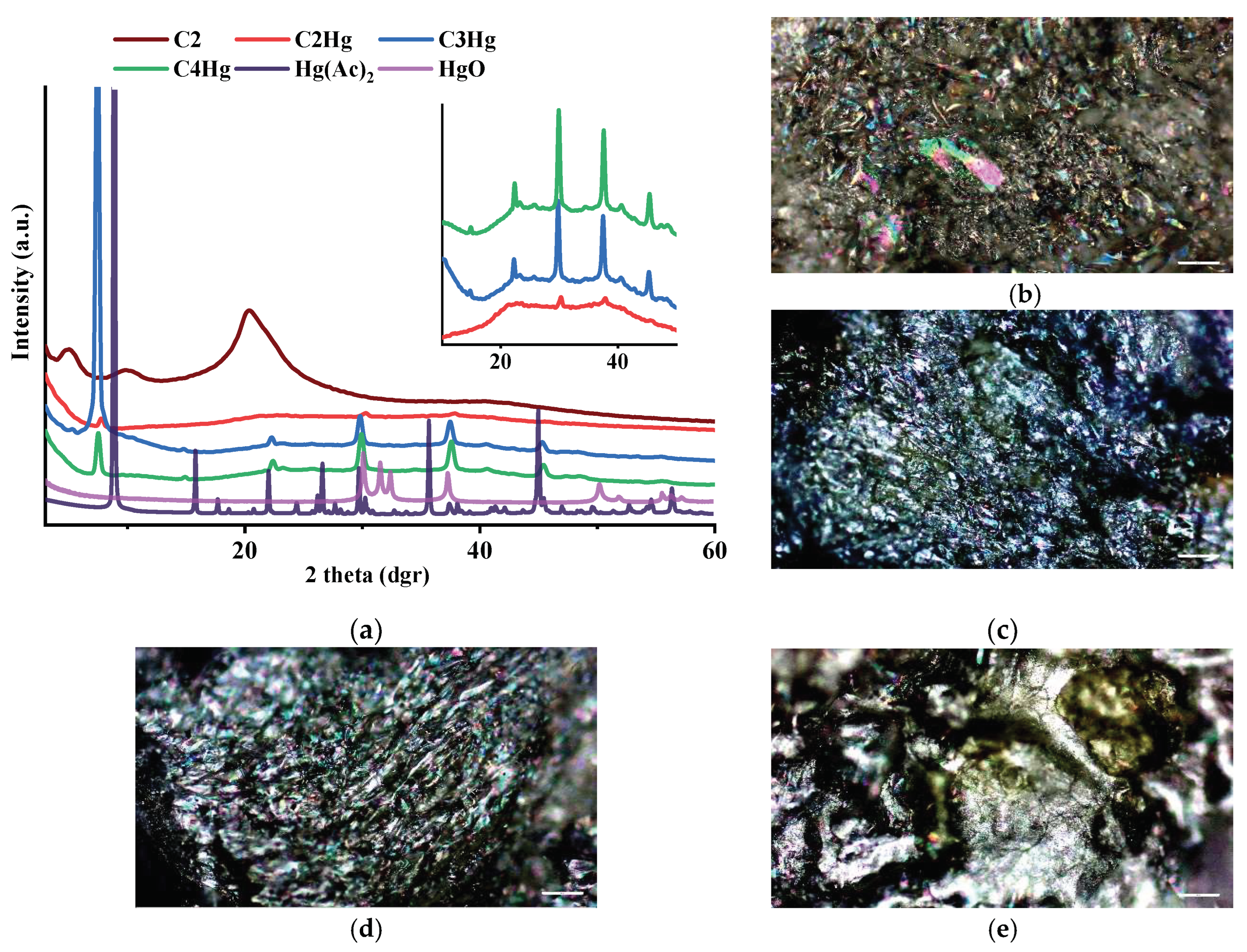
3.4. Wide-Angle X-ray Diffraction
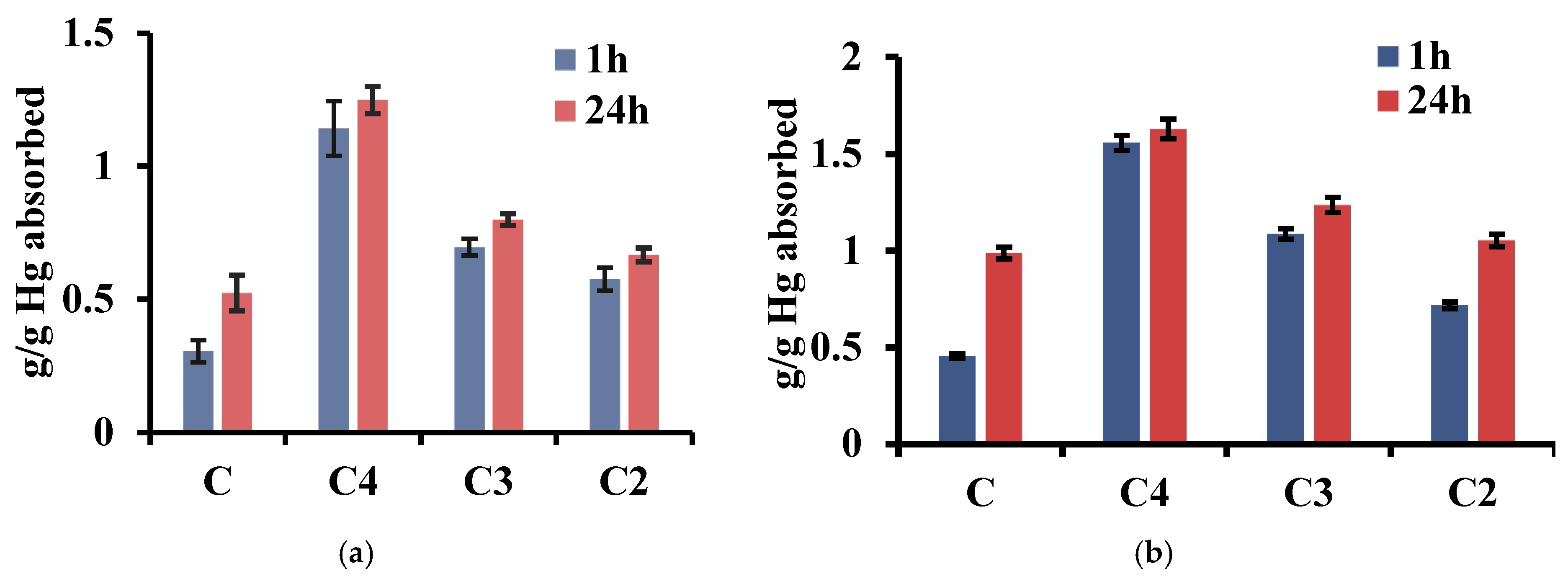
3.5. The Investigations of Mercury Absorption Capacity of the Xerogels
3.5.1. Gravimetric Measurements—The Water Uptake Capacity of the Xerogels and Stability in the Acidic Medium
3.5.2. The Stability of the Imine Linkage in an Acidic Aqueous Solution
3.5.3. The Evaluation of the Xerogels as Materials for Mercury Recovery
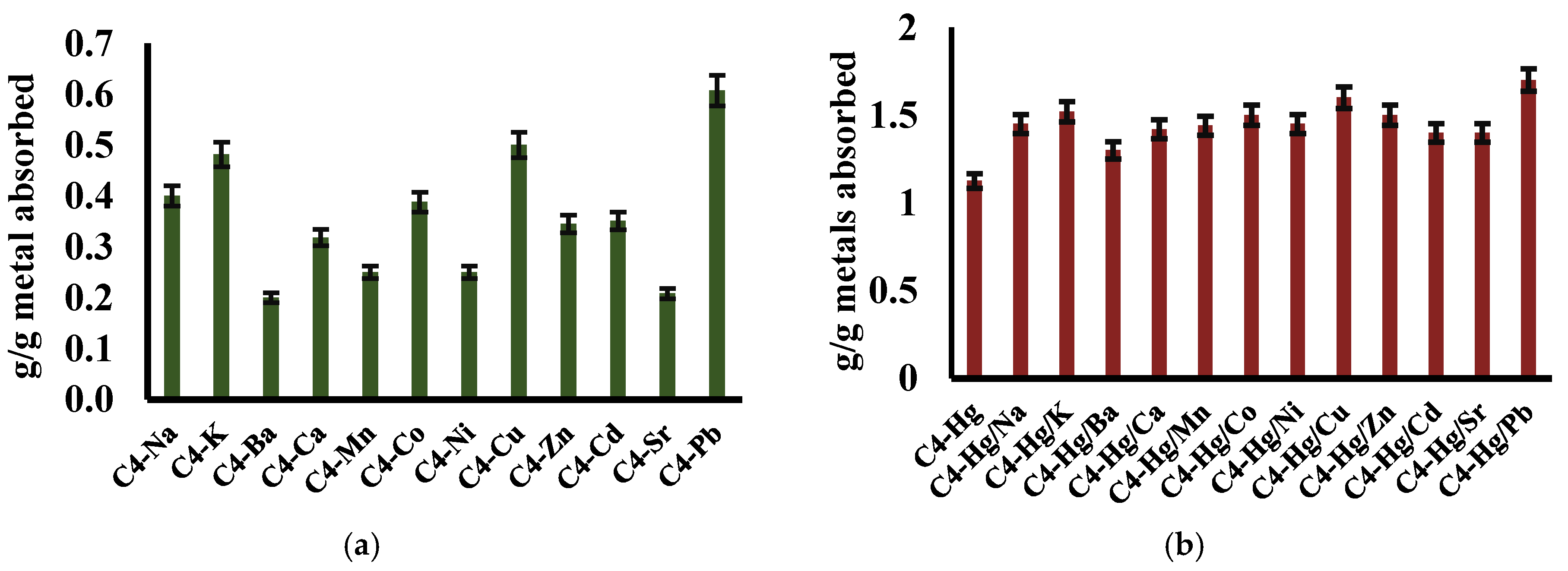
3.5.4. The Interference with Other Metals in the Process of Mercury Retention
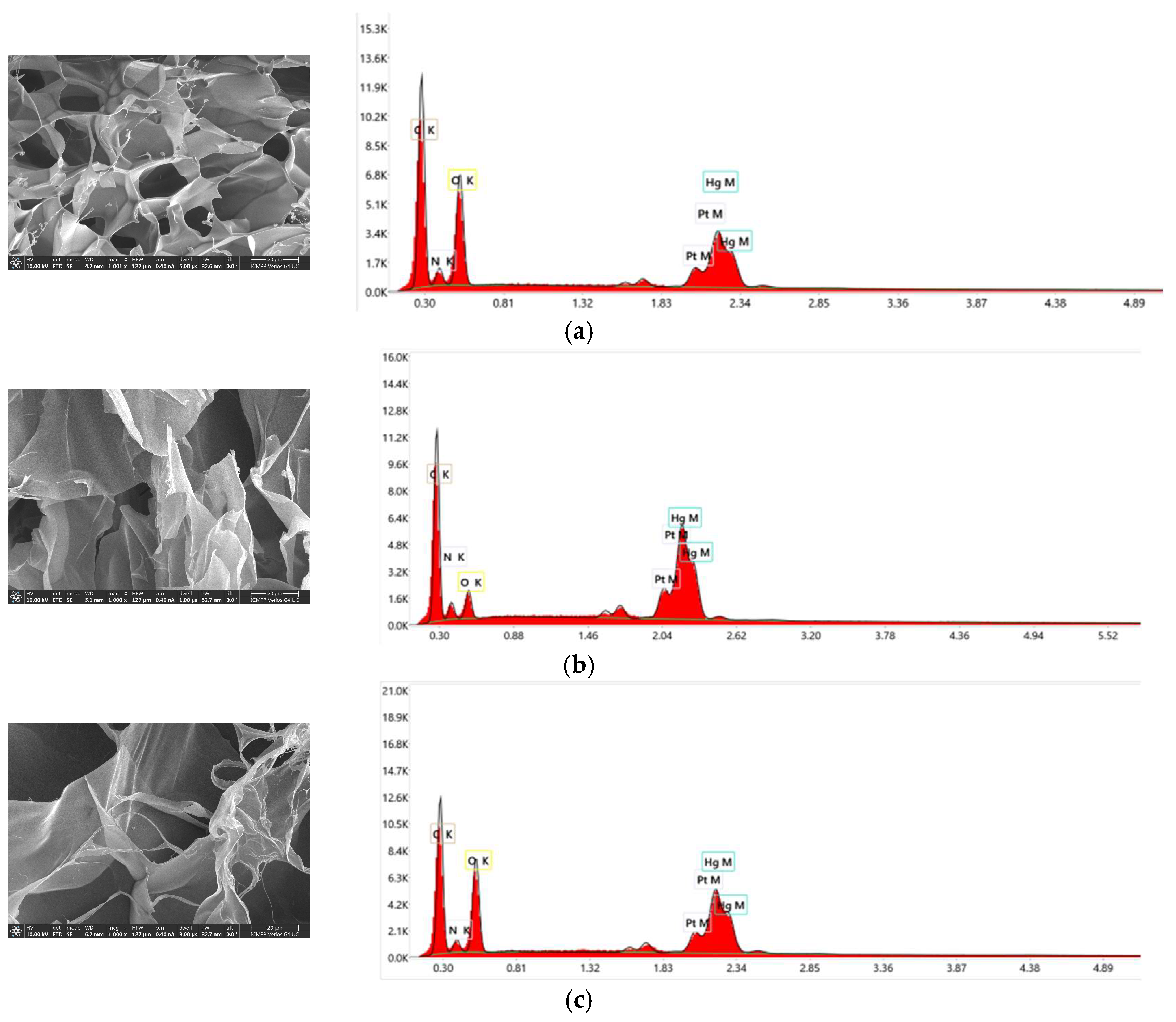
3.5.5. The Characterization of the Materials after Mercury Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, N.; Roy, K.; Barman, P.; Rabha, S.; Bora, H.K.; Khare, P.; Konwar, R.; Saikia, B.K. Chemical and Toxicological Studies on Black Crust Formed over Historical Monuments as a Probable Health Hazard. J. Hazard. Mater. 2024, 464, 132939. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 1 October 2023).
- Available online: https://www.healthline.com/health/mercury-poisoning#symptoms (accessed on 1 October 2023).
- Kester, M.; Karpa, K.D.; Vrana, K.E. Toxicology. In Elsevier’s Integrated Review Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 29–39. [Google Scholar]
- Bejan, A.; Doroftei, F.; Cheng, X.; Marin, L. Phenothiazine-Chitosan Based Eco-Adsorbents: A Special Design for Mercury Removal and Fast Naked Eye Detection. Int. J. Biol. Macromol. 2020, 162, 1839–1848. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, H.; Liu, W.; Ni, H.; Zhang, X.; Zhai, A.; Quan, Z.; Qu, Z.; Yan, N. One Step Interface Activation of ZnS Using Cupric Ions for Mercury Recovery from Nonferrous Smelting Flue Gas. Environ. Sci. Technol. 2019, 53, 4511–4518. [Google Scholar] [CrossRef]
- Jang, M.; Hong, S.M.; Park, J.K. Characterization and Recovery of Mercury from Spent Fluorescent Lamps. Waste Manag. 2005, 25, 5–14. [Google Scholar] [CrossRef]
- Cibotaru, S.; Ailincai, D.; Andreica, B.-I.; Cheng, X.; Marin, L. TEGylated Phenothiazine-Imine-Chitosan Materials as a Promising Framework for Mercury Recovery. Gels 2022, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Abdelwahab, M.S.; Ibrahim, G.A.A. The Design of SnO2-Crosslinked-Chitosan Nanocomposite for Microwave-Assisted Adsorption of Aqueous Cadmium and Mercury Ions. Sustain. Chem. Pharm. 2022, 28, 100731. [Google Scholar] [CrossRef]
- Lin, H.; Duan, Y.; Zhao, B.; Feng, Q.; Li, M.; Wei, J.; Zhu, Y.; Li, M. Efficient Hg(II) Removal to Ppb Level from Water in Wider PH Based on Poly-Cyanoguanidine/Graphene Oxide: Preparation, Behaviors, and Mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128467. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Yang, S.; Liu, H.; Liu, C.; Xie, X.; Xu, Z. Selective Recovery of Mercury from High Mercury-Containing Smelting Wastes Using an Iodide Solution System. J. Hazard. Mater. 2019, 363, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Koumentakou, I.; Liakos, E.V.; Lazaridou, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Adsorption of Uranium, Mercury, and Rare Earth Elements from Aqueous Solutions onto Magnetic Chitosan Adsorbents: A Review. Polymers 2021, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Anghel, N.; Dinu, M.V.; Vlad, S.; Bele, A.; Ciubotaru, B.I.; Verestiuc, L.; Pamfil, D. Development and Performance of Bioactive Compounds-Loaded Cellulose/Collagen/Polyurethane Materials. Polymers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Zhuang, J.; Chen, C.; Ouyang, K.; Xu, M.; Xu, Z. Characterization and Evaluation of the Adsorption Potential of Chitosan-Impregnated Cellulose Nanofiber Multi-Walled Carbon Nanotube Aerogel for Copper Ions. New J. Chem. 2022, 46, 3156–3167. [Google Scholar] [CrossRef]
- Humelnicu, D.; Dragan, E.S.; Ignat, M.; Dinu, M.V. A Comparative Study on Cu2+, Zn2+, Ni2+, Fe3+, and Cr3+ Metal Ions Removal from Industrial Wastewaters by Chitosan-Based Composite Cryogels. Molecules 2020, 25, 2664. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, D.; Lazar, M.M. Analysis of Copper(II), Cobalt(II) and Iron(III) Sorption in Binary and Ternary Systems by Chitosan-Based Composite Sponges Obtained by Ice-Segregation Approach. Gels 2021, 7, 103. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Zhang, Z.; Xiang, P.; Xia, S. Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge. Int. J. Environ. Res. Public Health 2020, 17, 1488. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Wang, M.; You, X. Critical Review of Magnetic Polysaccharide-Based Adsorbents for Water Treatment: Synthesis, Application and Regeneration. J. Clean. Prod. 2021, 323, 129118. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef] [PubMed]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I.; et al. Methods to Prepare Biosorbents and Magnetic Sorbents for Water Treatment: A Review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Maity, S.; Biswas, R.; Verma, S.K.; Sarkar, A. Natural Polysaccharides as Potential Biosorbents for Heavy Metal Removal. In Food, Medical, and Environmental Applications of Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 627–665. [Google Scholar]
- Kumari, S.; Kishor, R. Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Tamer, C.E.; Suna, S.; Özcan-Sinir, G. Toxicological Aspects of Ingredients Used in Nonalcoholic Beverages. In Non-Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–481. [Google Scholar]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Kurasova, M.N.; Dysin, A.P.; Kurliuk, A.V.; Shakola, T.V.; et al. Active Antibacterial Food Coatings Based on Blends of Succinyl Chitosan and Triazole Betaine Chitosan Derivatives. Food Packag. Shelf Life 2020, 25, 100534. [Google Scholar] [CrossRef]
- Ayme, J.-F.; Lehn, J.-M. Self-Sorting of Two Imine-Based Metal Complexes: Balancing Kinetics and Thermodynamics in Constitutional Dynamic Networks. Chem. Sci. 2020, 11, 1114–1121. [Google Scholar] [CrossRef]
- Yin, M.; Luo, F. Applications of Covalent Organic Framework–Based Nanomaterials as Superior Adsorbents in Wastewater Treatment. In Emerging Nanomaterials for Recovery of Toxic and Radioactive Metal Ions from Environmental Media; Elsevier: Amsterdam, The Netherlands, 2022; pp. 127–159. [Google Scholar]
- Iftime, M.M.; Morariu, S.; Marin, L. Salicyl-Imine-Chitosan Hydrogels: Supramolecular Architecturing as a Crosslinking Method toward Multifunctional Hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of Biocompatible Glycodynameric Hydrogels Joining Two Natural Motifs by Dynamic Constitutional Chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef]
- Ailincai, D.; Morariu, S.; Rosca, I.; Sandu, A.I.; Marin, L. Drug Delivery Based on a Supramolecular Chemistry Approach by Using Chitosan Hydrogels. Int. J. Biol. Macromol. 2023, 248, 125800. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostănaru-Iliescu, A.-C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Porzio, W.; Marin, L. Hydrogels Based on Imino-Chitosan Amphiphiles as a Matrix for Drug Delivery Systems. Polymers 2020, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Mititelu-Tartau, L.; Marin, L. Citryl-Imine-PEG-Ylated Chitosan Hydrogels—Promising Materials for Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 162, 1323–1337. [Google Scholar] [CrossRef]
- Ailincai, D.; Bercea, M.; Mititelu Tartau, L.; Marin, L. Biocompatible Drug Delivery Systems Able to Co-Deliver Antifungal and Antiviral Agents. Carbohydr. Polym. 2022, 298, 120071. [Google Scholar] [CrossRef]
- Ailincai, D.; Tartau Mititelu, L.; Marin, L. Drug Delivery Systems Based on Biocompatible Imino-Chitosan Hydrogels for Local Anticancer Therapy. Drug Deliv. 2018, 25, 1080–1090. [Google Scholar] [CrossRef]
- Ailincai, D.; Cibotaru, S.; Anisiei, A.; Coman, C.G.; Pasca, A.S.; Rosca, I.; Sandu, A.-I.; Mititelu-Tartau, L.; Marin, L. Mesoporous Chitosan Nanofibers Loaded with Norfloxacin and Coated with Phenylboronic Acid Perform as Bioabsorbable Active Dressings to Accelerate the Healing of Burn Wounds. Carbohydr. Polym. 2023, 318, 121135. [Google Scholar] [CrossRef]
- Sultan, J.S.; Lateaf, S.M.; Rashid, D.K. Synthesis, Characterization and Antibacterial Activity of Mixed Ligand (HL) Complexes Mn(ll), Co(ll), Ni(ll), Zn(ll), Cd(ll) and Hg(ll) with Azide (N3-). Open J. Inorg. Chem. 2015, 5, 102–111. [Google Scholar] [CrossRef]
- de Paiva, R.E.F.; Nakahata, D.H.; de Carvalho, M.A.; Bergamini, F.R.G.; Corbi, P.P. N, N′, N′′ versus N, N′, O Imine-Containing Coordination Motifs: Ligand-Directed Synthesis of Mononuclear and Binuclear Cu II Compounds. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1563–1567. [Google Scholar] [CrossRef]






| Code | NH2/CHO Ratio | mchito (mg) | VH2O (mL) | VCH3COOH (µL) | mald (mg) | Vet (mL) | mdried subs (mg) | mxerogel (mg) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| C | - | 300 | 15 | 105 | - | - | - | 300 | 100 |
| C2 | 2 | 300 | 15 | 105 | 116.5 | 11.6 | 416.5 | 416 | 99.9 |
| C3 | 3 | 300 | 15 | 105 | 77.6 | 7.76 | 377.6 | 377 | 99.8 |
| C4 | 4 | 300 | 15 | 105 | 58.8 | 5.88 | 358.8 | 358 | 99.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailincai, D.; Andreica, B.I. Citryl-Imino-Chitosan Xerogels as Promising Materials for Mercury Recovery from Waste Waters. Polymers 2024, 16, 19. https://doi.org/10.3390/polym16010019
Ailincai D, Andreica BI. Citryl-Imino-Chitosan Xerogels as Promising Materials for Mercury Recovery from Waste Waters. Polymers. 2024; 16(1):19. https://doi.org/10.3390/polym16010019
Chicago/Turabian StyleAilincai, Daniela, and Bianca Iustina Andreica. 2024. "Citryl-Imino-Chitosan Xerogels as Promising Materials for Mercury Recovery from Waste Waters" Polymers 16, no. 1: 19. https://doi.org/10.3390/polym16010019