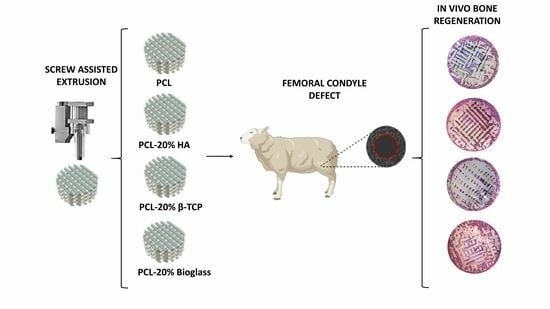
Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Fabrication of PCL Plugs
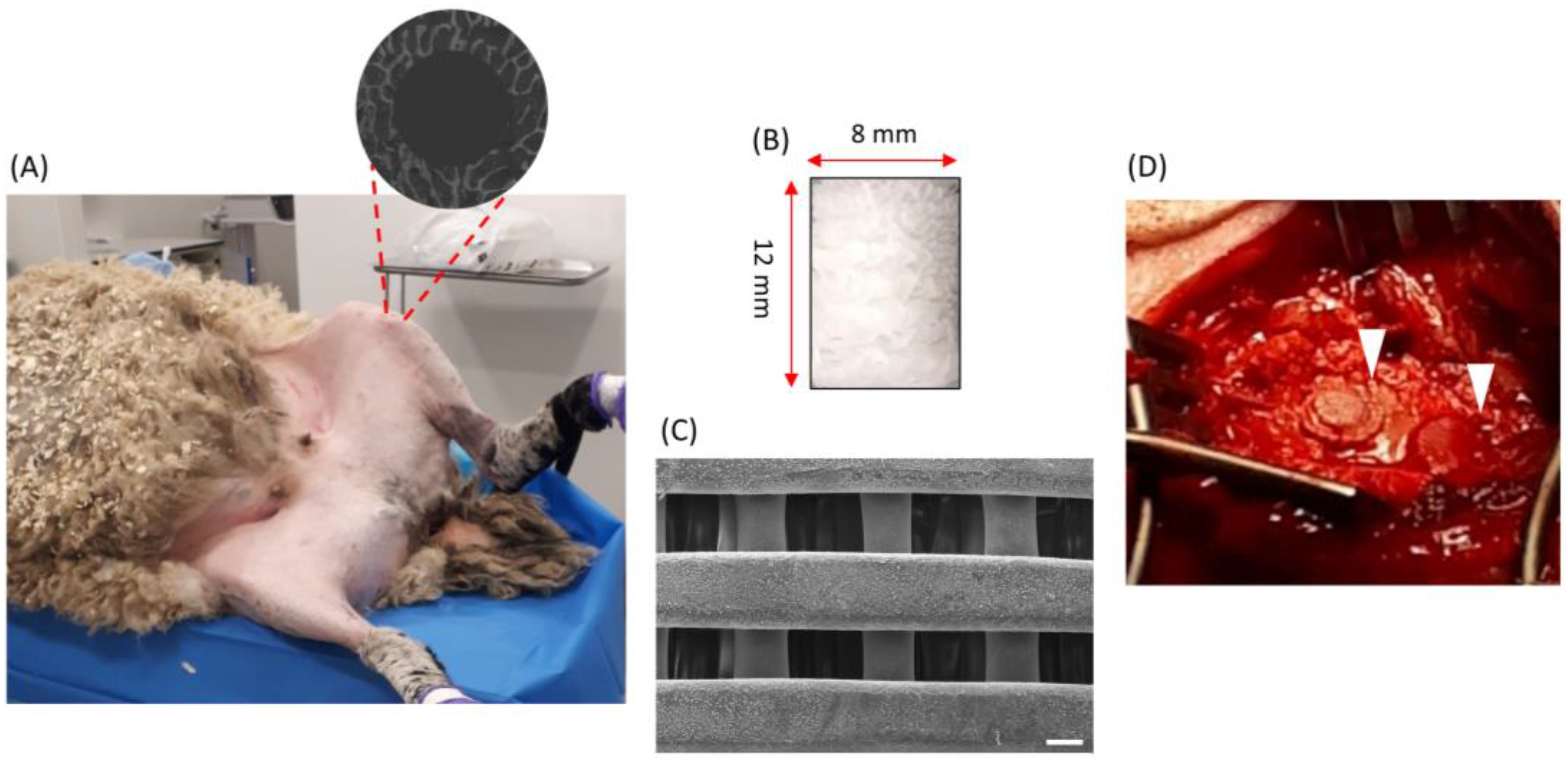
2.3. Surgical Procedure
2.4. X-ray Computed Tomography Imaging
2.5. Image Post-Processing
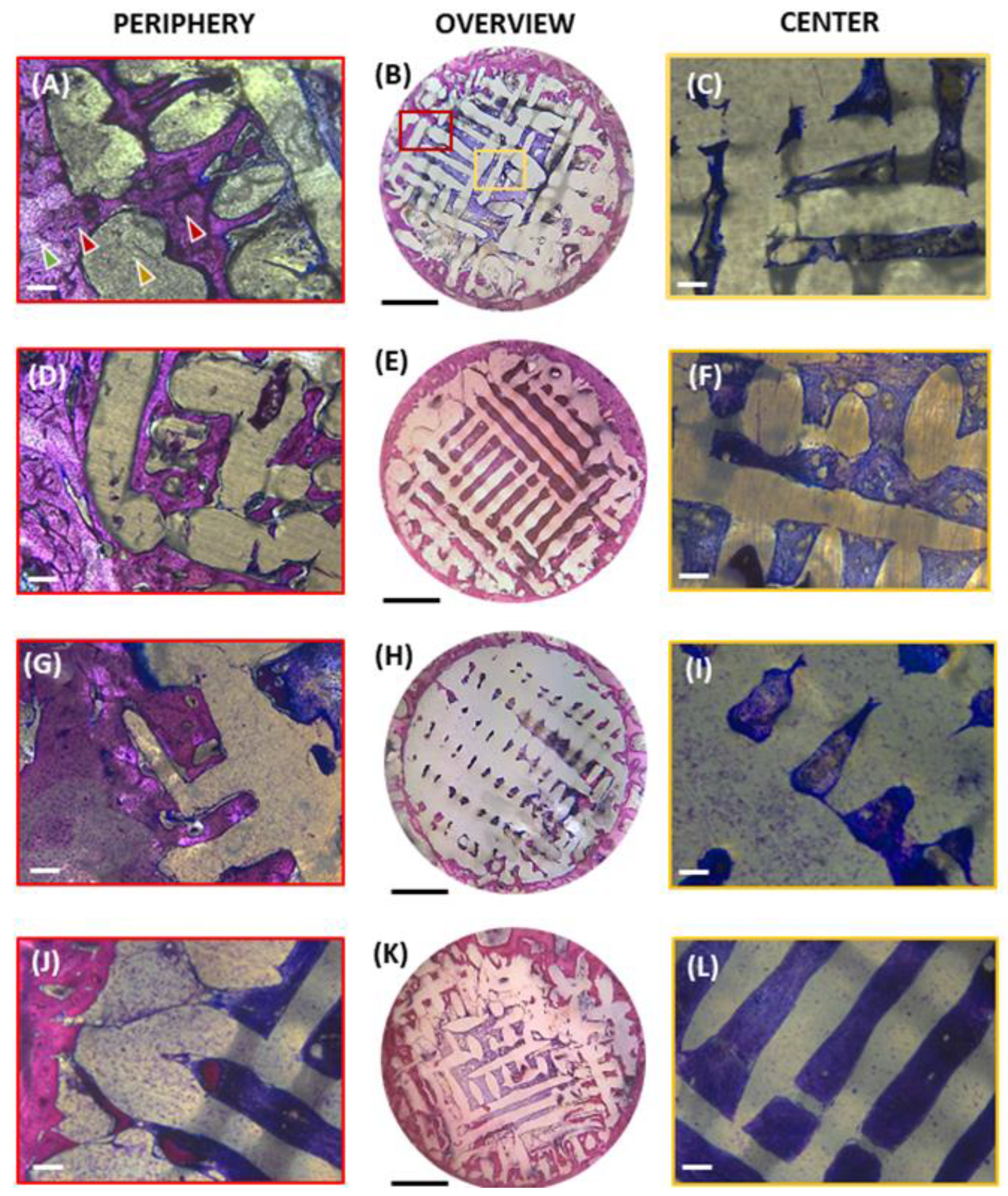
2.6. Histology
2.7. Backscattered Electron Microscopy
2.8. Statistical Analysis
3. Results
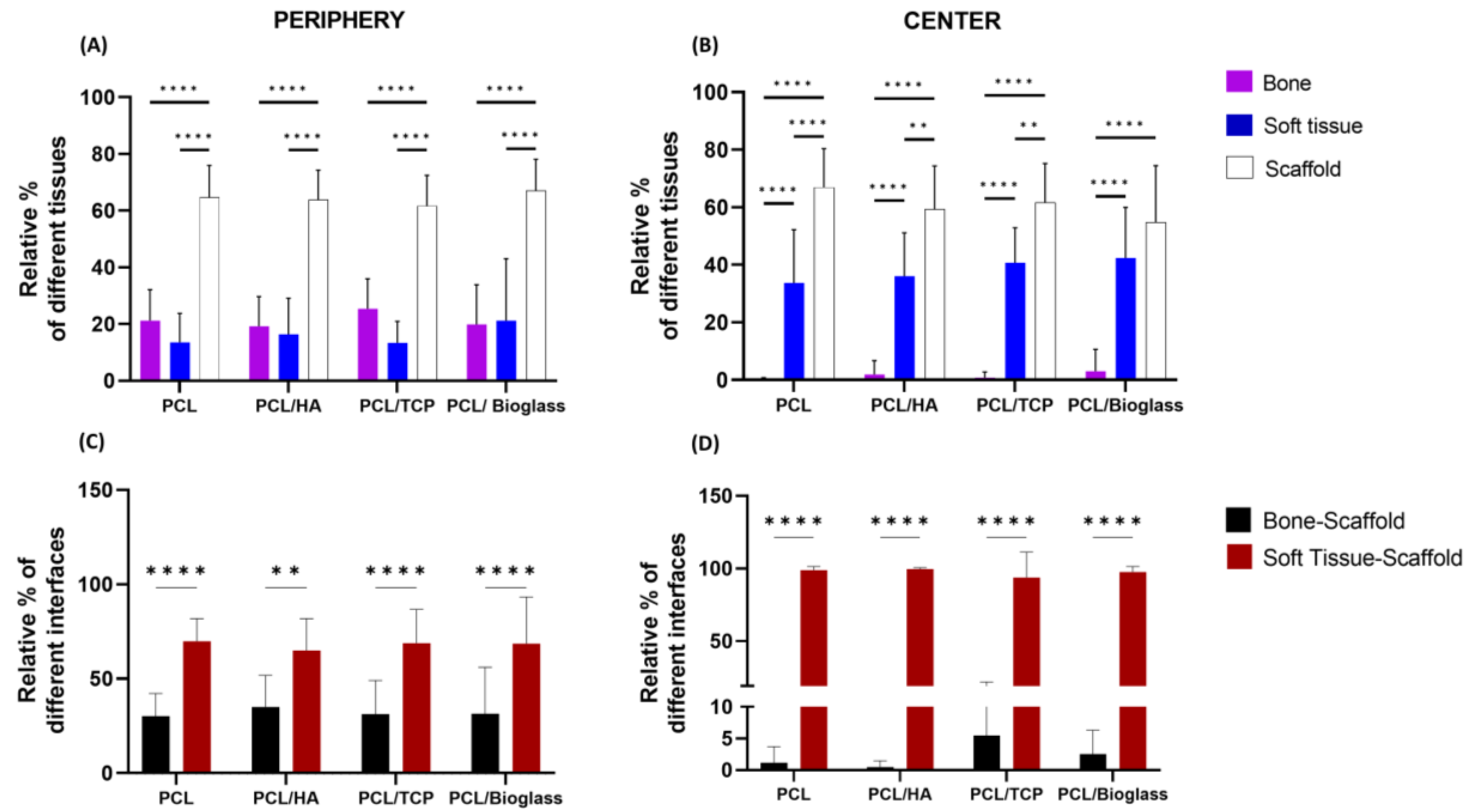
3.1. Histomorphometric Analysis
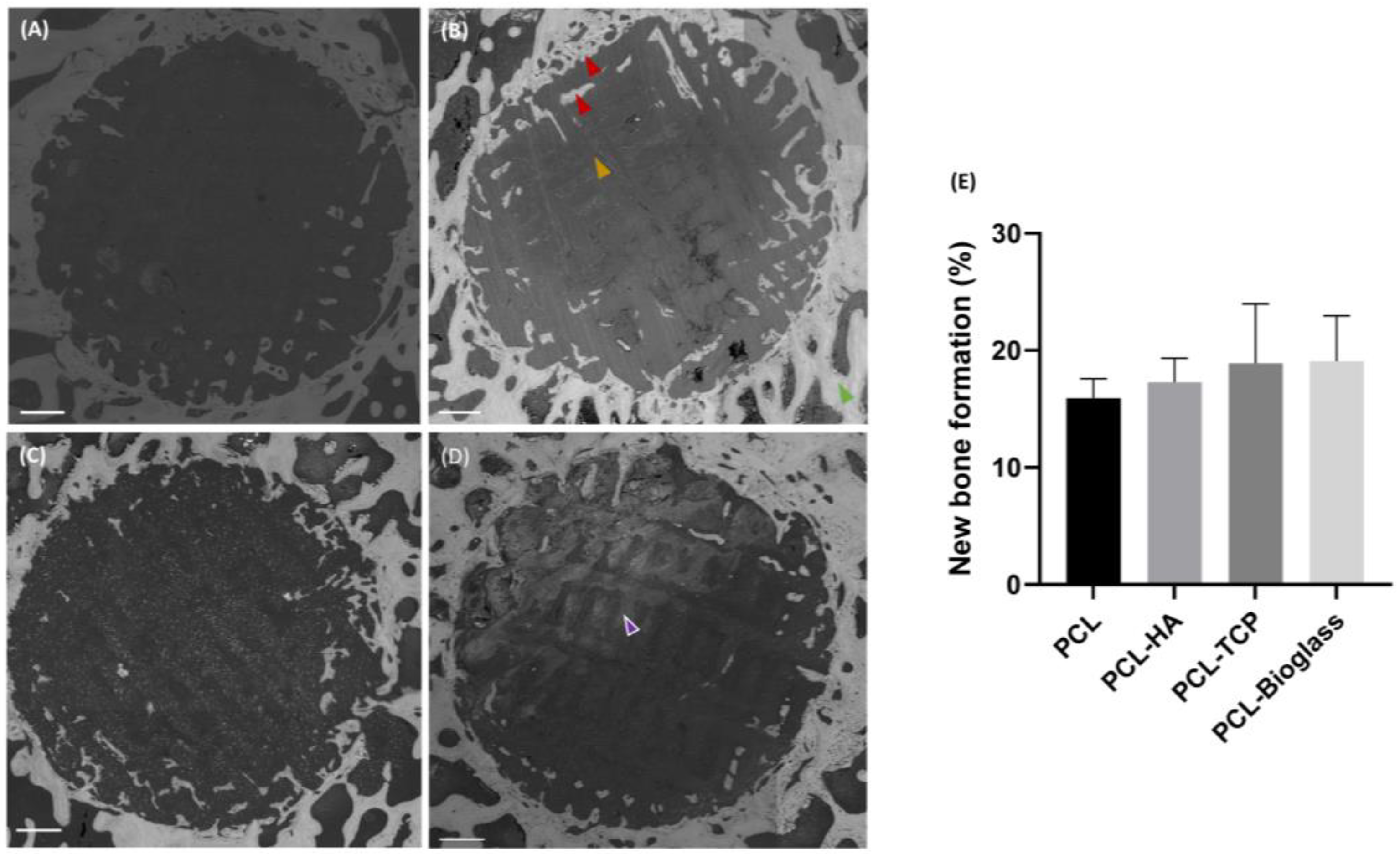
3.2. Backscattered Electron Microscopy
3.3. X-ray Computed Tomography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singaram, S.; Naidoo, M. The physical, psychological and social impact of long bone fractures on adults: A review. Afr. J. Prim. Health Care Fam. Med. 2019, 11, e1–e9. [Google Scholar] [CrossRef]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Clasper, J.; Ramasamy, A. Traumatic amputations. Br. J. Pain 2013, 7, 67–73. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.Y.; Gao, S.; Zhang, Y.W.; Zhou, R.B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Kazmers, N.H.; Fragomen, A.T.; Rozbruch, S.R. Prevention of pin site infection in external fixation: A review of the literature. Strateg. Trauma Limb Reconstr. 2016, 11, 75–85. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz, M.; Pawłowska, E.; et al. The Role of 3D Printing in Planning Complex Medical Procedures and Training of Medical Professionals-Cross-Sectional Multispecialty Review. Int. J. Environ. Res. Public Health 2022, 19, 3331. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tri-calcium phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef]
- Egan, P.F. Integrated design approaches for 3D printed tissue scaffolds: Review and outlook. Materials 2019, 12, 2355. [Google Scholar] [CrossRef]
- Patlolla, A.; Collins, G.; Livingston Arinzeh, T. Solvent-dependent properties of electrospun fibrous composites for bone tissue regeneration. Acta Biomater. 2010, 6, 90–101. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, H.; Kim, G. A surface-modified poly(ε-caprolactone) scaffold comprising variable nanosized surface-roughness using a plasma treatment. Tissue Eng. Part C Methods 2014, 20, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- De Mori, A.; Alasa, U.J.; Mühlhölzl, A.; Blunn, G. Slipper Limpet (Crepidula fornicata) Shells Support In Vitro Osteogenesis of Human Adipose-Derived Stem Cells. Mar. Drugs. 2023, 21, 248. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals. 2021, 14, 75. [Google Scholar] [CrossRef]
- Dashnyam, K.; El-Fiqi, A.; Buitrago, J.O.; Perez, R.A.; Knowles, J.C.; Kim, H.W. A mini review focused on the proangiogenic role of silicate ions released from silicon-containing biomaterials. J. Tissue Eng. 2017, 8, 204173141770733. [Google Scholar] [CrossRef]
- Dash, P.A.; Mohanty, S.; Nayak, S.K. A review on bioactive glass, its modifications and applications in healthcare sectors. J. Non-Cryst. Solids 2023, 614, 122404. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Yuan, H.; de Bruijn, J.D.; Zhang, X.; van Blitterswijk, C.A.; de Groot, K. Bone induction by porous glass ceramic made from Bioglass® (45S5). J. Biomed. Mater. Res. 2001, 58, 270–276. [Google Scholar] [CrossRef]
- Algarni, H.; AlShahrani, I.; Ibrahim, E.H.; Eid, R.A.; Kilany, M.; Ghramh, H.A.; Shaaban, E.R.; Reben, M. Nano and microstructure of bioglasses: In vitro and in vivo bioactivity properties. J. Non-Cryst. Solids 2019, 512, 72–80. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Roy, S.K.; Basu, D. In vivo response of porous hydroxyapatite and beta-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 217–227. [Google Scholar] [CrossRef]
- Przybilla, P.; Subkov, E.; Latorre, S.H.; Zankovic, S.; Mayr, H.O.; Killinger, A.; Schmal, H.; Seidenstuecker, M. Effect of 20 μm thin ceramic coatings of hydroxyapatite, bioglass, GB14 and Beta-Tricalciumphosphate with copper on the biomechanical stability of femoral implants. J. Mech. Behav. Biomed. Mater. 2023, 144, 105951. [Google Scholar] [CrossRef]
- Razavi, S.M.; Rismanchian, M.; Jafari-Pozve, N.; Nosouhian, S. Comparing the Efficacy of Three Different Nano-scale Bone Substitutes: In vivo Study. Adv. Biomed. Res. 2017, 6, 64. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Helaehil, J.V.; Lourenço, C.B.; Huang, B.; Helaehil, L.V.; de Camargo, I.X.; Chiarotto, G.B.; Santamaria-Jr, M.; Bártolo, P.; Caetano, G.F. In vivo investigation of polymer-ceramic pcl/ha and pcl/β-tcp 3d composite scaffolds and electrical stimulation for bone regeneration. Polymers 2022, 14, 65. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Hassan, M.H.; Omar, A.M.; Vyas, C.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; et al. In Vitro Evaluation of Pore Size Graded Bone Scaffolds with Different Material Composition. 3d Print. Addit. Manuf. 2022. ahead of printing. [Google Scholar] [CrossRef]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Cooper, G.; Weightman, A.; Bartolo, P. Rheological behaviour of different composite materials for additive manufacturing of 3D bone scaffolds. J. Mater. Res. Technol. 2023, 24, 3670–3682. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.A.; Liu, F.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koç, B.; et al. Bone Bricks: The Effect of Architecture and Material Composition on the Mechanical and Biological Performance of Bone Scaffolds. ACS Omega 2022, 7, 7515–7530. [Google Scholar] [CrossRef]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic Osteoinductivity of Porous Titanium Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef]
- Tamaddon, M.; Blunn, G.; Xu, W.; Domínguez, M.E.A.; Monzón, M.; Donaldson, J.; Skinner, J.; Arnett, T.R.; Wang, L.; Liu, C. Sheep condyle model evaluation of bone marrow cell concentrate combined with a scaffold for repair of large osteochondral defects Aims. Bone Jt. Res. 2021, 10, 677–689. [Google Scholar] [CrossRef]
- Autefage, H.; Allen, F.; Tang, H.M.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’Donnell, M.D.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef]
- Reznikov, N.; Boughton, O.R.; Ghouse, S.; Weston, A.E.; Collinson, L.; Blunn, G.W.; Jeffers, J.R.; Cobb, J.P.; Stevens, M.M. Individual response variations in scaffold-guided bone regeneration are determined by independent strain- and injury-induced mechanisms. Biomaterials 2019, 194, 183–194. [Google Scholar] [CrossRef]
- Jayesh, R.; Dhinakarsamy, V. Osteointegration. J. Pharm. Bioallied Sci. 2015, 7, 226. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chang, J.; Bai, F.; Sun, X.; Lu, J.; Lin, L.; Lei, C.; Dai, R. Role of the Porous Structure of the Bioceramic Scaffolds in Bone Tissue Engineering. Nat. Preced. 2010. [Google Scholar] [CrossRef]
- Wang, X.; Nie, Z.; Chang, J.; Lu, M.L.; Kang, Y. Multiple channels with interconnected pores in a bioceramic scaffold promote bone tissue formation. Sci. Rep. 2021, 11, 20447. [Google Scholar] [CrossRef]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly (ε-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2013, 98, 209–218. [Google Scholar] [CrossRef]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B.; Bartolo, P. Accelerated Degradation of Poly-ε;-caprolactone Composite Scaffolds for Large Bone Defects. Polymers 2023, 15, 670. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Westhauser, F.; Essers, C.; Karadjian, M.; Reible, B.; Schmidmaier, G.; Hagmann, S.; Moghaddam, A. Supplementation with 45S5 Bioactive Glass Reduces In Vivo Resorption of the β-Tricalcium-Phosphate-Based Bone Substitute Material Vitoss. Int. J. Mol. Sci. 2019, 20, 4253. [Google Scholar] [CrossRef]
- Moritz, N.; Vedel, E.; Ylänen, H.; Jokinen, M.; Hupa, M.; Yli-Urpo, A. Characterisation of bioactive glass coatings on titanium substrates produced using a CO2 laser. J. Mater. Sci. Mater. Med. 2004, 15, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Cheong, V.S.; Fromme, P.; Mumith, A.; Coathup, M.J.; Blunn, G.W. Novel adaptive finite element algorithms to predict bone ingrowth in additive manufactured porous implants. J. Mech. Behav. Biomed. Mater. 2018, 87, 230–239. [Google Scholar] [CrossRef]
- Luchman, N.A.; Wahab, R.M.A.; Ariffin, S.H.Z.; Nasruddin, N.S.; Lau, S.F.; Yazid, F. Comparison between hydroxyapatite and polycaprolactone in inducing osteogenic differentiation and augmenting maxillary bone regeneration in rats. PeerJ 2022, 10, e13356. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mori, A.; Karali, A.; Daskalakis, E.; Hing, R.; Da Silva Bartolo, P.J.; Cooper, G.; Blunn, G. Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration. Polymers 2024, 16, 66. https://doi.org/10.3390/polym16010066
De Mori A, Karali A, Daskalakis E, Hing R, Da Silva Bartolo PJ, Cooper G, Blunn G. Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration. Polymers. 2024; 16(1):66. https://doi.org/10.3390/polym16010066
Chicago/Turabian StyleDe Mori, Arianna, Aikaterina Karali, Evangelos Daskalakis, Richard Hing, Paulo Jorge Da Silva Bartolo, Glen Cooper, and Gordon Blunn. 2024. "Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration" Polymers 16, no. 1: 66. https://doi.org/10.3390/polym16010066
APA StyleDe Mori, A., Karali, A., Daskalakis, E., Hing, R., Da Silva Bartolo, P. J., Cooper, G., & Blunn, G. (2024). Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration. Polymers, 16(1), 66. https://doi.org/10.3390/polym16010066