Disposable Molecularly Imprinted Polymer-Modified Screen-Printed Electrodes for Rapid Electrochemical Detection of l-Kynurenine in Human Urine
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus
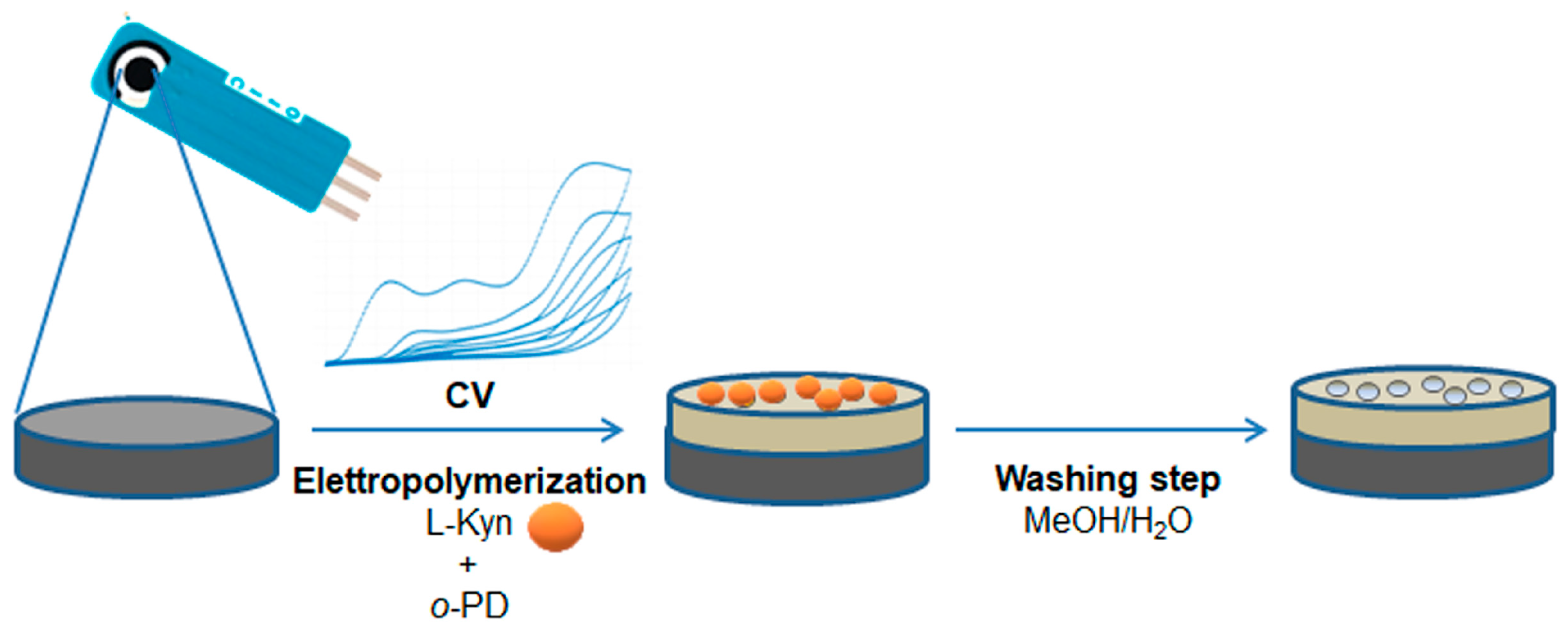
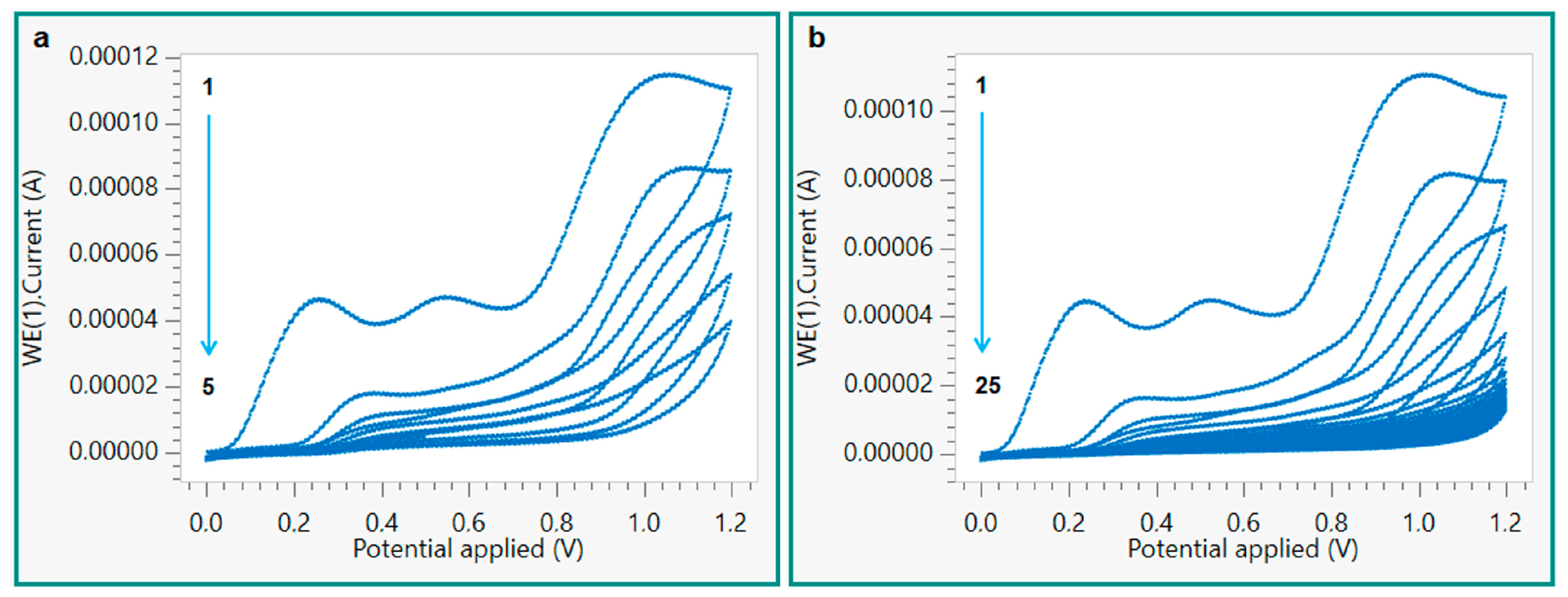
2.3. Preparation of kyn-MIP-SPCE
2.4. Electrochemical Measurements of l-Kyn Standard Solutions Using kyn-MIP-SPCE
2.5. Selectivity Studies
2.6. Human Urine Sample Preparation
2.7. Electrochemical Experiments in Human Urine
2.8. Method Validation
3. Results and Discussion
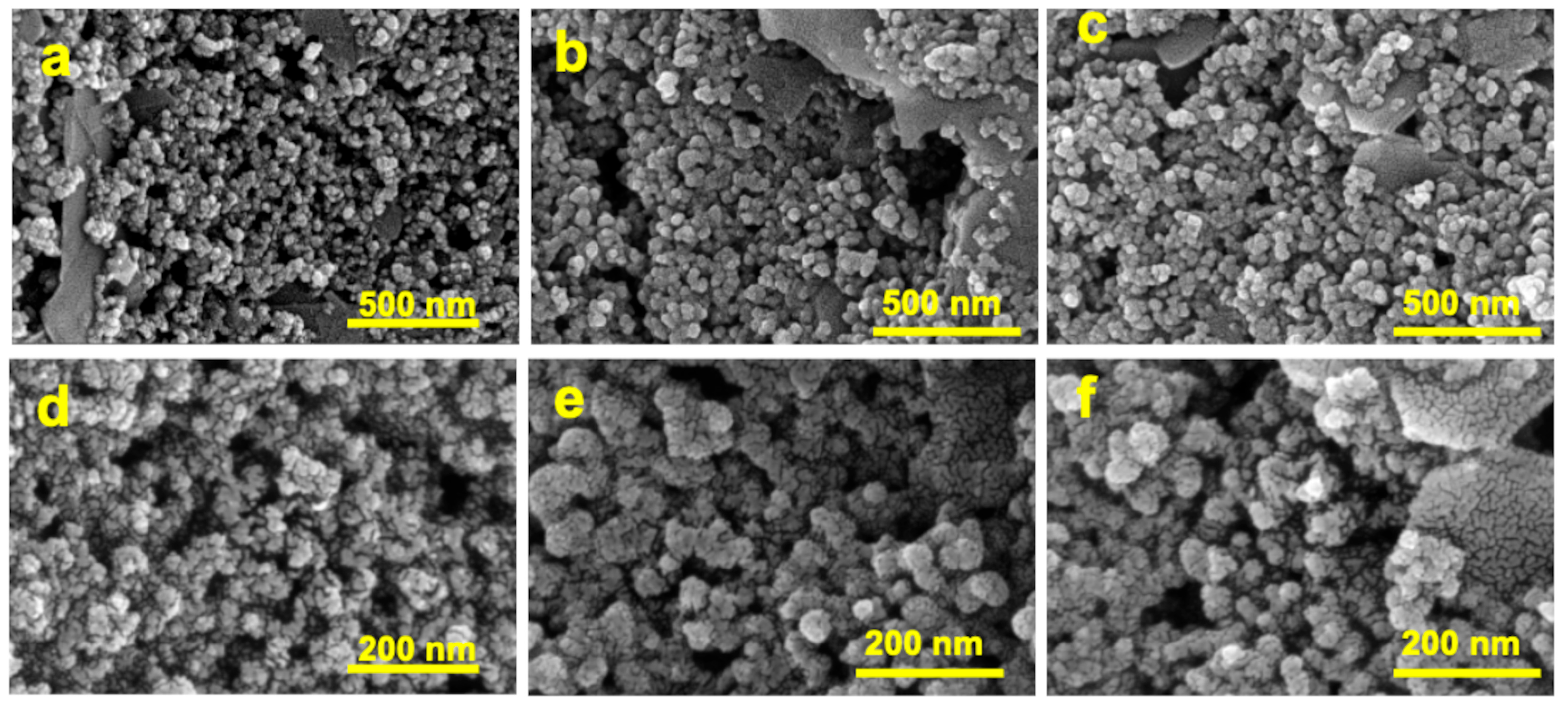
3.1. Preparation and Characterization of kyn-MIP-SPCE
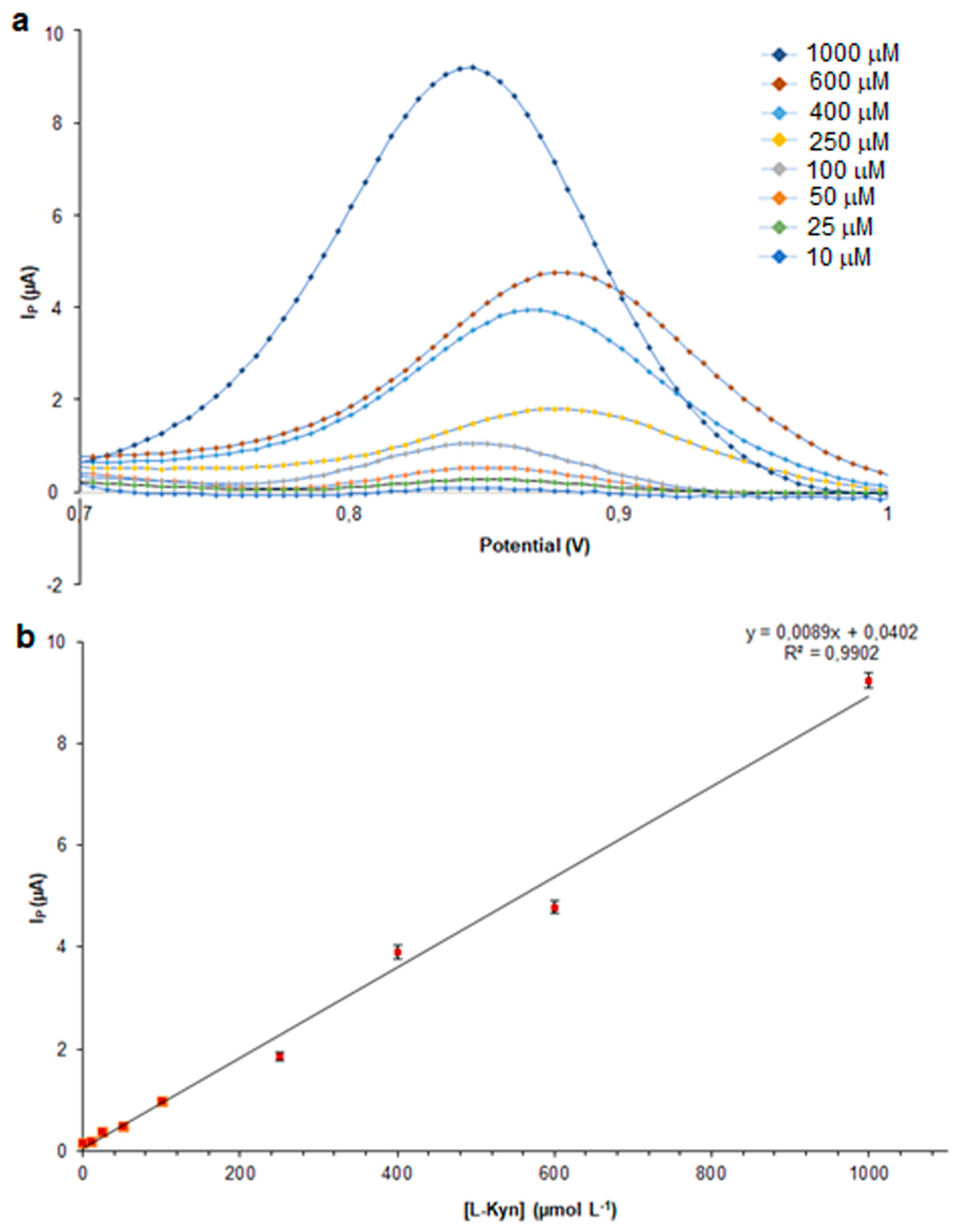
3.2. Analytical Performance of kyn-MIP-SPCE
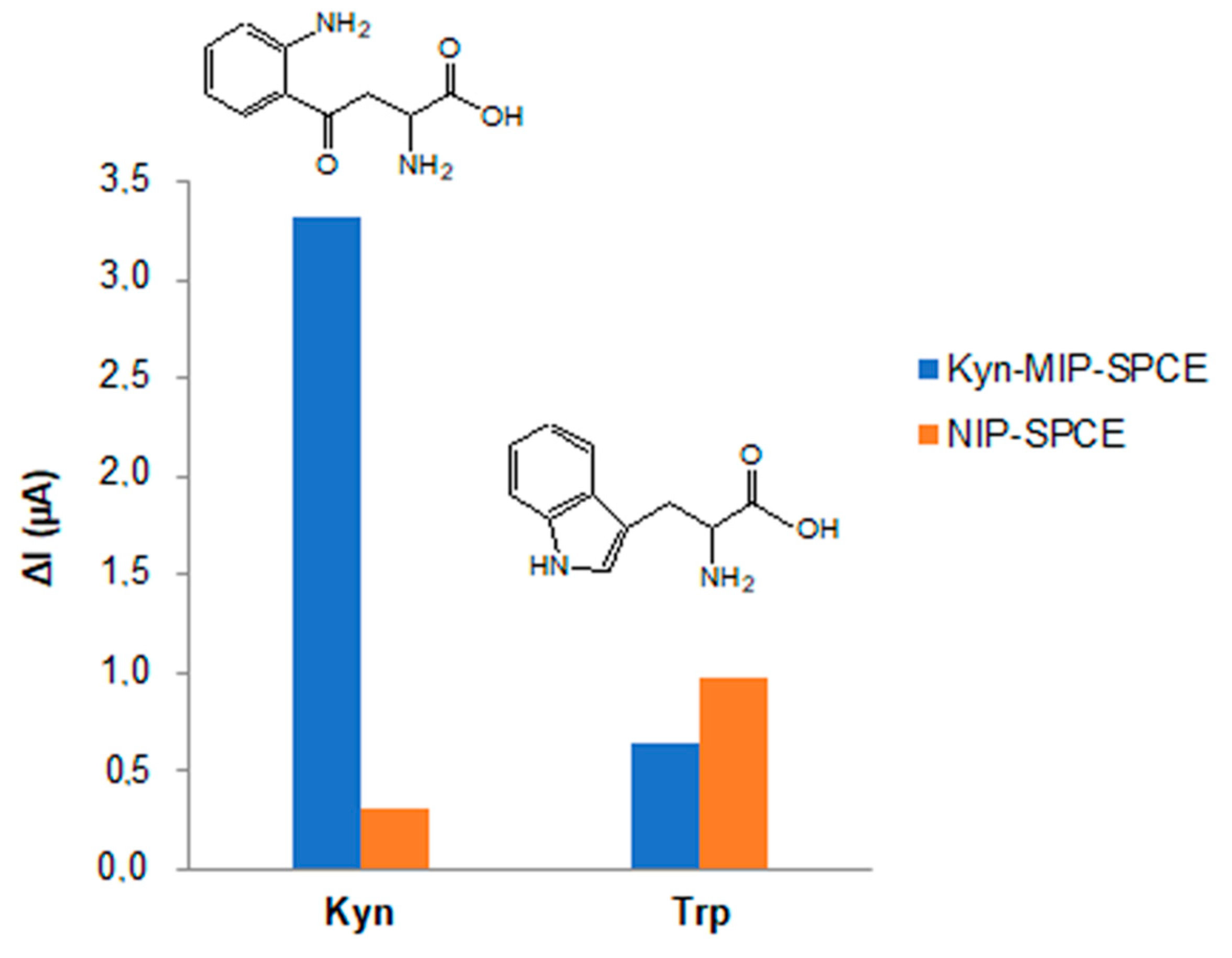
3.3. Selectivity Studies
3.4. Application of kyn-MIP-SPCE in Human Urine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows–Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845–107872. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.E.; Sun, J. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2017, 39, 307–325. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Mrštná, K.; Kujovská Krčmová, L.; Švec, F. Advances in kynurenine analysis. Clin. Chim. Acta 2023, 547, 117441. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Stangl, M.; Börner, N.; Bösch, F.; Durner, J.; Drunin, G.; Buhl, J.-L.; Abendroth, D. Kynurenine serves as useful biomarker in acute, Long- and Post-COVID-19 diagnostics. Front. Immunol. 2022, 13, 1004545. [Google Scholar] [CrossRef]
- Vignau, J.; Jacquemont, M.C.; Lefort, A.; Imbenotte, M.; Lhermitte, M. Simultaneous determination of tryptophan and kynurenine in serum by HPLC with UV and fluorescence detection. Biomed. Chromatogr. 2004, 18, 872–874. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Ding, M. Simultaneous determination of tryptophan and kynurenine in plasma samples of children patients with Kawasaki disease by high- performance liquid chromatography with programmed wavelength ultraviolet detection. J. Chromatogr. B 2009, 877, 1678–1682. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, G.; Yang, M.; Zheng, F.; Zheng, Z.; Zhang, S.; Zhong, S. Ultra- performance liquid chromatography-tandem mass spectrometry quantitative profiling of tryptophan metabolites in human plasma and its application to clinical study. J. Chromatogr. B 2019, 1128, 121745. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zhang, Y.; Wang, F.; Chen, Z. Determination of tryptophan and kynurenine in human plasma by liquid chromatography-electrochemical detection with multi-wall carbon nanotube-modified glassy carbon electrode. Biomed. Chromatogr. 2011, 25, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Staniszewska, M. Electrochemical determination of kynurenine pathway metabolites-challenges and perspectives. Sensors 2021, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Majidi, M.R.; Johari-Ahar, M.; Barar, J.; Omidi, Y. Development of screen-printed tryptophan-kynurenine immunosensor for in vitro assay of kynurenine-mediated immunosuppression effect of cancer cells on activated T-cells. Biosens. Bioelectron. 2017, 92, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Kamata, T.; Sumimoto, M. Electrochemical detection of tryptophan metabolites via kynurenine pathway by using nanocarbon sensors. Electroanalysis 2021, 25, 938–942. [Google Scholar] [CrossRef]
- Sadok, I.; Tyszczuk-Rotko, K.; Mroczka, R.; Staniszewska, M. Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta 2020, 209, 120574. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Tyszczuk-Rotko, K.; Mroczka, R.; Kozak, J.; Staniszewska, M. Improved voltammetric determination of kynurenine at the nafion covered glassy carbon electrode—Application in samples delivered from human cancer cells. Int. J. Tryptophan Res. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Patel, K.; Fernandez-Villamarin, M.; Ward, C.; Lord, J.M.; Tino, P.; Mendes, P.M. Establishing a quantitative fluorescence assay for the rapid detection of kynurenine in urine. Analyst 2022, 147, 1931. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Del Sole, R.; Mele, G.; Bloise, E.; Mergola, L. Green aspects in molecularly imprinted polymers by biomass waste utilization. Polymers 2021, 13, 2430. [Google Scholar] [CrossRef]
- Janczura, M.; Lulínski, P.; Sobiech, M. Imprinting technology for effective sorbent fabrication: Current state-of-art and future prospects. Materials 2021, 14, 1850. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Mergola, L.; Orabona, C.; Albini, E.; Vasapollo, G.; Scorrano, S.; Del Sole, R. Urinary l-kynurenine quantification and selective extraction through a molecularly imprinted solid-phase extraction device. J. Sep. Sci. 2018, 41, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Squissato, A.L.; Munoz, R.A.A.; Banks, C.E.; Richter, E.M. An overview of recent electroanalytical applications utilizing screen-printed electrodes within flow systems. ChemElectroChem 2020, 7, 2211–2221. [Google Scholar] [CrossRef]
- Seguro, I.; Pacheco, J.G.; Delerue-Matos, C. Low cost, easy to prepare and disposable electrochemical molecularly imprinted sensor for diclofenac detection. Sensors 2021, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Gonçalves, L.M.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Karadurmus, L.; Corman, M.E.; Uzun, L.; Ozkan, S.A. Enantioselective recognition of esomeprazole with a molecularly imprinted sol-gel-based electrochemical sensor. Microchim. Acta 2022, 189, 225. [Google Scholar] [CrossRef]
- Karami, X.P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef]
- Gomes, R.S.; Moreira, F.T.C.; Fernandes, R.; Sales, M.G.F. Sensing CA 15-3 in point-of-care by electropolymerizing O-phenylenediamine (oPDA) on Au-screen printed electrodes. PLoS ONE 2018, 13, e0196656. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Karami, P.; Ghanei, M.; Afkhami, A.; Bagheri, H. Development of a molecularly imprinted polymer tailored on disposable screen-printed electrodes for dual detection of EGFR and VEGF using nano-liposomal amplification strategy. Biosens. Bioelectron. 2018, 107, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Y.; Li, Y.-T.; Li, X.-J.; Zhang, L.; Fodj, E.K.; Han, S. Controllable in situ fabrication of portable AuNP/mussel-inspired polydopamine molecularly imprinted SERS substrate for selective enrichment and recognition of phthalate plasticizers. Chem. Eng. J. 2020, 402, 125179. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Syritski, V. Sulfamethizole-imprinted polymer on screen-printed electrodes: Towards the design of a portable environmental sensor. Sens. Actuators B Chem. 2020, 320, 128600. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhao, F.; Zhang, W.; Ye, Z. Molecularly imprinted polymer-based sensors for atrazine detection by electropolymerization of o-phenylenediamine. RSC Adv. 2015, 5, 56534–56540. [Google Scholar] [CrossRef]
- Fiore, L.; De Lellis, B.; Mazzaracchio, V.; Suprun, E.; Massoud, R.; Goffredo, B.M.; Moscone, D.; Arduini, F. Smartphone-assisted electrochemical sensor for reliable detection of tyrosine in serum. Talanta 2022, 237, 122869. [Google Scholar] [CrossRef]
- Garrido, E.M.P.J.; Garrido, J.M.P.J.; Milhazes, N.; Borges, F.; Oliveira-Brett, A.M. Electrochemical oxidation of amphetamine-like drugs and application to electro- analysis of ecstasy in human serum. Bioelectrochemistry 2010, 79, 77–83. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 1046. [Google Scholar] [CrossRef]
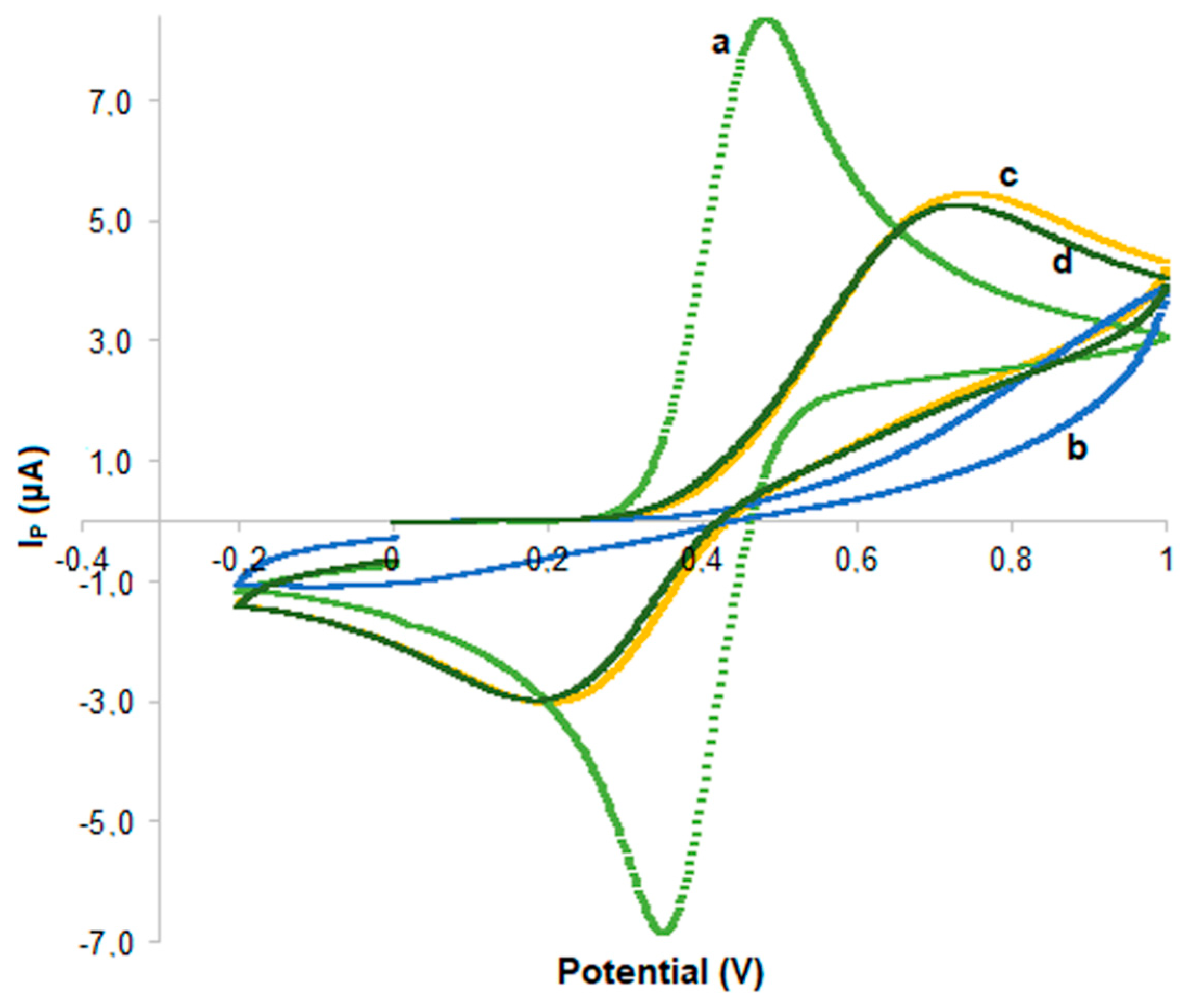
 ) and kyn-MIP-SPCE incubated with l-Kyn standard solutions at 10 µM (
) and kyn-MIP-SPCE incubated with l-Kyn standard solutions at 10 µM ( ), 50 µM (
), 50 µM ( ), and 100 µM (
), and 100 µM ( ).
).
 ) and kyn-MIP-SPCE incubated with l-Kyn standard solutions at 10 µM (
) and kyn-MIP-SPCE incubated with l-Kyn standard solutions at 10 µM ( ), 50 µM (
), 50 µM ( ), and 100 µM (
), and 100 µM ( ).
).

| SPE | Functional Monomer | Analyte | Matrix | LOD (µM) | LOQ (µM) | LR (µM) | Analytical Method | Incubation Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MIP-SPCE | o-PD | Ecstasy | Urine | 6.4 | 21 | Up to 0.2 | SWV | 10 | [27] |
| MIP-SPCE | o-PD | l-Kyn | Urine | 1.5 | 5 | 10–1000 | SWV | 10 | This work |
| GCE * | - | Amphetamine- like drugs | Serum | 2.4 | 8.3 | 12–45 | SWV | - | [36] |
| MIP-SPCE | Dopamine | Diclofenac | Water | 0.07 | 0.2 | 0.1–10 | DPV | 30 | [26] |
| SPE/rGO/AuNPs ** | - | Trp | Buffer solution | 0.39 | 1.32 | 0.5–500 | DPV | - | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Sole, R.; Stomeo, T.; Mergola, L. Disposable Molecularly Imprinted Polymer-Modified Screen-Printed Electrodes for Rapid Electrochemical Detection of l-Kynurenine in Human Urine. Polymers 2024, 16, 3. https://doi.org/10.3390/polym16010003
Del Sole R, Stomeo T, Mergola L. Disposable Molecularly Imprinted Polymer-Modified Screen-Printed Electrodes for Rapid Electrochemical Detection of l-Kynurenine in Human Urine. Polymers. 2024; 16(1):3. https://doi.org/10.3390/polym16010003
Chicago/Turabian StyleDel Sole, Roberta, Tiziana Stomeo, and Lucia Mergola. 2024. "Disposable Molecularly Imprinted Polymer-Modified Screen-Printed Electrodes for Rapid Electrochemical Detection of l-Kynurenine in Human Urine" Polymers 16, no. 1: 3. https://doi.org/10.3390/polym16010003
APA StyleDel Sole, R., Stomeo, T., & Mergola, L. (2024). Disposable Molecularly Imprinted Polymer-Modified Screen-Printed Electrodes for Rapid Electrochemical Detection of l-Kynurenine in Human Urine. Polymers, 16(1), 3. https://doi.org/10.3390/polym16010003






