Surface Degradation of DGEBA Epoxy Resins Cured with Structurally Different Amine Hardeners: Effects of UV Radiation
Abstract
:1. Introduction
2. Experimental Section
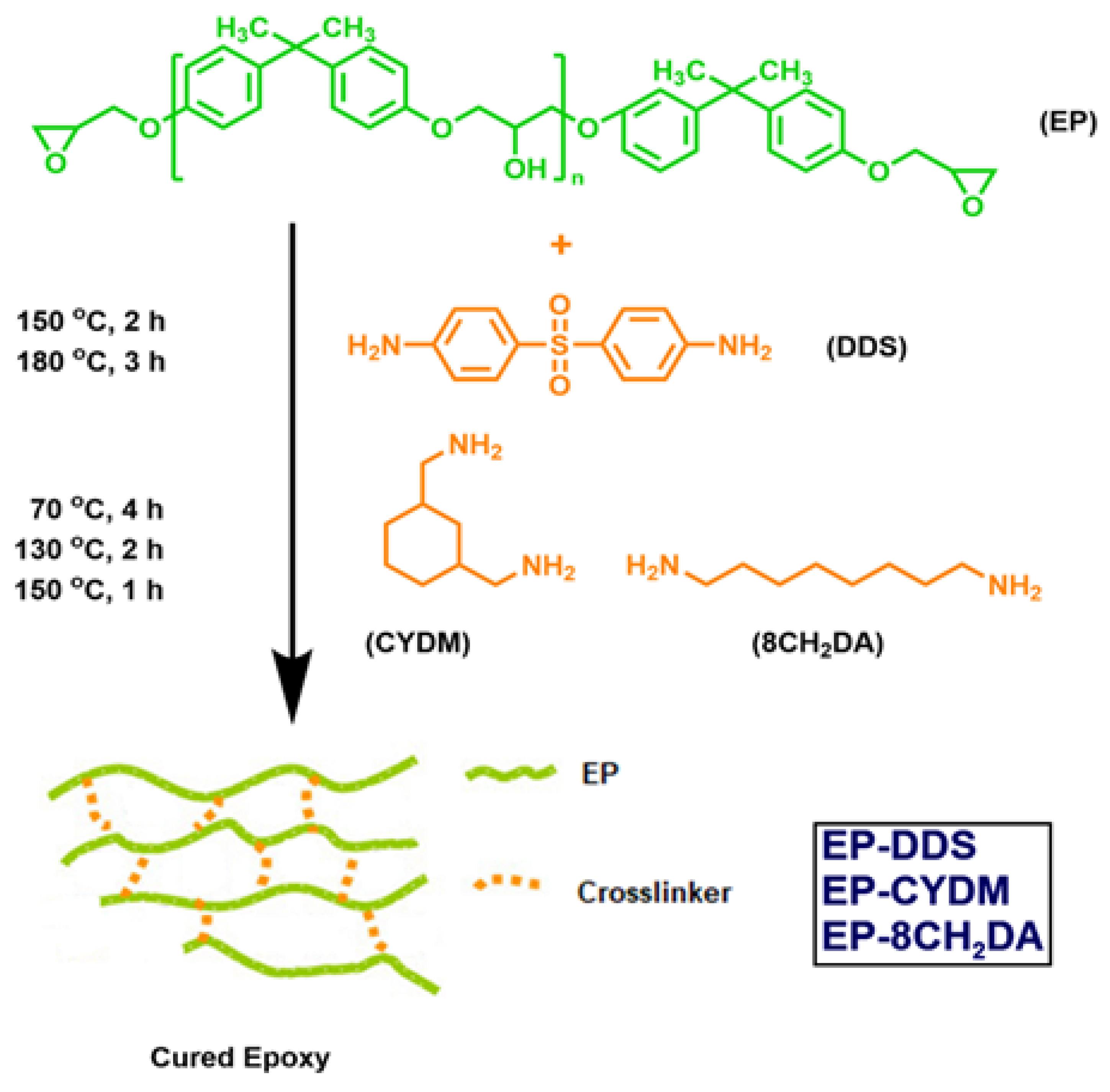
2.1. Materials
2.2. Obtaining the Cured Epoxy Resins
2.3. Measurements
2.3.1. Irradiation
2.3.2. Surface Color Change Studies
2.3.3. Contact Angle Measurements
2.3.4. Structural Modifications by FT-IR during Photoirradiation
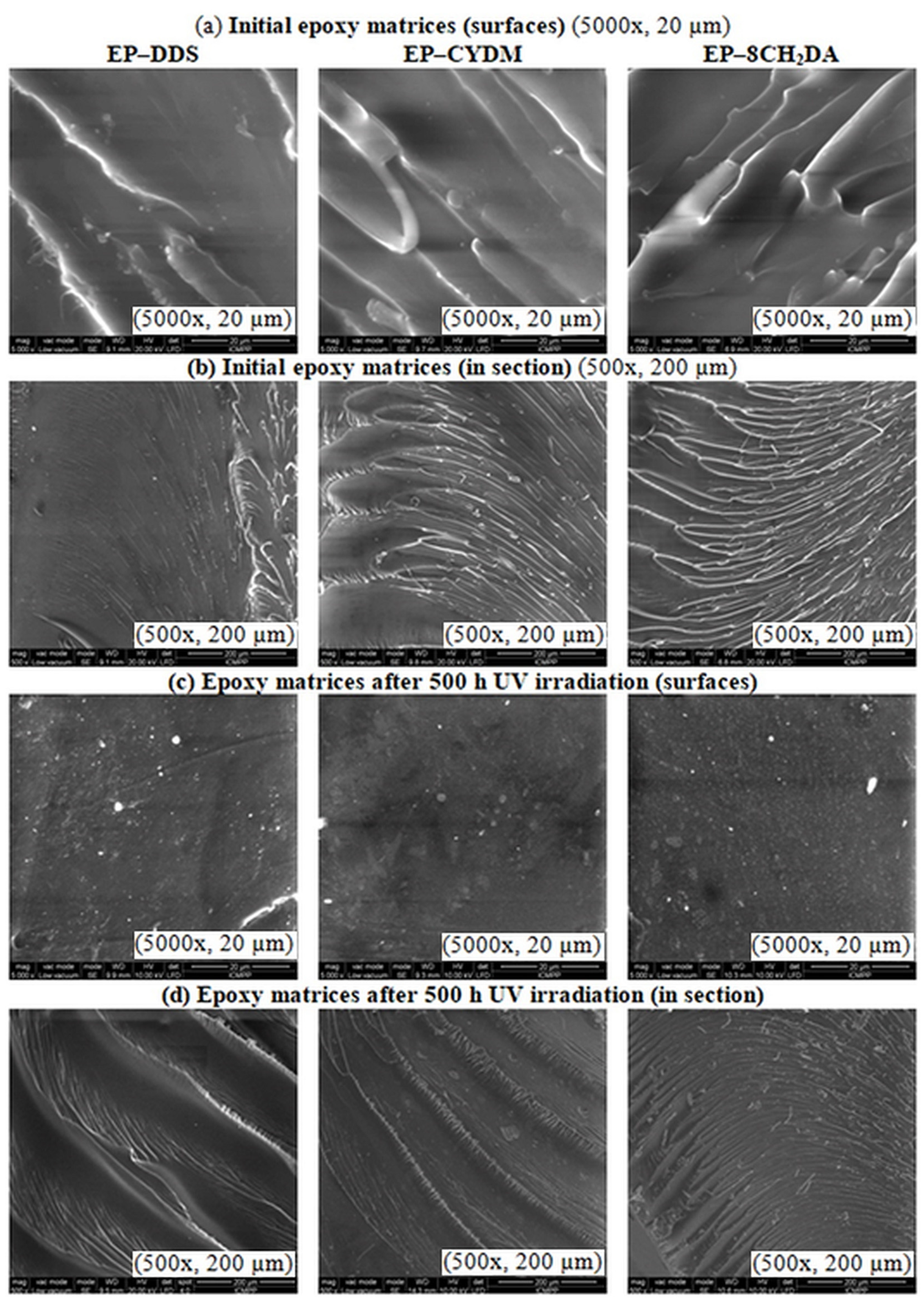
2.3.5. Morphological Study
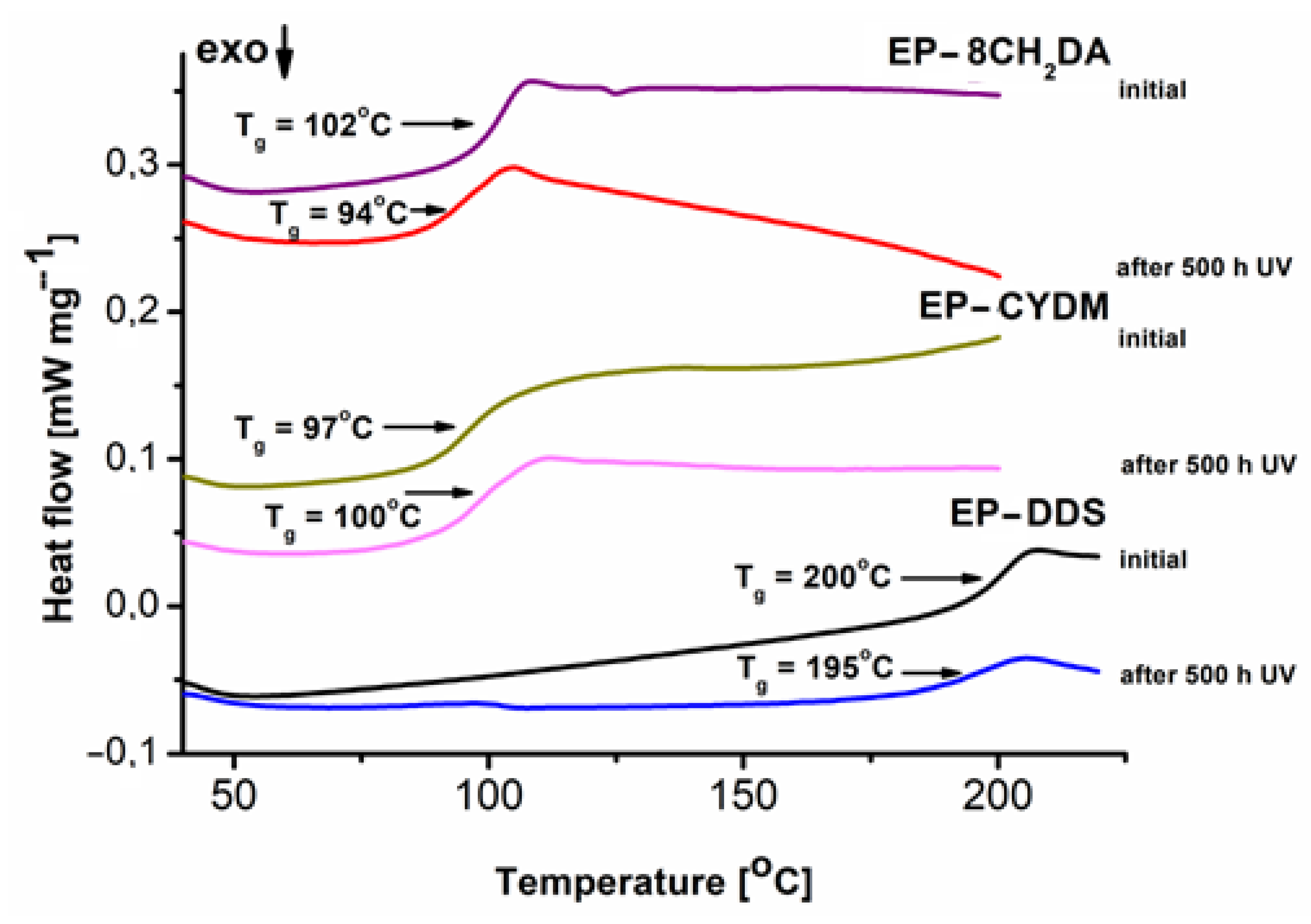
2.3.6. Differential Scanning Calorimetry
2.3.7. Water Absorption Behavior
3. Results and Discussion
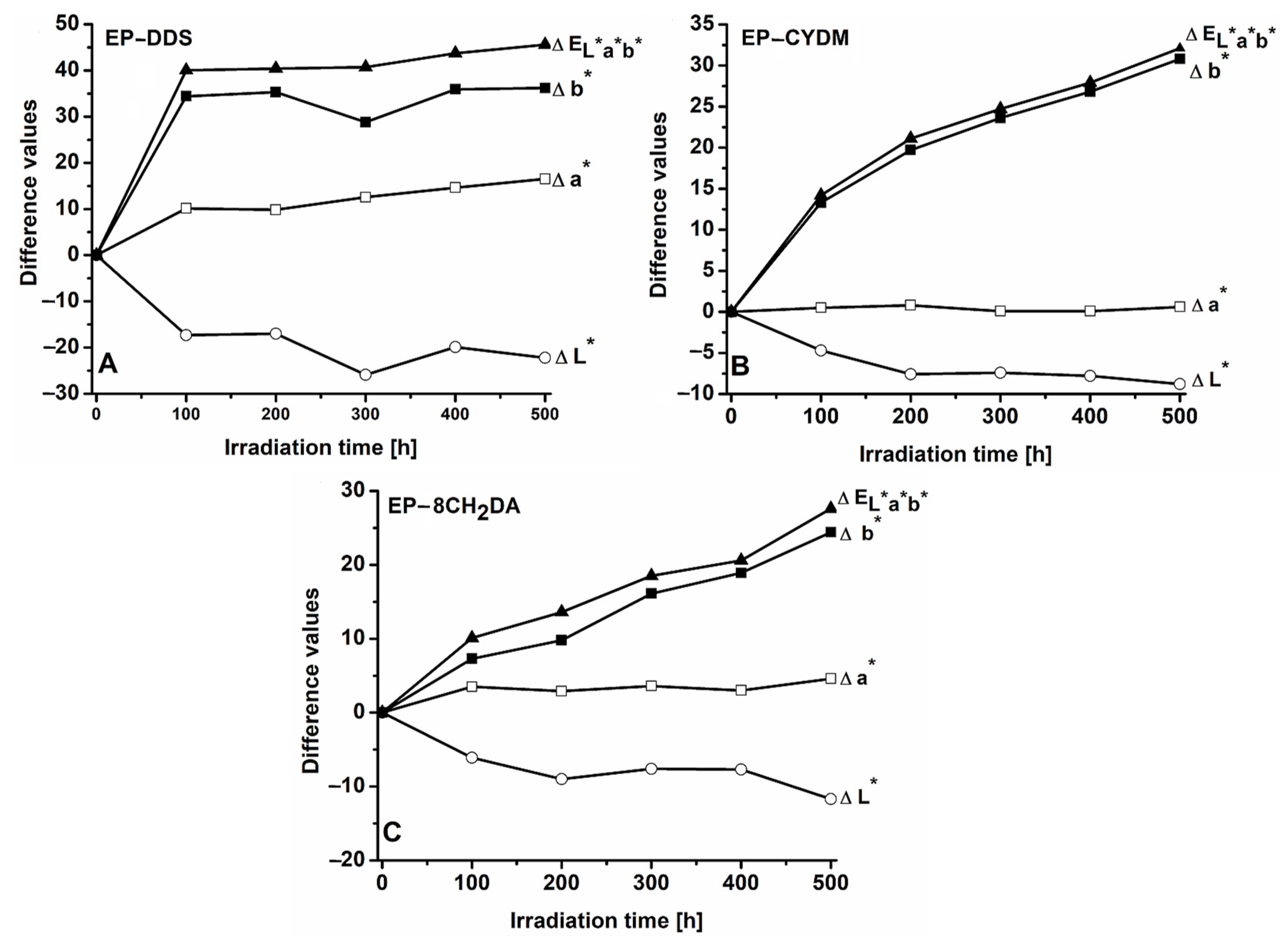
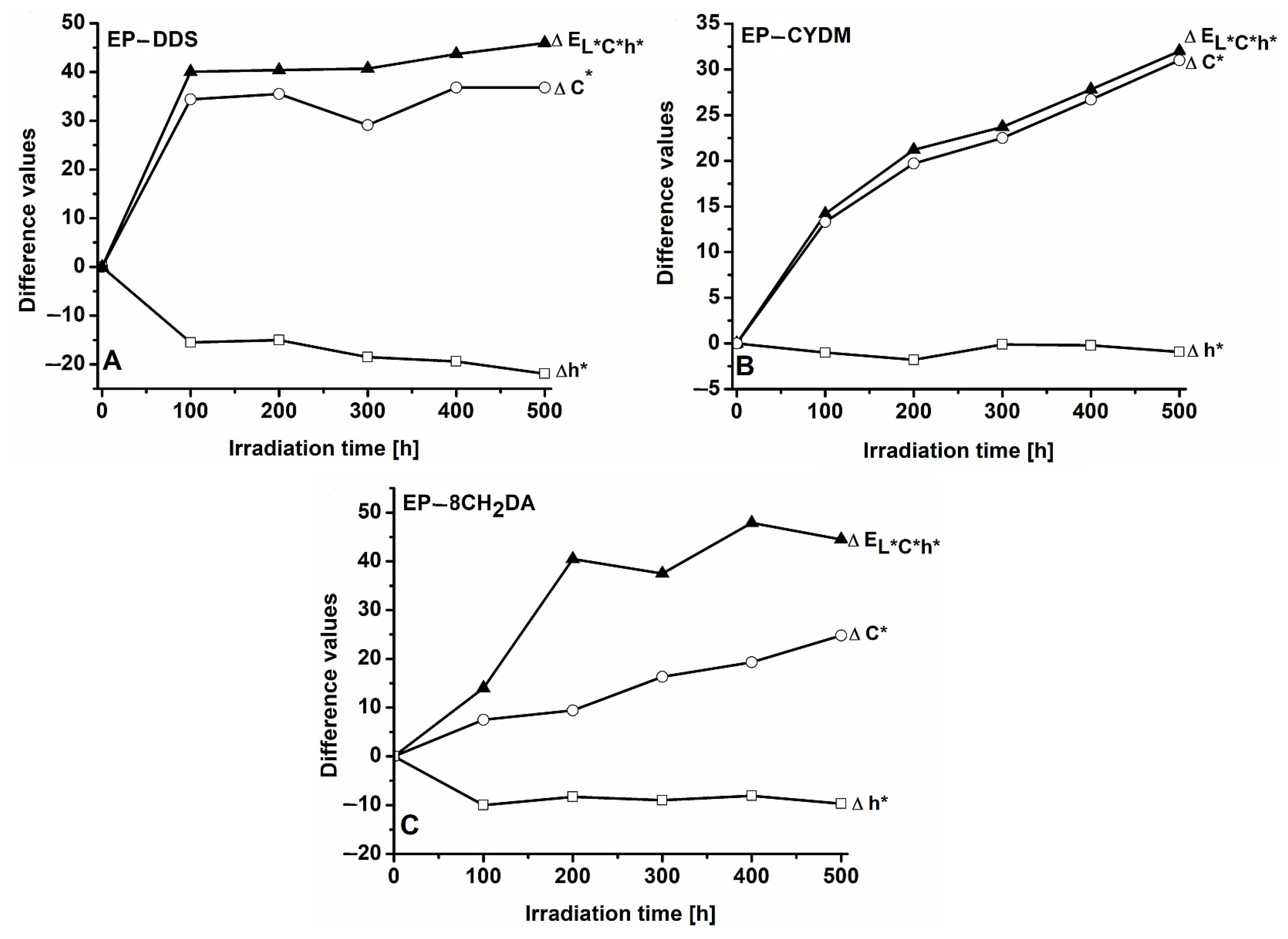
3.1. Color Modifications during UV Irradiation
3.1.1. Color Modifications of the EP–DDS Cured Resin
3.1.2. Color Modifications of the EP–CYDM Cured Resin
3.1.3. Color Modifications of the EP–8CH2DA Cured Resin
3.1.4. Concluding General Remarks on Color Modifications of the Cured Epoxy Resins
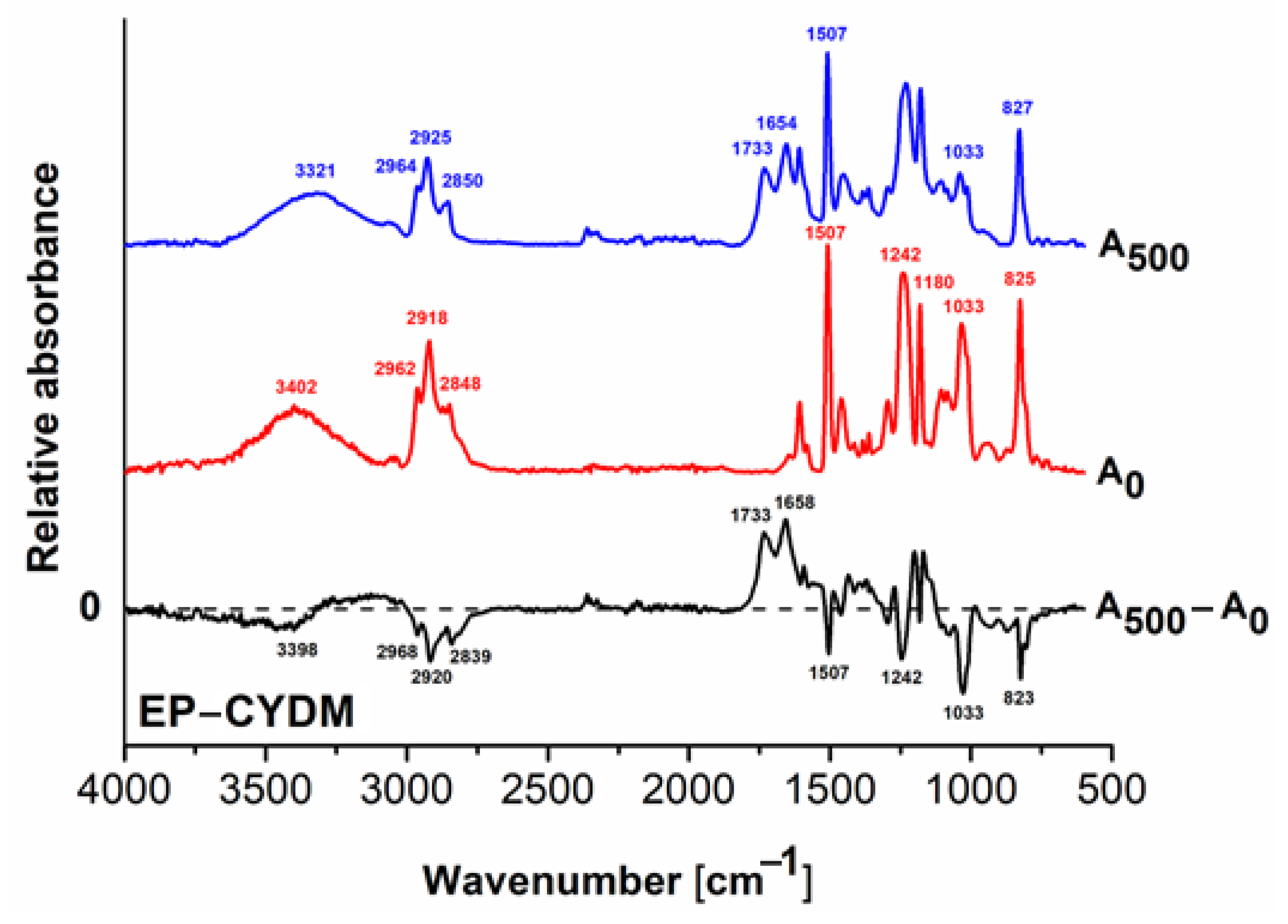
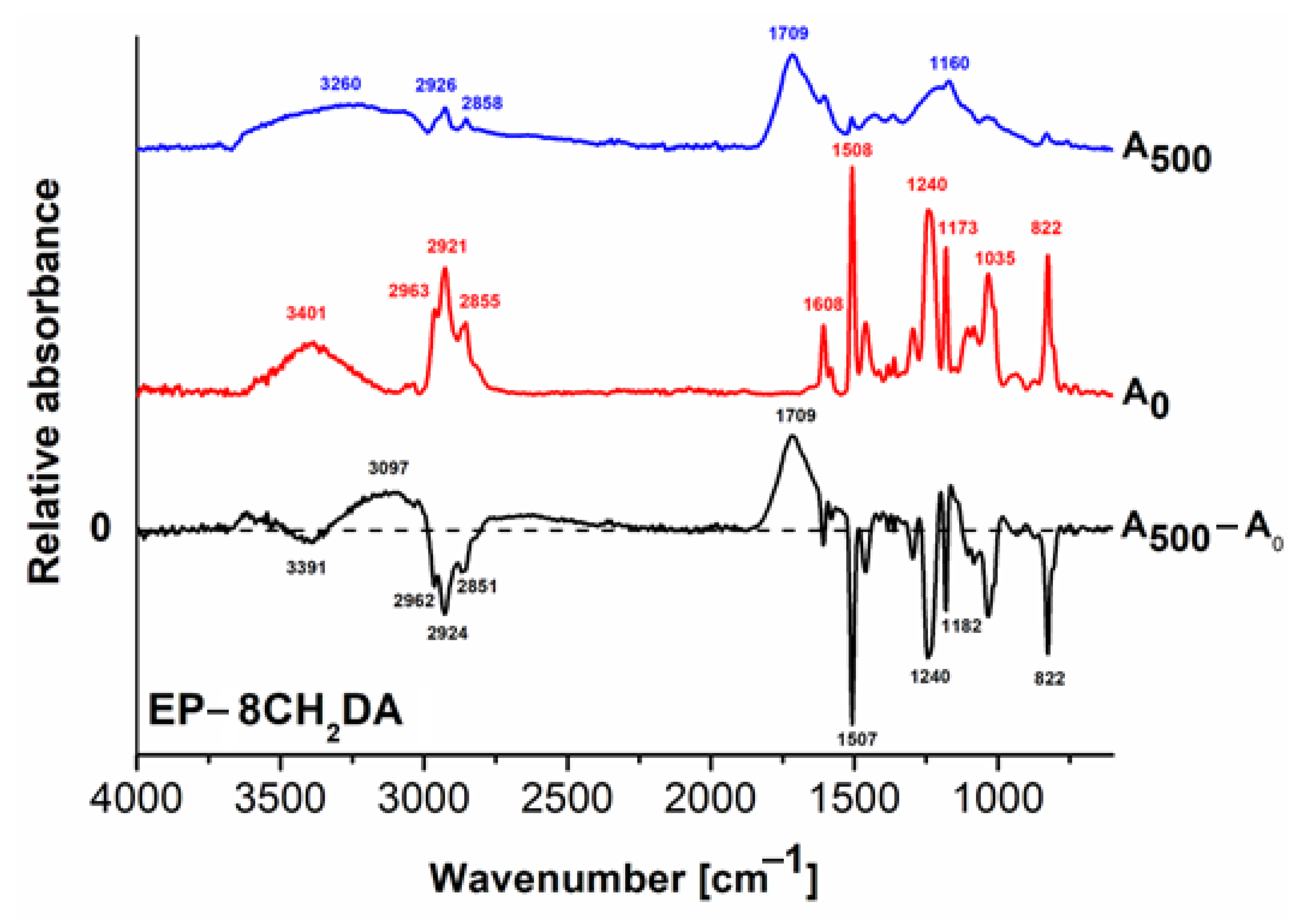
3.2. Structural Modifications
FT–IR Analysis
3.3. Morphological and Thermal Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Vijayan, P.; Thomas, S. Recycling of Thermosetting Polymers. In Recent Developments in Polymer Recycling; Transworld Research Network: Trivandrum, India, 2011; pp. 122–129. [Google Scholar]
- Mustata, F.; Rosu, D.; Varganici, C.D.; Rosu, L.; Rosca, I.; Tudorachi, N. Assessing the Thermal and Fungal Behavior of Eco-Friendly Epoxy Thermosets Derived From Vegetable Oils for Wood Protective Coatings. Prog. Org. Coat. 2022, 163, 106612. [Google Scholar] [CrossRef]
- Irfan, M.H. Chemistry and Technology of Thermosetting Polymers in Construction Applications; Springer Science + Business Media, B.V.: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Rosu, L.; Mustata, F.; Rosu, D.; Varganici, C.D.; Rosca, I.; Rusu, T. Bio-Based Coatings From Epoxy Resins Crosslinked with a Rosin Acid Derivative for Wood Thermal and Anti–fungal Protection. Prog. Org. Coat. 2021, 151, 106008. [Google Scholar] [CrossRef]
- Hayaty, M.; Honarkar, H.; Beheshty, M.H. Curing Behavior of Dicyandiamide/Epoxy Resin System Using Different Accelerators. Iran. Polym. J. 2013, 22, 591–598. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Jašo, V.M.; Radičević, R.Ž.; Ilić, O.Z.; Simendić, J.K.B. Investigation of the Curing Kinetics of Alkyd–Melamine–Epoxy Resin System. Prog. Org. Coat. 2012, 73, 415–424. [Google Scholar] [CrossRef]
- Skeist, I. Handbook of Adhesives; Springer Inc.: New York, NY, USA, 2011. [Google Scholar]
- Schwaiger, M.; Resch–Fauster, K. Mechanical Flexible Epoxy Resins with 100% Bio-Based Carbon Content Based on Epoxidized Vegetable Oils. J. Appl. Polym. Sci. 2022, 139, e53233. [Google Scholar] [CrossRef]
- Miracle, D.B.; Donaldson, S.L. ASM Handbook Volume 21: Composites; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Ashcroft, W.; Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Li, K.; Huo, N.; Liu, X.; Cheng, J.; Zhang, J. Effects of the furan ring in epoxy resin on the thermomechanical properties of highly cross-linked epoxy networks: A molecular simulation study. RSC Adv. 2016, 6, 769–777. [Google Scholar] [CrossRef]
- Ehlers, J.-E.; Rondan, N.G.; Huynh, L.K.; Pham, H.; Marks, M.; Truong, T.N. Theoretical Study on Mechanisms of the Epoxy−Amine Curing Reaction. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Klose, L.; Meyer-Heydecke, N.; Wongwattanarat, S.; Chow, J.; García, P.P.; Carré, C.; Streit, W.; Antranikian, G.; Romero, A.M.; Liese, A. Review Towards Sustainable Recycling of Epoxy-Based Polymers: Approaches and Challenges of Epoxy Biodegrada-tion. Polymers 2023, 15, 2653. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An Overview of Multifunctional Epoxy Nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Toldy, A.; Szabó, A.; Novák, C.; Madarász, J.; Tóth, A.; Marosi, G. Intrinsically Flame Retardant Epoxy Resin-Fire Performance and Background–Part II. Polym. Degrad. Stab. 2008, 93, 2007–2013. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, L.H.; Ma, L.C.; Ma, L.N.; Yang, X.B.; Huang, Y.D.; Ryu, J.E.; Shankar, A.; Li, T.X.; Yan, C.; et al. Layer–by–Layer Grafting CNTs Onto Carbon Fibers Surface for Enhancing the Interfacial Properties of Epoxy Resin Composites. Compos. Sci. Technol. 2018, 154, 28–36. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, L.; Lehner, S.; Hamciuc, C.; Jovic, M.; Rosu, D.; Mustata, F.; Gaan, S. Semi–Interpenetrating Networks Based on Epoxy Resin and Oligophosphonate: Comparative Effect of Three Hardeners on the Thermal and Fire Properties. Mater. Des. 2021, 212, 110237. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, L.; Rosu, D.; Hamciuc, C.; Rosca, I.; Vasiliu, A.L. Effect of Hardener Type on the Photochemical and Antifungal Performance of Epoxy and Oligophosphonate S–IPNs. Polymers 2022, 14, 3784. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Q.; Liu, X.; Feng, P. A Comprehensive Review on Mechanical Properties of Pultruded FRP Composites Subjected to Long-Term Environmental Effects. Compos. B Eng. 2020, 191, 107958. [Google Scholar] [CrossRef]
- Wu, C.; Chen Meng, B.; Tam, L.; He, L. Yellowing Mechanisms of Epoxy and Vinyl Ester Resins Under Thermal, UV and Natural Aging Conditions and Protection Methods. Polym. Test. 2022, 114, 107708. [Google Scholar] [CrossRef]
- Wan, X.; Demir, B.; An, M.; Walsh, T.R.; Yang, N. Thermal conductivities and mechanical properties of epoxy resin as a function of the degree of cross-linking. Int. J. Heat Mass Transf. 2021, 180, 121821. [Google Scholar] [CrossRef]
- Weisbrodt, M.; Kowalczyk, A.; Kowalczyk, K. Structural Adhesives Tapes Based on a Solid Epoxy Resin and Multifunctional Acrylic Telomers. Polymers 2021, 13, 3561. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Liu, C.-C.; Shiau, Y.-C.; Lin, W.-C. Discussion on the Reinforcement of Reinforced Concrete Slab Structures. Sustainability 2019, 11, 1756. [Google Scholar] [CrossRef]
- Chand, N.; Fahim, M. Tribology of Natural Fiber Polymer Composites, 2nd ed.; Woodhead Publishing: Cambridge, MA, USA, 2021. [Google Scholar]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-Based Polymer Nanocomposite for High-Performance Energy Storage Applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Khan, M.M.; Tan, Y.H.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Rahman, M.E. Comprehensive Review on Carbon Nanotubes Embedded in Different Metal and Polymer Matrix: Fabrications and Applications. Crit. Rev. Solid State Mater. Sci. 2022, 47, 837–864. [Google Scholar] [CrossRef]
- Pan, D.; Luo, S.; Feng, Y.; Zhang, X.; Su, F.; Liu, H.; Liu, C.; Mai, X.; Naik, N.; Guo, Z. Highly Thermally Conductive 3D BN/MWCNTs/C Spatial Network Composites with Improved Electrically Insulating and Flame Retardancy Prepared by Biological Template Assisted Method. Compos. Part B Eng. 2021, 222, 109039. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer Nanocomposites Based on Silylated-Montmorillonite: A Review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Islam, M.H.; Afroj, S.; Uddin, M.A.; Andreeva, D.V.; Novoselov, K.S.; Karim, N. Graphene and CNT-Based Smart Fiber-Reinforced Composites: A Review. Adv. Funct. Mater. 2022, 32, 2205723. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and Adhesive Properties of Filled PIB-Based Pressure-Sensitive Adhesives. I. Rheology and Shear Resistance. J. Adhes. Sci. Technol. 2015, 29, 1831–1848. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Brantseva, T.V.; Gorbunova, I.Y.; Antonov, S.V.; Korolev, Y.M.; Kerber, M.L. Epoxy Reinforcement with Silicate Particles: Rheological and Adhesive Properties—Part I: Characterization of Composites with Natural and Organically Modified Montmorillonites. Int. J. Adhes. Adhes. 2015, 61, 127–136. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy Crude Oil Asphatenes as a Nanofiller for Epoxy Resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Sinha, R.K.; Ka, S.; Purohit, R.; Malviya, R.K. Effect of Nano SiO2 on Properties of Natural Fiber Reinforced Epoxy Hybrid Composite: A Review. Mater. Today Proc. 2020, 26, 3183–3186. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, A.; Rana, S.; Guadagno, L. An Overview on Carbon Fiber-Reinforced Epoxy Composites: Effect of Graphene Oxide Incorporation on Composites Performance. Polymers 2022, 14, 1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Y.; Yu, Y.; Zhang, M.; Li, L.; Ma, L. Effect of Nano-SiO2 Modification on Mechanical and Insulation Properties of Basalt Fiber Reinforced Composites. Polymers 2022, 14, 3353. [Google Scholar] [CrossRef]
- Sapiai, N.; Jumahat, A.; Jawaid, M.; Midani, M.; Khan, A. Tensile and Flexural Properties of Silica Nanoparticles Modified Unidirectional Kenaf and Hybrid Glass/Kenaf Epoxy Composites. Polymers 2020, 12, 2733. [Google Scholar] [CrossRef]
- Reinprecht, L.; Hulla, M. Colour Changes in Beech Wood Modified With Essential Oils Due to Fungal and Ageing-Fungal Attacks with Coniophora Puteana. Drewno 2015, 58, 37–48. [Google Scholar] [CrossRef]
- Musto, P.; Ragosta, G.; Mascia, L. Vibrational Spectroscopy Evidence for the Dual Nature of Water Sorbed into Epoxy Resins. Chem. Mater. 2000, 12, 1331–1341. [Google Scholar] [CrossRef]
- Cascaval, C.N.; Ciobanu, C.; Rosu, D.; Rosu, L. Polyurethane Epoxy Maleate of Bisphenol A Semi-Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 2002, 83, 138–144. [Google Scholar] [CrossRef]
- Poletto, M. Comparative Study of Wood Flour Photodegradation of Two Wood Species Submitted to Artificial Weathering. Maderas Cienc. Tecnol. 2017, 19, 141–148. [Google Scholar] [CrossRef]
- Rosu, D.; Mustata, F.R.; Rosu, L.; Varganici, C.-D. Photochemical Aging of Eco-Friendly Wood Coatings Derived from Vegetable Oils. ACS Appl. Polym. Mater. 2021, 3, 6303–6314. [Google Scholar] [CrossRef]
- Gu, X.; Stanley, D.; Byrd, W.E.; Dickens, B.; Vaca-Trigo, I.; Meeker, W.Q.; Nguyen, T.; Chin, J.W.; Martin, J.W. Linking Accelerated Laboratory Test with Outdoor Performance Results for a Model Epoxy Coating System. In Service Life Prediction of Polymeric Materials–Global Perspectives; Springer Science and Business Media: Boston, MA, USA, 2009; pp. 3–28. [Google Scholar]
- Rosu, D.; Rosu, L.; Mustata, F.; Varganici, C.-D. Effect of UV Radiation on Some Semi-interpenetrating Polymer Networks Based on Polyurethane and Epoxy Resin. Polym. Degrad. Stab. 2012, 97, 1261–1269. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2004; p. 366. [Google Scholar]
- Balaban, A.T.; Banciu, M.; Pogany, I. Applications of Physical Methods in Organic Chemistry; Scientific and Encyclopedic Publishing: Bucharest, Romania, 1983. [Google Scholar]
- Bruni, M.C.; Ponterini, G.; Scoponi, M. Photophysics and Photochemistry of Diphenyl Sulfone. 2. Investigation of the S1 Decay Pathways. J. Phys. Chem. 1989, 93, 678–683. [Google Scholar] [CrossRef]
- Ling, Y.; Yao, S.; Chen, Y.; Hu, D.D.; Xi, Z.; Zhao, L. Synergetic Effect Between Curing Reaction and CO2 Diffusion for Microcellular Epoxy Foam Preparation in Supercritical CO2. J. Supercrit. Fluids 2022, 180, 105424. [Google Scholar] [CrossRef]
- Larché, J.F.; Bussière, P.O.; Thérias, S.; Gardette, J.L. Photooxidation of Polymers: Relating Material Properties to Chemical Changes. Polym. Degrad. Stabil. 2012, 97, 25–34. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. Effect of Ultraviolet Radiation on Vinyl Ester Network Based on Bisphenol A. J. Photochem. Photobiol. A Chem. 2008, 194, 275–282. [Google Scholar] [CrossRef]
- Field, L.D.; Li, H.L.; Magill, A.M. Organic Structures from Spectra, 6th ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Nandiyanto, A.B.D.; Okitani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.S.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Delor-Jestin, F.; Drouin, D.; Cheval, P.Y.; Lacoste, J. Thermal and Photochemical Ageing of Epoxy Resin—Influence of Curing Agents. Polym. Degrad. Stab. 2006, 91, 1247–1255. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varganici, C.-D.; Rosu, L.; Rosu, D.; Rosca, I.; Ignat, M.-E.; Ignat, L. Surface Degradation of DGEBA Epoxy Resins Cured with Structurally Different Amine Hardeners: Effects of UV Radiation. Polymers 2024, 16, 67. https://doi.org/10.3390/polym16010067
Varganici C-D, Rosu L, Rosu D, Rosca I, Ignat M-E, Ignat L. Surface Degradation of DGEBA Epoxy Resins Cured with Structurally Different Amine Hardeners: Effects of UV Radiation. Polymers. 2024; 16(1):67. https://doi.org/10.3390/polym16010067
Chicago/Turabian StyleVarganici, Cristian-Dragos, Liliana Rosu, Dan Rosu, Irina Rosca, Maurusa-Elena Ignat, and Leonard Ignat. 2024. "Surface Degradation of DGEBA Epoxy Resins Cured with Structurally Different Amine Hardeners: Effects of UV Radiation" Polymers 16, no. 1: 67. https://doi.org/10.3390/polym16010067
APA StyleVarganici, C.-D., Rosu, L., Rosu, D., Rosca, I., Ignat, M.-E., & Ignat, L. (2024). Surface Degradation of DGEBA Epoxy Resins Cured with Structurally Different Amine Hardeners: Effects of UV Radiation. Polymers, 16(1), 67. https://doi.org/10.3390/polym16010067








