A Review on Barrier Properties of Cellulose/Clay Nanocomposite Polymers for Packaging Applications
Abstract
:1. Introduction
2. Preparation of Nanocellulose
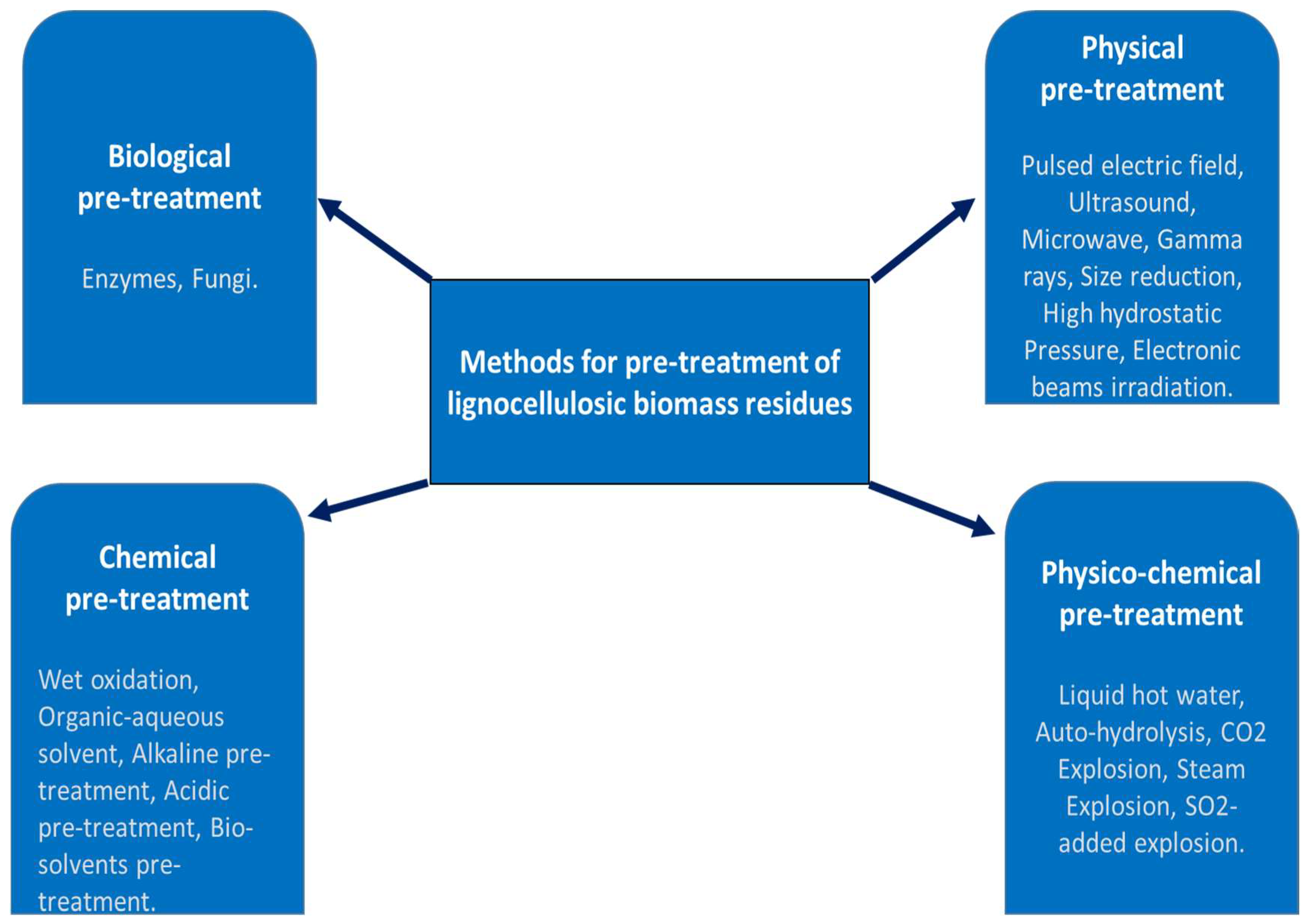
2.1. Pretreatment Methods of Lignocellulosic Biomass
2.1.1. Biological Methods
2.1.2. Physical Methods
2.1.3. Physicochemical Methods
2.1.4. Chemical Methods
2.2. Bleaching
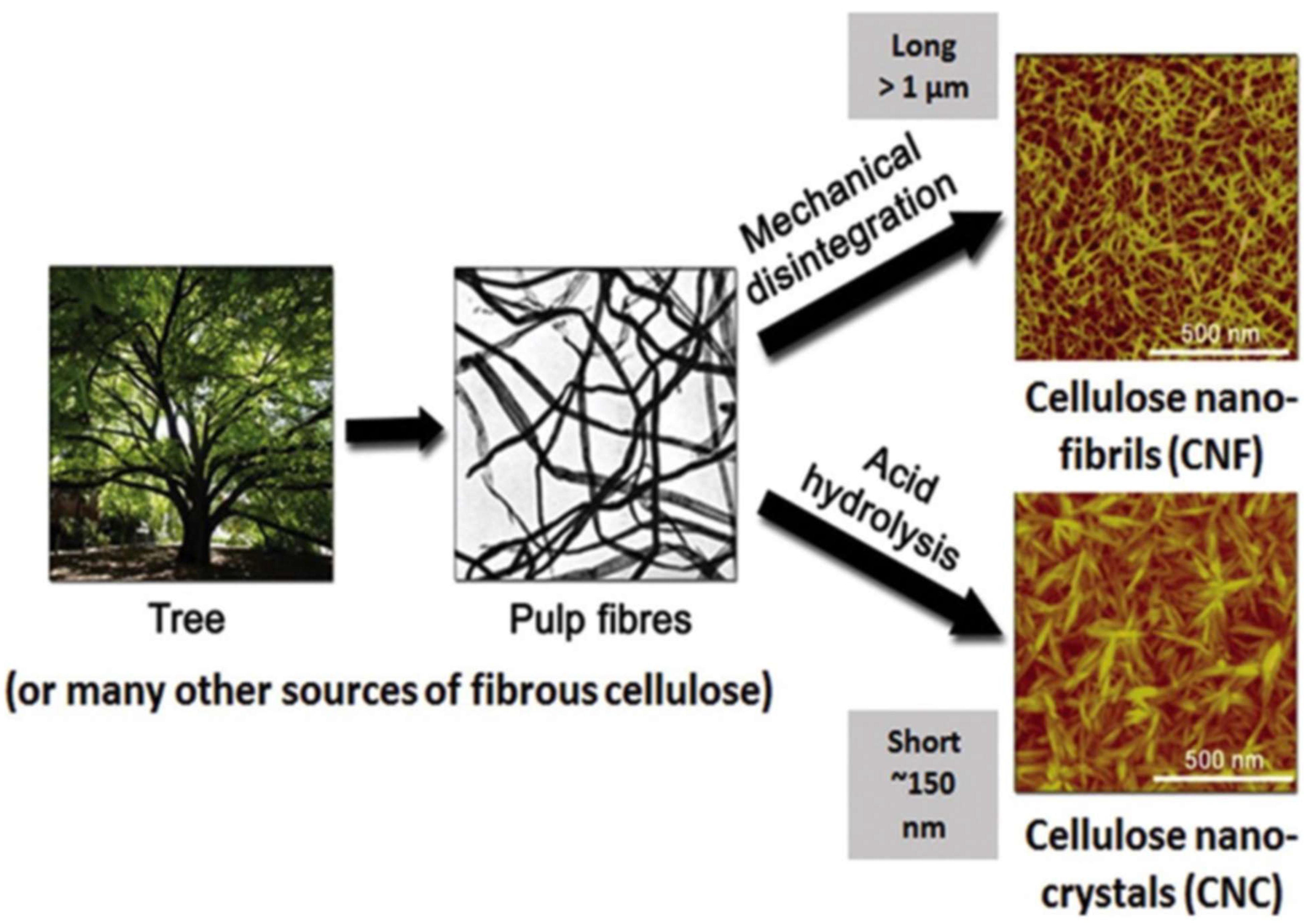
2.3. Methods for Cellulose Nanoparticle Isolation
2.3.1. Physical Methods
2.3.2. Enzymatic Hydrolysis
2.3.3. Acid Hydrolysis
3. Modification of Cellulose Fibers
3.1. Physical Modification of Cellulose Fibers
3.1.1. Heat Treatment
3.1.2. UV Modification
3.2. Chemical Modification of Cellulose Fibers
3.2.1. TEMPO-Mediated Oxidation
3.2.2. Acetylation
3.2.3. Periodate Oxidation
4. Nanofillers
Nanoclays
5. Nanocomposite Preparation Methods
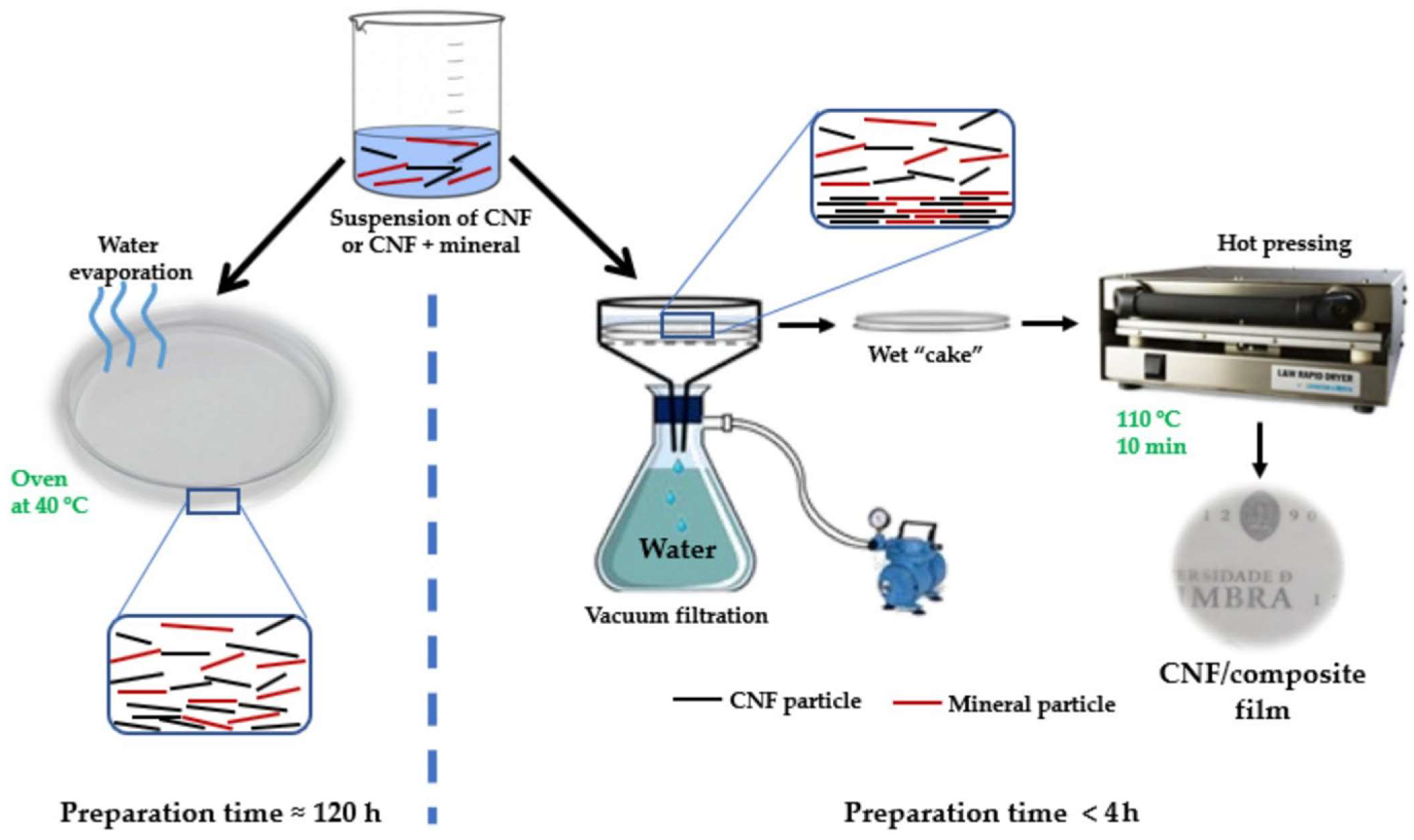
5.1. The Solvent-Casting Method
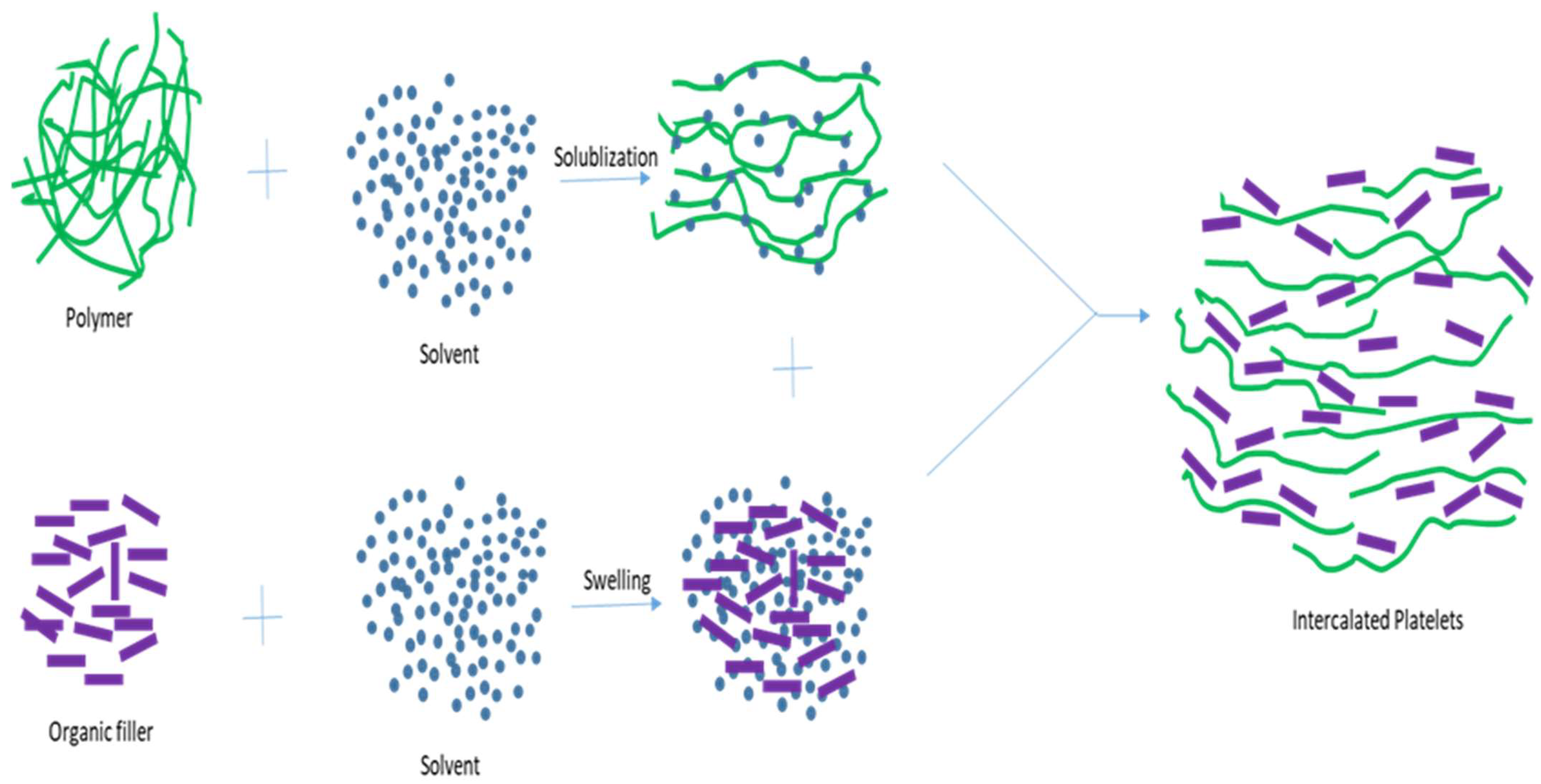
5.2. The Melt Intercalation Process
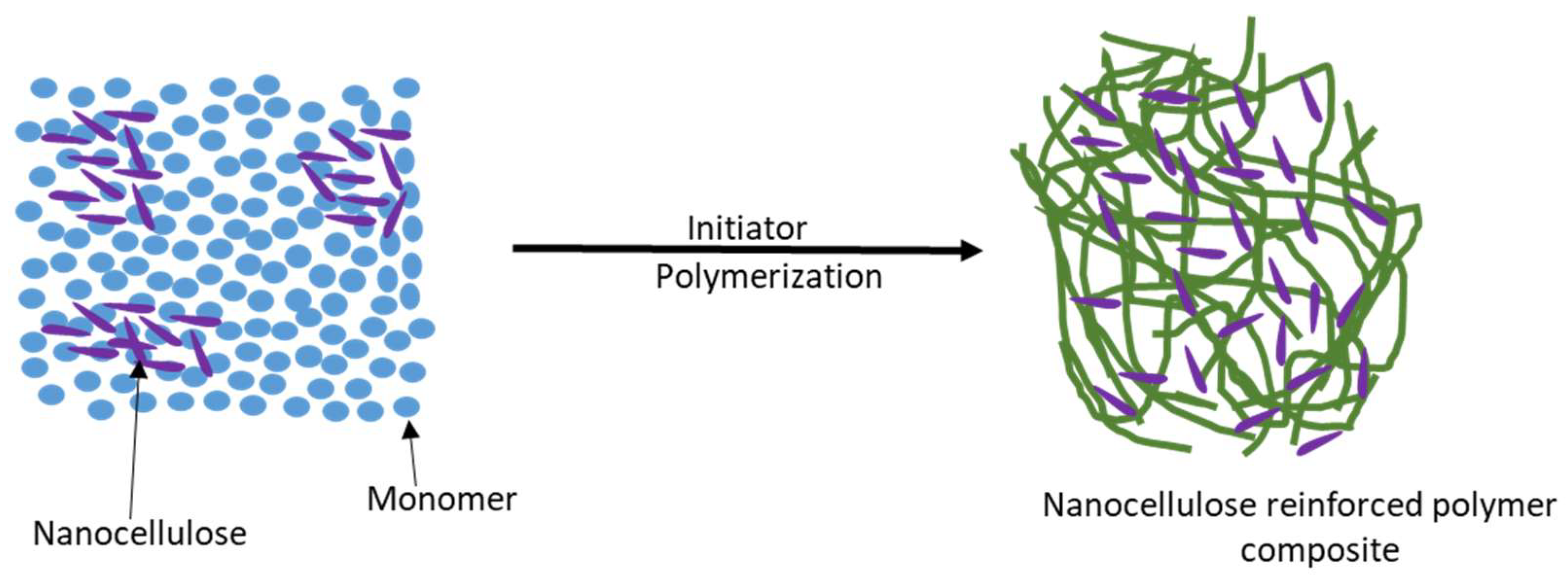
5.3. In Situ Polymerization
6. Barrier Performance
7. Processing Features and Challenges
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindström, T.; Österberg, F. Evolution of biobased and nanotechnology packaging—A review. Nord. Pulp Pap. Res. J. 2020, 35, 491–515. [Google Scholar] [CrossRef]
- Bardet, R.; Reverdy, C.; Belgacem, N.; Leirset, I.; Syverud, K.; Bardet, M.; Bras, J. Substitution of nanoclay in high gas barrier films of cellulose nanofibrils with cellulose nanocrystals and thermal treatment. Cellulose 2015, 22, 1227–1241. [Google Scholar] [CrossRef]
- Abelti, A.L.; Teka, T.A.; Fikreyesus Forsido, S.; Tamiru, M.; Bultosa, G.; Alkhtib, A.; Burton, E. Bio-based smart materials for fish product packaging: A review. Int. J. Food Prop. 2022, 25, 857–871. [Google Scholar] [CrossRef]
- Palali, S.N. Cellulose Nanocrystals: Potential Replacement for Food Packaging. Science 2019, 39–73. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Allen, D.; Berguson, B.; DellaPenna, D.; Shachar-Hill, Y.; Stymne, S. Driving on biomass. Science 2009, 324, 1019–1020. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Biopolymer-Based Films Reinforced with Green Synthesized Zinc Oxide Nanoparticles. Polymers 2022, 14, 5202. [Google Scholar] [CrossRef]
- Cakmak, H.; Dekker, M. Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films. Foods 2022, 11, 3932. [Google Scholar] [CrossRef]
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-based films and their emerging applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764. [Google Scholar] [CrossRef]
- Li, J.; Song, Z.; Li, D.; Shang, S.; Guo, Y. Cotton cellulose nanofiber-reinforced high density polyethylene composites prepared with two different pretreatment methods. Ind. Crops Prod. 2014, 59, 318–328. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobization of lignocellulosic materials part III: Modification with polymers. Cellulose 2022, 29, 5943–5977. [Google Scholar] [CrossRef]
- Boluk, Y.; Lahiji, R.; Zhao, L.; McDermott, M.T. Suspension viscosities and shape parameter of cellulose nanocrystals (CNC). Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 297–303. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Hua, K.; Carlsson, D.O.; Ålander, E.; Lindström, T.; Strømme, M.; Mihranyan, A.; Ferraz, N. Translational study between structure and biological response of nanocellulose from wood and green algae. RSC Adv. 2014, 4, 2892–2903. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, C.D.M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. [Google Scholar] [CrossRef]
- Wang, C.; Shi, J.; He, M.; Ding, L.; Li, S.; Wang, Z.; Wei, J. High strength cellulose/ATT composite films with good oxygen barrier property for sustainable packaging applications. Cellulose 2018, 25, 4145–4154. [Google Scholar] [CrossRef]
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable cellulose film prepared from banana pseudo-stem using an ionic liquid for mango preservation. Front. Plant Sci. 2021, 12, 625878. [Google Scholar] [CrossRef]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Sethi, J.; Wågberg, L.; Larsson, P.A. Water-resistant hybrid cellulose nanofibril films prepared by charge reversal on gibbsite nanoclays. Carbohydr. Polym. 2022, 295, 119867. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J.C. Chitin-and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Hussin, F.N.N.M.; Wahab, R.A.; Attan, N. Nanocellulose and nanoclay as reinforcement materials in polymer composites: A review. Malays. J. Fundam. Appl. Sci. 2020, 16, 145–153. [Google Scholar] [CrossRef]
- Mdletshe, G.P. Extraction and Characterisation of Cellulose Nanocrystals (CNCs) from Sugarcane Bagasse Using Ionic Liquids. Ph.D. Thesis, Durban University of Technoloy, Durban, South Africa, 2019. [Google Scholar]
- Beig, B.; Riaz, M.; Naqvi, S.R.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.; Chi, N.T.L. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Kumar Mohanty, M.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefin. 2021, 13, 1503–1527. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A.; Lata. Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and barriers of advanced biofuel technologies: A review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.S.S.; Silva, A.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; Barros, R.R.O.; Ferrara, M.A.; Bon, E.P.S. Biomass pretreatment: A critical choice for biomass utilization via biotechnological routes. BMC Proc. 2014, 8, O34. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Gáspár, M.; Kálmán, G.; Réczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem. 2007, 42, 1135–1139. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, X.; Zhang, S. Effects of cationic structure on cellulose dissolution in ionic liquids: A molecular dynamics study. ChemPhysChem 2012, 13, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.-L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Aita, G.M.; Kim, M. Pretreatment Technologies for the Conversion of Lignocellulosic Materials to Bioethanol. In Sustainability of the Sugar and Sugar−Ethanol Industries; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 117–145. [Google Scholar]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Singh, L.K.; Chaudhary, G.; Majumder, C.; Ghosh, S. Utilization of hemicellulosic fraction of lignocellulosic biomaterial for bioethanol production. Adv. Appl. Sci. Res. 2011, 2, 508–521. [Google Scholar]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Hazwan Hussin, M.; Trache, D.; Chuin, C.T.H.; Nurul Fazita, M.; Mohamad Haafiz, M.; Hossain, M. Extraction of cellulose nanofibers and their eco-friendly polymer composites. In Sustainable Polymer Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 653–691. [Google Scholar]
- Pereira, A.L.S.; Nascimento, D.M.; Cordeiro, E.M.S.; Morais, J.P.S.; Sousa, M.d.S.M.; Rosa, M.d.F. Characterization of Lignocellulosic Materials Extracted from the Banana Pseudostem. 2010. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/34240/1/AT10096.pdf (accessed on 5 April 2023).
- Aridi, A.S.; Chin, N.L.; Ishak, N.A.; Yusof, N.N.M.; Kadota, K.; Manaf, Y.N.; Yusof, Y.A. Effect of Sodium Hypochlorite Concentration during Pre-treatment on Isolation of Nanocrystalline Cellulose from Leucaena leucocephala (Lam.) Mature Pods. BioResources 2021, 16, 3137. [Google Scholar] [CrossRef]
- Shin, H.K.; Pyo Jeun, J.; Bin Kim, H.; Hyun Kang, P. Isolation of cellulose fibers from kenaf using electron beam. Radiat. Phys. Chem. 2012, 81, 936–940. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Marakana, P.G.; Dey, A.; Saini, B. Isolation of nanocellulose from lignocellulosic biomass: Synthesis, characterization, modification, and potential applications. J. Environ. Chem. Eng. 2021, 9, 106606. [Google Scholar] [CrossRef]
- Tahir, D.; Karim, M.R.A.; Hu, H.; Naseem, S.; Rehan, M.; Ahmad, M.; Zhang, M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers 2022, 14, 4468. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Souza, A.G.; Nunes, L.L.; Shahi, N.; Rangari, V.K.; dos Santos Rosa, D. Use of ball mill to prepare nanocellulose from eucalyptus biomass: Challenges and process optimization by combined method. Mater. Today Commun. 2020, 22, 100755. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2014, 2, 421–449. [Google Scholar] [CrossRef]
- Ragoubi, M.; George, B.; Molina, S.; Bienaimé, D.; Merlin, A.; Hiver, J.M.; Dahoun, A. Effect of corona discharge treatment on mechanical and thermal properties of composites based on miscanthus fibres and polylactic acid or polypropylene matrix. Compos. Part A Appl. Sci. Manuf. 2012, 43, 675–685. [Google Scholar] [CrossRef]
- Cao, Y.; Sakamoto, S.; Goda, K. Effects of heat and alkali treatments on mechanical properties of kenaf fibers. In Proceedings of the 16th International Conference on Composite Materials, Kyoto, Japan, 8–13 July 2007; pp. 8–13. [Google Scholar]
- Ahmad, R.; Hamid, R.; Osman, S.A. Physical and Chemical Modifications of Plant Fibres for Reinforcement in Cementitious Composites. Adv. Civ. Eng. 2019, 2019, 5185806. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, X.; Nair, S.S.; Ragauskas, A.; Zhu, J.; Deng, Y. Thermally enhanced high performance cellulose nano fibril barrier membranes. RSC Adv. 2014, 4, 45136–45142. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, Z.; Liu, W.; Li, V.C.F.; Cao, Y.; Zhang, W.; Deng, Y. Highly transparent 100% cellulose nanofibril films with extremely high oxygen barriers in high relative humidity. Cellulose 2018, 25, 4057–4066. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, S.; Chen, X.; Sheng, L. Nanocomposite films based on TEMPO-mediated oxidized bacterial cellulose and chitosan. Cellulose 2014, 21, 2757–2772. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2019, 55, 829–892. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Hartman, K. Esterified cellulose nanocrystals as reinforcement in poly(lactic acid) nanocomposites. Cellulose 2019, 26, 2349–2362. [Google Scholar] [CrossRef]
- Visanko, M.; Liimatainen, H.; Sirviö, J.A.; Mikkonen, K.S.; Tenkanen, M.; Sliz, R.; Hormi, O.; Niinimäki, J. Butylamino-functionalized cellulose nanocrystal films: Barrier properties and mechanical strength. RSC Adv. 2015, 5, 15140–15146. [Google Scholar] [CrossRef]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, K.S.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Films with High Oxygen Barrier Properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.A.; Kochumalayil, J.J.; Wågberg, L. Oxygen and water vapour barrier films with low moisture sensitivity fabricated from self-cross-linking fibrillated cellulose. In Proceedings of the 15th Fundamental Research Symposium: Advances in Pulp and Paper Research, Cambridge, UK, 9–13 September 2013; The Pulp and Paper Fundamental Research Society: Cambridge, UK, 2013; pp. 851–866. [Google Scholar]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Jamroz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Pires, J.; Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the Barrier and Mechanical Behavior of Different Nanofillers in Chitosan Films for Food Packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; R, S.; Pal, K.; Sarkar, P. Nanoclay-based active food packaging systems: A review. Food Packag. Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Akhila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins based packaging film with added nanoparticles: A review. Int. J. Biol. Macromol. 2022, 203, 515–525. [Google Scholar]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef]
- Wu, C.N.; Saito, T.; Fujisawa, S.; Fukuzumi, H.; Isogai, A. Ultrastrong and high gas-barrier nanocellulose/clay-layered composites. Biomacromolecules 2012, 13, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Gmoshinski, I.V.; Bagryantseva, O.V.; Arnautov, O.V.; Khotimchenko, S.A. Nanoclays in food products: Benefits and possible risks (literature review). Health Risk Anal. 2020, 1, 142–164. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.a.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.-V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
- Bharimalla, A.; Deshmukh, S.; Vigneshwaran, N.; Patil, P.; Prasad, V. Nanocellulose-polymer composites for applications in food packaging: Current status, future prospects and challenges. Polym. Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Silva, F.A.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose bio-based composites for food packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Ng, P.K. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Singha, S.; Hedenqvist, M.S. A review on barrier properties of poly (lactic acid)/clay nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef]
- Bharimalla, A.; Patil, P.; Mukherjee, S.; Yadav, V.; Prasad, V. Nanocellulose-polymer composites: Novel materials for food packaging applications. In Polymers for Agri-Food Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 553–599. [Google Scholar]
- Garusinghe, U.M.; Varanasi, S.; Raghuwanshi, V.S.; Garnier, G.; Batchelor, W. Nanocellulose-montmorillonite composites of low water vapour permeability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 233–241. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Puttegowda, M.; Mavinkere Rangappa, S.; Siengchin, S. Influence of nanofillers on biodegradable composites: A comprehensive review. Polym. Compos. 2021, 42, 5691–5711. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl Cellulose/Gelatin Hydrogel Films Loaded with Zinc Oxide Nanoparticles for Sustainable Food Packaging Applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2017, 6, 49–70. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, C.N.; Saito, T.; Isogai, A. Cellulose-clay layered nanocomposite films fabricated from aqueous cellulose/LiOH/urea solution. Carbohydr Polym 2014, 100, 179–184. [Google Scholar] [CrossRef]
- Yang, Q.; Fukuzumi, H.; Saito, T.; Isogai, A.; Zhang, L. Transparent cellulose films with high gas barrier properties fabricated from aqueous alkali/urea solutions. Biomacromolecules 2011, 12, 2766–2771. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Xu, D.; Cai, J.; Qiu, Y.; Ren, J.; Zhang, L. Facile construction of cellulose/montmorillonite nanocomposite biobased plastics with flame retardant and gas barrier properties. Cellulose 2015, 22, 3799–3810. [Google Scholar] [CrossRef]
- Farmahini-Farahani, M.; Bedane, A.H.; Pan, Y.; Xiao, H.; Eic, M.; Chibante, F. Cellulose/nanoclay composite films with high water vapor resistance and mechanical strength. Cellulose 2015, 22, 3941–3953. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Dairi, N. Green nanocomposite films based on cellulose acetate and biopolymer-modified nanoclays: Studies on morphology and properties. Iran. Polym. J. 2014, 23, 917–931. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Wahit, M.U.; Ismail, A.; Yussuf, A. Preparation of regenerated cellulose/montmorillonite nanocomposite films via ionic liquids. Carbohydr. Polym. 2012, 88, 1251–1257. [Google Scholar] [CrossRef]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose–nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef]
- Liimatainen, H.; Ezekiel, N.; Sliz, R.; Ohenoja, K.; Sirvio, J.A.; Berglund, L.; Hormi, O.; Niinimaki, J. High-strength nanocellulose–talc hybrid barrier films. ACS Appl. Mater. Interfaces 2013, 5, 13412–13418. [Google Scholar] [CrossRef]
- Yao, K.; Huang, S.; Tang, H.; Xu, Y.; Buntkowsky, G.; Berglund, L.A.; Zhou, Q. Bioinspired interface engineering for moisture resistance in nacre-mimetic cellulose nanofibrils/clay nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 20169–20178. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Zahed-Karkaj, S.; Peighambardoust, S.H.; Ebrahimi, Y.; Peressini, D. Characterization of carboxymethyl cellulose-based active films incorporating non-modified and Ag or Cu-modified Cloisite 30B and montmorillonite nanoclays. Iran. Polym. J. 2020, 29, 1087–1097. [Google Scholar] [CrossRef]
- Gamelas, J.; Ferraz, E. Composite films based on nanocellulose and nanoclay minerals as high strength materials with gas barrier capabilities: Key points and challenges. BioResources 2015, 10, 6310–6313. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Gamelas, J.A.F. Composites of nanofibrillated cellulose with clay minerals: A review. Adv. Colloid Interface Sci. 2019, 272, 101994. [Google Scholar] [CrossRef]
- Mondal, S. Review on nanocellulose polymer nanocomposites. Polym. Plast. Technol. Eng. 2018, 57, 1377–1391. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of Nanocellulose and Its Application in Nanocomposite Packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]




| Product Name | Oxygen Permeance (cm3 STP/m2·day·Pa) 23 °C | WVTR (g/m2·day) 23 °C | Ref. |
|---|---|---|---|
| Dairy products | 10−2 | 0.2–8 | [86] |
| Nuts, snacks, chips | 10−5 | 0.093–3.0 | [86] |
| Hard cheese | 10−3 | 50 | [86] |
| Meat and meat-based products | 10−1 | 2–100 | [86] |
| Retorted food | 10−5 | 0.40–7.6 | [86] |
| Fruits, vegetables, fresh salads | 10−1–2 | 10–4000 | [86] |
| Fats | 10−4 | 5.2–9.2 | [86] |
| S. No | Solvent | Methods | Cellulose Chemical Modification | Cellulose/Film Physical Modification | Nanoclay Modification | Key Findings | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Solvent casting | TEMPO-mediated oxidation | Heat treatment | Excellent oxygen permeability at 50% RH | [2] | ||
| 2 | Solvent casting | TEMPO-mediated oxidation | Mechanical | Oxygen permeability is lower than ethylene−vinylalcohol at 0% RH | [81] | ||
| 3 | LiOH/urea/H2O | Solvent casting | Oxygen permeability is lower than ethylene−vinylalcohol at 0% RH | [94] | |||
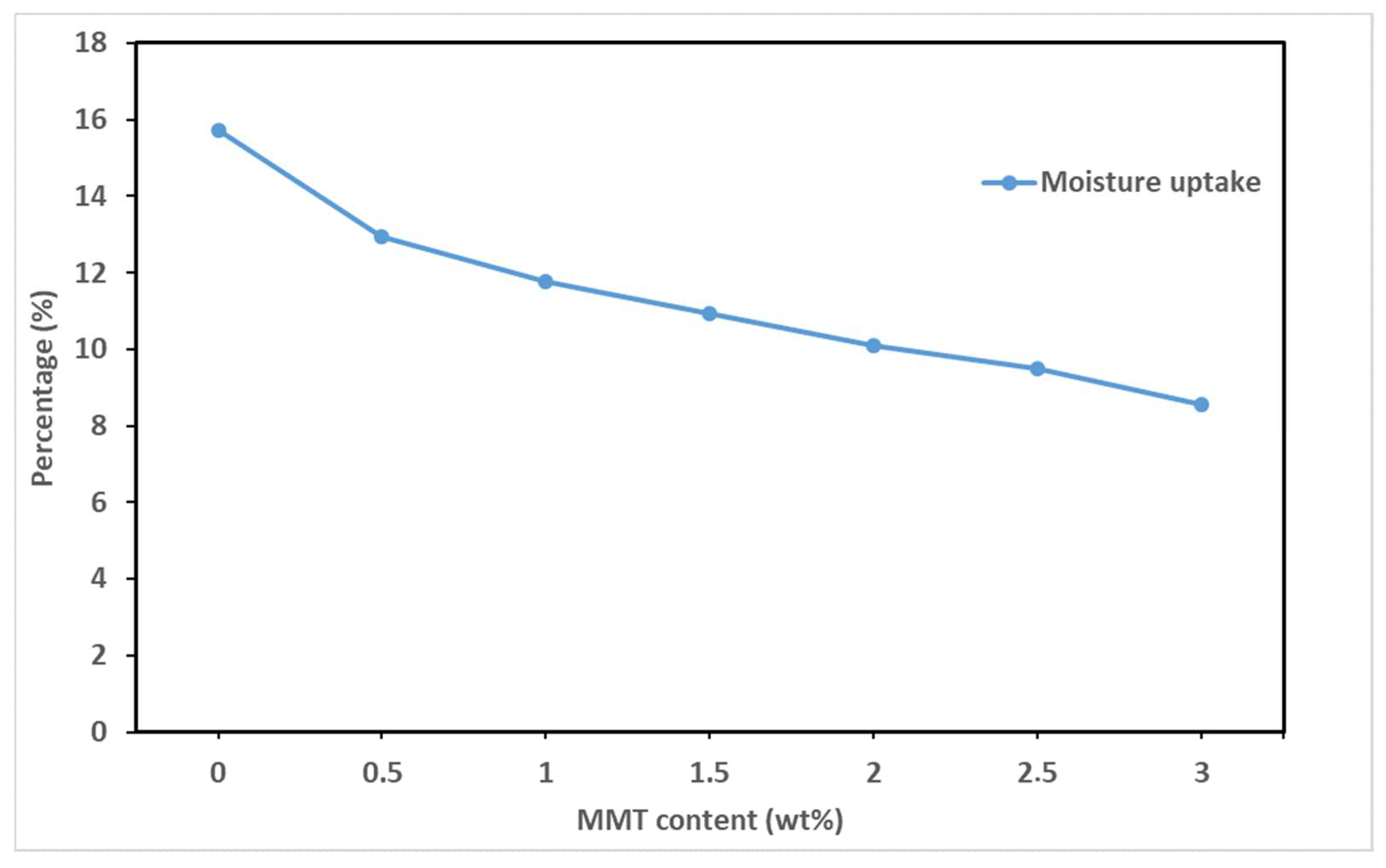
| 4 | Solvent casting | Decrease in WVP as clay content increases | [90] | ||||
| 5 | LiOH/urea/H2O | Solvent casting | Permeation of gases like N2, H2, CO2, and CH4 decreased with increase in MMT content | [96] | |||
| 6 | LiOH/urea/H2O | Solvent casting | Improved water-vapor permeability via the addition of nanoclay | [97] | |||
| 7 | Acetic acid/water | Solvent casting | Acetylation | Nanoclay organo-modified with gelatin or chitosan | Decrease in water-vapor transition rate for 5 wt% MMT film compared to neat cellulose film | [98] | |
| 8 | BMIMCl | Solvent casting | 33% reduction in oxygen and 31% in carbon dioxide permeability via 6 wt% MMT loading | [99] | |||
| 9 | Solvent casting | Nanoclay modified via cationic exchange with delaminated citrate ions and lithium salts | 20 wt% nanoclay film showed lower permeability compared than neat cellulose film | [100] | |||
| 10 | Water | Solvent casting | Nanoclay modified with Cu and Ag ions | Cu-modified Cloisite 30B clay had a higher reduction in WVP compared to Ag-modified clay | [103] | ||
| 11 | Solvent casting | Composite film with DA-CNF/MMT had lower oxygen transmission rate | [102] | ||||
| 12 | Solvent casting | Gibbsite/cellulose film had a factor of 36 lower oxygen permeability compared to neat cellulose | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jali, S.; Mohan, T.P.; Mwangi, F.M.; Kanny, K. A Review on Barrier Properties of Cellulose/Clay Nanocomposite Polymers for Packaging Applications. Polymers 2024, 16, 51. https://doi.org/10.3390/polym16010051
Jali S, Mohan TP, Mwangi FM, Kanny K. A Review on Barrier Properties of Cellulose/Clay Nanocomposite Polymers for Packaging Applications. Polymers. 2024; 16(1):51. https://doi.org/10.3390/polym16010051
Chicago/Turabian StyleJali, Sandile, Turup Pandurangan Mohan, Festus Maina Mwangi, and Krishnan Kanny. 2024. "A Review on Barrier Properties of Cellulose/Clay Nanocomposite Polymers for Packaging Applications" Polymers 16, no. 1: 51. https://doi.org/10.3390/polym16010051
APA StyleJali, S., Mohan, T. P., Mwangi, F. M., & Kanny, K. (2024). A Review on Barrier Properties of Cellulose/Clay Nanocomposite Polymers for Packaging Applications. Polymers, 16(1), 51. https://doi.org/10.3390/polym16010051







