Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review)
Abstract
1. Introduction
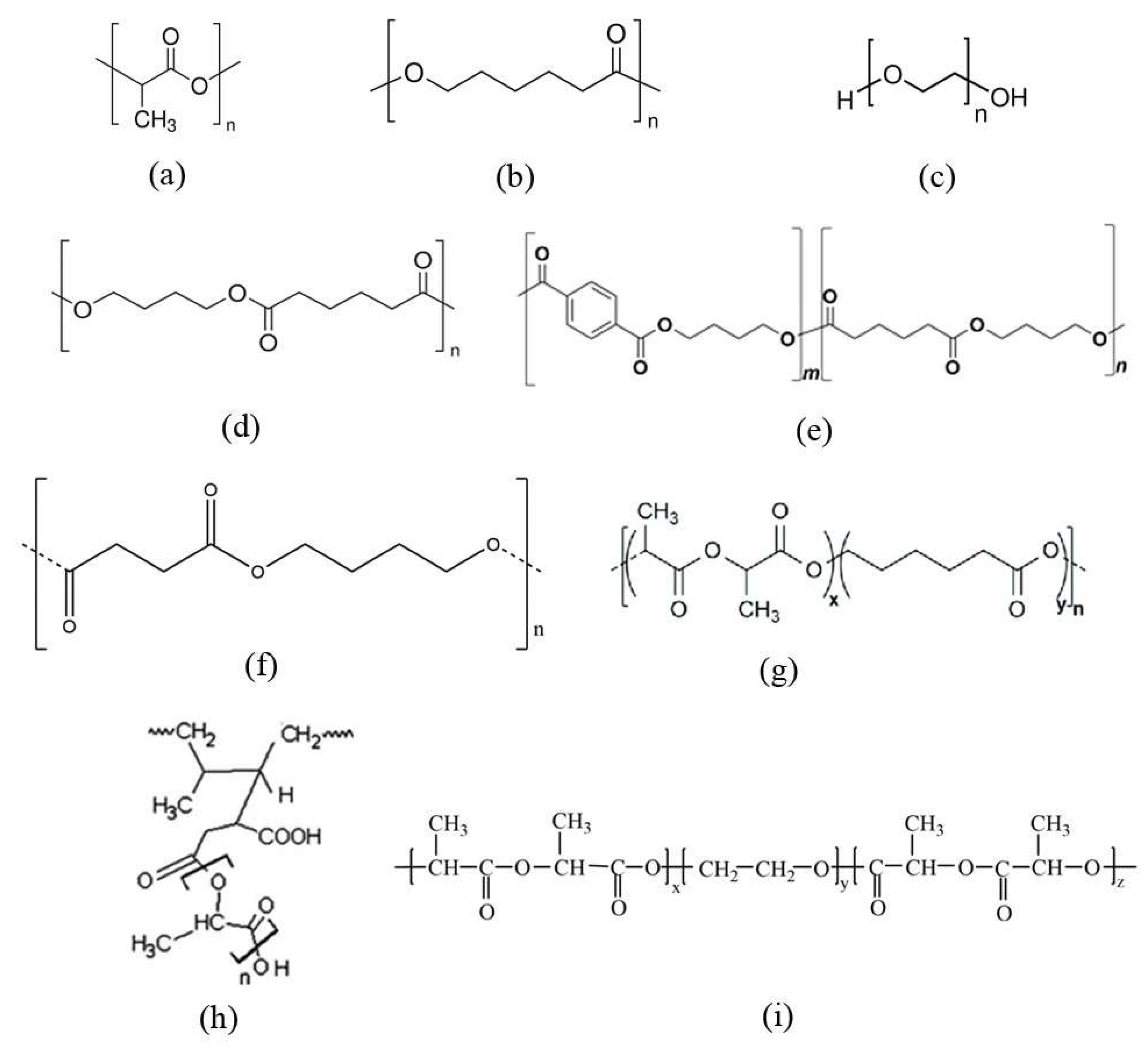
2. Copolymerization with Flexible Polymers
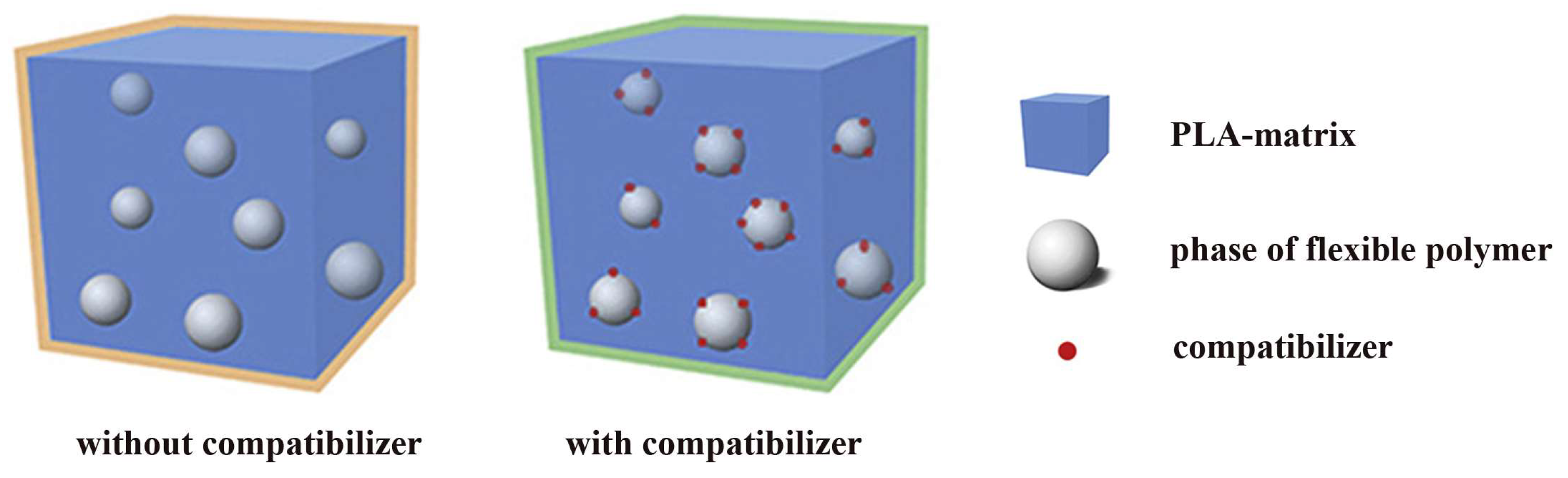
3. Compounding with Flexible Polymers (Blends)
4. Oligomers and Low-Molecular Weightadditives (Plasticizers)
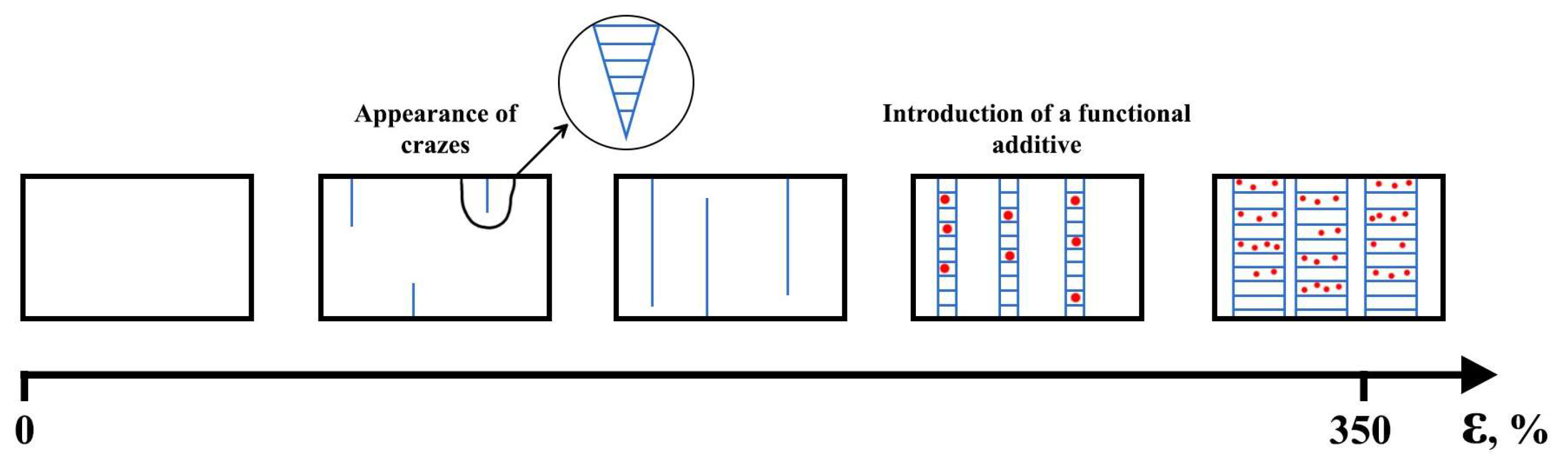
5. Structural Rearrangement of PLA Chains
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly(lactic acid) production for tissue engineering. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-based blends: A comprehensive review. Polymers 2023, 13, 5148. [Google Scholar] [CrossRef]
- Sin, L.T.; Tueen, B.S. Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Cappiello, G.; Gisario, A. Compatibilization strategies and analysis of morphological features of poly(butylene adipate-co-terephthalate) (PBAT)/poly(lactic acid) PLA blends: A state-of-art review. Eur. Polym. J. 2022, 173, 111304. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the degradation of poly(lactid acid) during melt processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef]
- Jenkins, M.; Stamboulis, A. Durability and Reliability of Medical Polymers; Elsevier: Amsterdam, The Netherlands, 2012; Available online: https://research.birmingham.ac.uk/en/publications/durability-and-reliability-of-medical-polymers (accessed on 13 December 2023).
- Olkhov, A.A.; Mastalygina, E.E.; Ovchinnikov, V.A.; Kurnosov, A.A.; Popov, A.A.; Iordanskii, A.L. Biological and oxidative degradation of ultrathin-fibrous nonwovens based on poly(lactic acid)/poly(3-hydroxybutyrate) blends. Int. J. Mol. Sci. 2023, 24, 7979. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- Zang, J.-B.; Li, K.-A.; Du, A.-K. Compatibilization strategies in poly(lactic acid)-based blends. RSC Adv. 2015, 5, 32546–32565. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Olkhov, A.A.; Voronstov, N.V.; Kiselev, N.V.; Khaidarov, T.B.; Khaydarov, B.B.; Kolesnkikov, E.A.; Burmistrov, I.N. Influence of copper-based fillers on structural and mechanical properties of polylactic acid composites. J. Compos. Sci. 2022, 6, 386. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(lactic acid) (PLA)/poly(butylene succinate-co-adipate) (PBSA) compatibilized binary biobased blends: Melt fluidity, morphological, thermos-mechanical and micromechanical analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.A.; Terzopoulou, Z.; Zamboulis, A.; Valera, M.A.; Mangas, A.; Kyritsis, A.; Pissis, P.; Bikiaris, D.N. Direct and indirect effects on molecular mobility in renewable polylactide-poly(propylene adipate) block copolymers as studied via dielectric spectroscopy and calorimetry. Soft Matter 2022, 18, 3725–3737. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.A.; Evangelopoulou, A.; Terzopoulou, Z.; Zamboulis, A.; Valera, M.A.; Mangas, A.; Kyritsis, A.; Bikiaris, D.N. Revisiting non-conventional crystallinity-induced effects on molecular mobility in sustainable deblock copolymers of poly(propylene adipate) and polylactide. Molecules 2022, 27, 7449. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.R. Pol(Lactic Acid) Block Copolymers—Synthesis, Characterization, and Structure-Property Relationships. Doctoral Thesis, Technical University of Berlin, Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Thepthawat, A.; Srikulkit, K. Improving the properties of polylactic acid by blending with low molecular weight polylactic acid-g-natural rubber. Polym. Eng. Sci. 2014, 54, 2770–2776. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Bikiaris, N.D.; Margellou, A.; Valera, M.A.; Mangas, A.; Koltsakidis, S.; Tsongas, K.; Tzetzis, D.; Triantafyllidis, K. Properties of PLA-co-PBSu copolymers rapidly synthesized by reactive processing. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Coudane, J.; Van Den Berghe, H.; Mouton, J.; Garric, X.; Nottelet, B. Poly(lactic acid)-based graft copolymers: Syntheses strategies and improvement of properties for biomedical and environmentally friendly applications: A review. Molecules 2022, 27, 4135. [Google Scholar] [CrossRef]
- Augé, M.-O.; Roncucci, D.; Bourbigot, S.; Bonnet, F.; Gaan, S.; Fontaine, G. Recent advances on reactive extrusion of poly(lactic acid). Eur. Polym. J. 2023, 184, 111727. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. Single-step reactive extrusion of PLLA in a corotating twin-screw extruder promoted by 2-ethylhexanoic acid tin (II) salt and triphenylphosphine. Polymer 2000, 41, 3395–3403. [Google Scholar] [CrossRef]
- Liu, M.-J.; Chen, S.-C.; Yang, K.-K.; Wang, Y.-Z. Biodegradable polylactide based materials with improved crystallinity, mechanical properties and rheological behaviour by introducing a long-chain branched copolymer. RSC Adv. 2015, 5, 42162–42173. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Wang, H.; Jin, W.; Li, J. Synthesis of multiblock thermoplastic elastomers based on biodegradable poly (lactic acid) and polycaprolactone. Mater. Sci. Eng. C 2009, 29, 889–893. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.-S.; Lee, Y.M.; Ihn, K.J.; Kim, S.H.; Kim, Y.H. Morphology of elastic Poly(l-lactide-co-ε-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules 2004, 5, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Nishimura, Y.; Tanaka, T.; Oonishi, M.; Kanematsu, W. Solid state NMR analysis of poly(L-lactide) random copolymer with poly(ε-caprolactone) and its reactive extrusion process. J. Appl. Polym. Sci. 2012, 123, 1865–1873. [Google Scholar] [CrossRef]
- Naddeo, M.; Sorrentino, A.; Pappalardo, D. Thermo-rheological and shape memory properties of block and random copolymers of lactide and ε-caprolactone. Polymers 2021, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Chen, S.; Qin, J.; Chen, X.; Zhou, D.; Wu, H. Synthesis, characterization, and crystallization behaviors of poly(D-lactic acid)-based triblock copolymer. Sci. Rep. 2020, 10, 3627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Su, J.-J.; Han, J.; Zhang, B.; Ou, L. Optimizing the balance between stiffness and flexibility by tuning the compatibility of a poly(lactic acid)/ethylene copolymer. RSC Adv. 2017, 7, 23065–23072. [Google Scholar] [CrossRef]
- Mohammad, N.N.B.; Arsad, A.; Rahmat, A.R.; Talib, M.S.; Mat Desa, M.S.Z. Influence of compatibilizer on mechanical properties of polylactic acid/natural rubber blends. Appl. Mech. Mater. 2014, 554, 81–85. [Google Scholar] [CrossRef]
- Fekete, I.; Ronkay, F.; Lendavi, L. Highly toughened blends of poly(lactic acid) (PLA) and natural rubber (NR) for FDM-based 3D printing applications: The effect of composition and infill pattern. Polym. Test. 2021, 99, 107205. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Baimark, Y.; Srisuwan, Y. Thermal and mechanical properties of highly flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastics: Effects of poly(ethylene glycol) block length and chain extender. J. Elastomers Plast. 2020, 52, 142–258. [Google Scholar] [CrossRef]
- Wu, W. Triblock Copolymers of PLLA-PEG-PLLA for Nerve Guidance Channel Scaffolds via 3D Printing. Master’s Thesis, Northeastern University, Boston, MA, USA, 2017. Available online: https://repository.library.northeastern.edu/files/neu:cj82qq07g/fulltext.pdf (accessed on 13 December 2023).[Green Version]
- Choi, K.-M.; Choi, M.-C.; Han, D.-H.; Park, T.-S.; Ha, C.-S. Plasticization of poly(lactic acid) (PLA) through chemical grafting of poly(ethylene glycol) (PEG) via in situ reactive blending. Eur. Polym. J. 2013, 49, 2356–2364. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Bikiaris, D.N.; Valera, M.A.; Mangas, A. Synthesis, properties, and enzymatic hydrolysis of poly(lactic acid)-co-poly(propylene adipate) block copolymers prepared by reactive extrusion. Polymers 2021, 13, 4121. [Google Scholar] [CrossRef] [PubMed]
- Mendelkern, L. Crystallization of Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; Volume 2. [Google Scholar] [CrossRef]
- Yeh, J.-T.; Tsou, C.-H.; Huang, C.-Y.; Chen, K.-N.; Wu, C.-S.; Chai, W.-L. Compatible and crystallization properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J. Appl. Polym. Sci. 2010, 116, 680–687. [Google Scholar] [CrossRef]
- Jiao, J.; Zeng, X.; Huang, X. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Mishra, J.; Tiwari, S.K.; Abolhasani, M.M.; Azimi, S.; Nayak, G.C. Fundamental of polymer blends and its thermodynamics. In Composites Science and Engineering: Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publ.: Cambridge, UK, 2017; pp. 27–55. [Google Scholar] [CrossRef]
- Su, S. Prediction of the miscibility of PBAT/PLA blends. Polymers 2021, 13, 2339. [Google Scholar] [CrossRef] [PubMed]
- Antoine, S.; Geng, Z.; Zofchak, E.S.; Chwatko, M.; Fredrickson, G.H.; Ganesan, V.; Hawker, C.J.; Lynd, N.A.; Segalman, R.A. Non-intuitive trends in Flory-Huggins interaction parameters in polyether-based polymers. Macromolecules 2021, 54, 6670–6677. [Google Scholar] [CrossRef]
- Budtri, N.; Aekrum, S.; Lertsiriyothin, W. The compatibility of polylactides and polybutylene succinate in PLA blends based on thermal, mechanical, and rheological properties. Orient. J. Chem. 2017, 33, 2766–2775. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Role of specific interfacial area in controlling properties of immiscible blends of biodegradable polylactide and poly[(butylene succinate)-co-adipate]. ACS Appl. Mater. Interfaces 2012, 4, 6690–6701. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened poly (lactic acid)-PLA formulations by binary blends with poly(butylene succinate-co-adipate)-PBSA and their shape memory behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, J.B.; Lee, D.Y.; Seo, K.H. Plasticization effect of poly(lactic acid) in the poly(butylene adipate–co–terephthalate) blown film for tear resistance improvement. Polymers 2020, 12, 1904. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xiao, A.; Yu, B.; Bhat, G.; Zhu, F. Effect of PCL and compatibilizer on the tensile and barrier properties of PLA/PCL films. Polymer 2017, 41, 181–188. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W. Characterization and processing of Biodegradable polymer blends of poly(lactic acid) with poly(butylene succinate adipate). Korea-Aust. Rheol. J. 2005, 17, 71–77. [Google Scholar]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly(lactic acid) blends. J. Nanomater. 2010, 2010, 287082. [Google Scholar] [CrossRef]
- Jeong, H.; Rho, J.; Shin, J.Y.; Lee, D.Y.; Hwang, T.; Kim, K.J. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed. Eng. Lett. 2018, 8, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Chen, Y.; Lin, Z.; Zhang, J.; Shi, X. Manipulating phase structure of biodegradable PLA/PBAT system: Effects on dynamic rheological responses and 3D printing. Compos. Sci. Technol. 2020, 200, 108399. [Google Scholar] [CrossRef]
- Rizzuto, M.; Marinetti, L.; Caretti, D.; Mugica, A.; Zubitur, M.; Müller, A.J. Can poly(ε-caprolactone) crystals nucleate glassy polylactide? CrystEngComm 2017, 19, 3178–3191. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Wang, L.F.; Rhim, J.W.; Hong, S.I. Preparation of poly(lactide)/poly(butylene adipate-co-terephthalate) blend films using a solvent casting method and their food packaging application. LWT—Food Sci. Technol. 2016, 68, 454–461. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Maggiore, I.D.; Bertoldo, M.; Signori, F.; Bronco, S.; Ciardelli, F. Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate) blending and acetyl tributyl citrate plasticization. J. Appl. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Bastioli, C.; Floridi, G.; Del Tredici, G. Biodegradable Multiphase Compositions Based on. Starch. Patent No. CA2662105C, 3 April 2008. Available online: https://patents.google.com/patent/CA2662105C/en?oq=CA2662105C (accessed on 13 December 2023).
- Bastioli, C.; Del Tredici, G.; Ponti, R.; Tosin, M. Highly Breathable Biodegradable Film. Bag. Patent No. US8747971B2, 10 June 2014. Available online: https://patents.google.com/patent/US8747971B2/en?oq=US8747971B2 (accessed on 13 December 2023).
- Yu, Y.; Cheng, Y.; Ren, J.; Cao, E.; Fu, X.; Guo, W. Plasticizing effect of poly(ethylene glycol)s with different molecular weights in poly(lactic acid)/starch blends. J. Appl. Polym. Sci. 2015, 132, 41808. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Kozlowski, M.; Masirek, R.; Piorkowska, E.; Gazicki-Lipman, M. Biodegradable blends of poly(L-lactide) and starch. J. Appl. Polym. Sci. 2007, 105, 269–277. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Vladimirov, L.V.; Berlin, A.A. Biodegradable polymer materials based on polylactide. Russ. J. Phys. Chem. B 2019, 13, 812–818. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Aleksanyan, K.V.; Krasheninnikov, V.G.; Perepelitsina, E.O.; Shashkin, D.P.; Berlin, A.A. Composites based on starch and polylactide. Polym. Sci. Ser. B 2019, 61, 334–340. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Loginova, A.A.; Ivanushkina, N.E.; Vladimirov, L.V.; Prut, E.V.; Berlin, A.A. Influence of PEG on mechanical properties and biodegradability of composites based on PLA and starch. Starch Stärke 2018, 70, 1700268. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Grachev, A.V.; Berlin, A.A.; Prut, E.V. Investigation of mechanical and thermophysical properties of biodegradable compositions of polylactide with ethyl cellulose and chitosan containing poly(ethylene glycol). Mendeleev Commun. 2015, 25, 361–363. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Long- and short-term stability of plasticized poly(lactic acid): Effects of plasticizers type on thermal, mechanical and morphological properties. Polym. Bull. 2019, 76, 423–445. [Google Scholar] [CrossRef]
- Takhulee, A.; Takahashi, Y.; Vao-soongnern, V. Molecular simulation and experimental studied of the miscibility of polylactic acid/polyethylene glycol blends. J. Polym. Res. 2017, 24, 8. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Liu, M.; Han, M.; Liu, Y.; Ji, S. Effect of molecular weight of poly(ethylene glycol) on plasticization of poly(L-lactic acid). Polymer 2021, 223, 123720. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ma, X.; Fang, J. Influence of carbon black on the properties of plasticized poly(lactic acid) composites. Polym. Degrad. Stab. 2008, 93, 1044–1052. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable micro and nano additives for controlling the migration of a biobased plasticizer from PLA-based flexible films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Cheng, W.; Song, W. The influence of poly(maleic anhydride-co-vinyl acetate) on polylactide/wood flour/calcium carbonate composites. Polym. Test. 2023, 120, 107945. [Google Scholar] [CrossRef]
- Rafie, M.A.F.; Marsilla, K.K.; Rusli, A.; Abdullah, M.K. Enhanced mechanical properties of plasticized polylactic acid filament for fused deposition modelling: Effect of in situ heat treatment. Prog. Rubber Plast. Recycl. Technol. 2020, 36, 131–142. [Google Scholar] [CrossRef]
- Kanabenja, W.; Passarapark, K.; Subchokpool, T.; Nawaaukkaratharnant, N.; Román, A.J.; Osswald, T.A.; Aumnate, C.; Potiyaraj, P. 3D printing filaments from plasticized polyhydroxybutyrate/polylactic acid blends reinforced with hydroxyapatite. Addit. Manuf. 2022, 59, 103130. [Google Scholar] [CrossRef]
- Gomez-Caturla, J.; Dominguez-Candela, I.; Medina-Casas, M.P.; Ivorra-Martinez, J.; Moreno, V.; Balart, R.; Garcia-Garcia, D. Improvement of poly(lactide) ductile properties by plasticization with biobased tartaric acid ester. Macromol. Mater. Eng. 2023, 308, 2200694. [Google Scholar] [CrossRef]
- Barandiaran, A.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Lascano, D.; Angel Selles, M.; Moreno, V.; Fenollar, O. Esters of cinnamic acid as green plasticizers for polylactide formulations with improved ductility. Macromol. Mater. Eng. 2023, 308, 2300022. [Google Scholar] [CrossRef]
- Mustapa, I.R.; Shanks, R.A.; Kong, I.; Daud, N. Morphological structure and thermomechanical properties of hemp fibre reinforced poly(lactic acid) Nanocomposites plasticized with tributyl citrate. Mater. Today Proc. 2018, 5, 3211–3218. [Google Scholar] [CrossRef]
- Harte, I.; Birkinshaw, C.; Jones, E.; Kennedy, J.; DeBarra, E. The effect of citrate ester plasticizers on the thermal and mechanical properties of poly(DL-lactide). J. Appl. Polym. Sci. 2013, 127, 1997–2003. [Google Scholar] [CrossRef]
- Ljundberg, N.; Wesselén, B. Tributyl citrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Gálvez, J.; Correa Aguirre, J.P.; Hidalgo Salazar, M.A.; Vera Mondragón, B.; Wagner, E.; Caicedo, C. Effect of extrusion screw speed and plasticizer proportions on the rheological, thermal, mechanical, morphological and superficial properties of PLA. Polymers 2020, 12, 2111. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.H.; Suen, M.C.; Yao, W.H.; Yeh, J.T.; Wu, C.S.; Tsou, C.Y.; Chiu, S.H.; Chen, J.C.; Wang, R.Y.; Lin, S.M.; et al. Preparation and characterization of bioplastic-based green renewable composites from tapioca with acetyl tributyl citrate as a plasticizer. Materials 2014, 7, 5617–5632. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, N.; Ma, X. Fabrication and characterization of poly(lactic acid)/acetyl tributyl citrate/carbon black as conductive polymer composites. Biomacromolecules 2008, 9, 1050–1057. [Google Scholar] [CrossRef]
- Jing, J.; Qiao, Q.; Jin, Y.; Ma, C.; Cai, H.; Meng, Y.; Cai, Z.; Feng, D. Molecular and mesoscopic dynamics simulations on the compatibility of PLA/plasticizer blends. Chin. J. Chem. 2012, 30, 133–138. [Google Scholar] [CrossRef]
- Shirai, M.A.; Grossmann, M.V.E.; Mali, S.; Yamashita, F.; Garcia, P.S.; Müller, C.M.O. Development of biodegradable flexible films of starch and poly(lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Lv, S.; Liu, X.; Gu, J.; Jiang, Y.; Tan, H.; Zhang, Y. Effect of glycerol introduced into PLA based composites on the UV weathering behavior. Constr. Build. Mater. 2017, 144, 525–531. [Google Scholar] [CrossRef]
- Müller, P.; Bere, J.; Fekete, E.; Móczó, J.; Nagy, B.; Kállay, M.; Gyarmati, B.; Pukánszky, B. Interactions, structure and properties in PLA/plasticized starch blends. Polymer 2016, 103, 9–18. [Google Scholar] [CrossRef]
- Preechawong, D.; Peesan, M.; Supaphol, P.; Rujiravanit, R. Preparation and characterization of starch/poly(L-lactic acid) hybrid foams. Carbohydr. Polym. 2005, 59, 329–337. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Avolio, R.; Castaldo, R.; Gentile, G.; Ambrogi, V.; Fiori, S.; Avella, M.; Cocca, M.; Errico, M.E. Plasticization of poly(lactic acid) through blending with oligomers of lactic acid: Effect of the physical aging on properties. Eur. Polym. J. 2015, 66, 533–542. [Google Scholar] [CrossRef]
- Volpe, V.; De Feo, G.; De Marco, I.; Pantani, R. Use of sunflower seed fried oil as an ecofriendly plasticizer for starch and application of this thermoplastic starch as a filler for PLA. Ind. Crop. Prod. 2018, 122, 545–552. [Google Scholar] [CrossRef]
- Santos, E.F.; Oliveira, R.V.B.; Reiznautt, Q.B.; Samois, D.; Nachtigall, S.M.B. Sunflower-oil biodiesel-oligoesters/polylactide blends: Plasticizing effect and ageing. Polym. Test. 2014, 39, 23–29. [Google Scholar] [CrossRef]
- Nagy, B.; Miskolczi, N.; Eller, Z. Improving mechanical properties of PLA/starch blends using masterbatch containing vegetable oil based active ingredients. Polymers 2021, 13, 2981. [Google Scholar] [CrossRef] [PubMed]
- Bouti, M.; Irinislimane, R.; Belhaneche-Bensemra, N. Properties investigation of epoxidized sunflower oil as bioplasticizer for poly(lactic acid). J. Polym. Environ. 2022, 30, 232–245. [Google Scholar] [CrossRef]
- Wadhi, M.M.; Weliam, R. Effect of epoxidized sunflower oil on polylactic acid properties. Res. Chem. Intermed. 2014, 40, 399–406. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crop. Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Pawlak, F.; Aldas, M.; Parres, F.; López-Martínez, J.; Arrieta, M.P. Silane-functionalized sheep wool fibers from dairy industry waste for the development of plasticized PLA composites with maleinized linseed oil for injection-molded parts. Polymers 2020, 12, 2523. [Google Scholar] [CrossRef]
- Pawlak, F.; Aldas, M.; López-Martínez, J.; Samper, M.D. Effect of different compatibilizers on injection-molded green fiber-reinforced polymers based on poly(lactic acid)-maleinized linseed oil system and sheep wool. Polymers 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Mysiukiewicz, O.; Barczewski, M. Crystallization of polylactide-based green composites filled with oil-rich waste fillers. J. Polym. Res. 2020, 27, 374. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Dolores Samper, M.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind. Crop. Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Boronat, T.; Quiles-Carrillo, L.; Fenollar, O.; Dominici, F.; Torre, L. Valorization of cotton industry byproducts in green composites with polylactide. J. Polym. Environ. 2020, 28, 2039–2053. [Google Scholar] [CrossRef]
- Dai, X.; Xiong, Z.; Na, H.; Zhu, J. How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos. Sci. Technol. 2014, 90, 9–15. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Tyan, W.-Q.; Zhang, H.; Weng, Y.-H.; Li, Y.-D.; Zeng, J.-B. Fully biobased polylactide/epoxidized soybean oil resin blends with balanced stiffness and toughness by dynamic vulcanization. Polym. Test. 2019, 78, 105981. [Google Scholar] [CrossRef]
- Aydın, R.S.T.; Akyol, E.; Hazer, B. Influence of soybean oil blending with polylactic acid (PLA) films: In vitro and in vivo evaluation. J. Am. Oil Chem. Soc. 2017, 94, 413–424. [Google Scholar] [CrossRef]
- Aydın, R.S.T.; Akyol, E.; Hazer, B. Soybean oil based polylactic acid membranes: Synthesis and degradation characteristics. J. Polym. Environ. 2018, 26, 1262–1271. [Google Scholar] [CrossRef]
- Goswami, S.R.; Nair, S.S.; Zhang, X.; Tanguy, N.; Yan, N. Starch maleate/epoxidized soybean oil/polylactic acid films with improved ductility and biodegradation potential for packaging fatty foods. ACS Sustain. Chem. Eng. 2022, 10, 14185–14194. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, J.; Fei, M.-E.; Qiu, R.; Sakai, E. Manufacturing of thermally remoldable blends from epoxidized soybean oil and poly(lactic acid) via dynamic cross-linking in a twin-screw extruder. Ind. Eng. Chem. Res. 2018, 57, 7516–7524. [Google Scholar] [CrossRef]
- Zych, A.; Perotto, G.; Trojanowska, D.; Tedeschi, G.; Bertolacci, L.; Francini, N.; Athanassiou, A. Super tough polylactic acid plasticized with epoxidized soybean oil methyl ester for flexible food packaging. ACS Appl. Polym. Mater. 2021, 3, 5087–5095. [Google Scholar] [CrossRef]
- He, Y.; Zhao, T.-H.; Li, Y.-D.; Wang, M.; Zeng, J.-B. Toughening polylactide by dynamic vulcanization with castor oil and different types of diisocyanates. Polym. Test. 2017, 59, 470–477. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Arantes, I.V.S.; Whittingham, M.J.; Sigley, E.; Kalinke, C.; Janegitz, B.C.; Bonacin, J.A.; Paixão, T.R.L.C.; Banks, C.E. Utilising bio-based plasticiser castor oil and recycled PLA for the production of conductive additive manufacturing feedstock and detection of bisphenol A. Green Chem. 2023, 25, 5591–5600. [Google Scholar] [CrossRef]
- Perez-Nakai, A.; Lerma-Canto, A.; Dominguez-Candela, I.; Ferri, J.M.; Fombuena, V. Novel epoxidized brazil nut oil as a promising plasticizing agent for PLA. Polymers 2023, 15, 1997. [Google Scholar] [CrossRef] [PubMed]
- Malwela, T.; Ray, S.S. Enzymatic degradation behavior of nanoclayreinforcedbiodegradable PLA/PBSA blend composites. Int. J. Biol. Macromol. 2015, 77, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Effect of modified starch and nanoclay particles on biodegradabilityand mechanical properties of cross-linked poly lactic acid. Carbohydr. Polym. 2015, 124, 237–244. [Google Scholar] [CrossRef]
- Wokadala, O.C.; Ray, S.S.; Bandyopadhay, J.; Wesley-Smith, J.; Emmambux, N.M. Morphology, thermal properties and crystallization kinetics of ternary blends of the polylactide and starch biopolymers and nanoclay: The role of nanoclay hydrophobicity. Polymer 2015, 71, 82–92. [Google Scholar] [CrossRef]
- Ayana, B.; Suin, S.; Khatua, B.B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lacticacid)(PLA)/clay nanocomposites using unmodified nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar] [CrossRef]
- Eğri, Ö.; Salimi, K.; Eğri, S.; Pişkin, E.; Rzayev, Z.M.O. Fabrication and characterization of novel starch-grafted poly l-lacticacid/montmorillonite organoclay nanocomposites. Carbohydr. Polym. 2016, 137, 111–118. [Google Scholar] [CrossRef]
- Tanoue, S.; Hasook, A.; Iemoto, Y.; Unryu, T. Preparation of poly(lactic acid)/poly(ethylene glycol)/organoclay nanocomposites by melt compounding. Polym. Compos. 2006, 27, 256–263. [Google Scholar] [CrossRef]
- Ludwiczak, J.; Frąckowiak, S.; Leluk, K. Study of thermal, mechanical and barrier properties of biodegradable PLA/PBAT films with highly oriented MMT. Materials 2021, 14, 7189. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guel, M.; Cabello-Alvarado, C.; Romero-Huitzil, R.L.; Rodríguez-Fernández, O.S.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Medellín-Banda, D.I.; Gallardo-Vega, C.; Cepeda-Garza, J. Nanocomposite PLA/C20A nanoclay by ultrasound-assisted melt extrusion for adsorption of uremic toxins and methylene blue dye. Nanomaterials 2021, 11, 2477. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Morreale, M.; La Mantia, F.P. The effects of nanoclay on the mechanical properties, carvacrol release and degradation of a PLA/PBAT blend. Materials 2020, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Coppola, B.; Cappetti, N.; Di Maio, L.; Scarfato, P.; Incarnato, L. 3D printing of PLA/clay nanocomposites: Influence of printing temperature on printed samples properties. Materials 2018, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Solarski, S.; Mahjoubi, F.; Ferreira, M.; Devaux, E.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da Silva Ferreira, A.; et al. (Plasticized) Polylactide/clay nanocomposite textile: Thermal, mechanical, shrinkage and fire properties. J. Mater. Sci. 2007, 42, 5105–5117. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solid Polymers, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 379–447. [Google Scholar]
- Deblieck, R.A.C.; van Beek, D.J.M.; Remerie, K.; Ward, I.M. Failure mechanisms in polyolefins: The role of crazing, shear yielding and the entanglement network. Polymer 2011, 52, 2979–2990. [Google Scholar] [CrossRef]
- Venkatesan, S.; Basu, S. Investigations into crazing in glassy amorphous polymers through molecular dynamics simulations. J. Mech. Phys. Solids 2015, 77, 123–145. [Google Scholar] [CrossRef]
- Volynskii, A.L.; Bakeev, N.F. Solvent Crazing of Polymers; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1995. [Google Scholar]
- Volynskii, L.; Bakeev, N.F. Surface Phenomena in the Structural and Mechanical Behaviour of Solid Polymers, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Moskvina, M.A.; Ivanova, O.A.; Potseleev, V.V.; Demina, V.A.; Nikonorova, N.I.; Bakirov, A.V.; Sedush, N.G.; Chvalun, S.N. Porous polylactide prepared by the delocalized crazing as a template for nanocomposite materials. Mendeleev Commun. 2020, 30, 171–173. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Nikonorova, N.I.; Moskvina, M.A.; Efimov, A.V.; Khavpachev, M.A.; Volynskii, A.L. Influence of liquid media on the craze initiation in amorphous polylactide. Polymer 2018, 142, 43–47. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Efimov, A.V.; Moskvina, M.A.; Ivanova, O.A.; Nikonorova, N.I.; Zezin, S.B.; Bakirov, A.V.; Volynskii, A.L. Nanocomposites based on porous polylactide, obtained by crazing mechanism in water-ethanol solution, and calcium phosphates. Polym. Sci. Ser. A 2018, 60, 845–853. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Moskvina, M.A.; Nikonorova, N.I.; Efimov, A.V.; Garina, E.S.; Grokhovskaya, T.E.; Ivanova, O.A.; Bakirov, A.V.; Sedush, N.G.; Chvalun, S.N. Hydrolytic degradation of polylactide films deformed by the environmental crazing mechanism. Eur. Polym. J. 2020, 139, 110000. [Google Scholar] [CrossRef]
- Khavpachev, M.A.; Trofimchuk, E.S.; Nikonorova, N.I.; Garina, E.S.; Moskvina, M.A.; Efimov, A.V.; Demina, V.A.; Bakirov, A.V.; Sedush, N.G.; Potseleev, V.V.; et al. Bioactive polylactide fibrous materials prepared by crazing mechanism. Macromol. Mater. Eng. 2020, 205, 2000163. [Google Scholar] [CrossRef]
- Potseleev, V.V.; Trofimchuk, E.S.; Nikonorova, N.I. Kinetics of the release of brilliant green from nanoporous polylactide obtained by a crazing mechanism. Mendeleev Commun. 2021, 31, 515–516. [Google Scholar] [CrossRef]
- Zhou, H.; Song, Z.; Cai, S. Toughening of poly(lactide acid) with low crystallinity through biaxial poststretching. J. Polym. Sci. 2020, 58, 3488–3495. [Google Scholar] [CrossRef]
- Cui, L.; Imre, B.; Tatraaljai, D.; Pukánszky, B. Physical ageing of Poly(Lactic acid): Factors and consequences for practice. Polymer 2020, 186, 122014. [Google Scholar] [CrossRef]
- Stoclet, G.; Lefebvre, J.M.; Séguéla, R.; Vanmansart, C. In-situ SAXS study of the plastic deformation behavior of polylactide upon cold-drawing. Polymer 2014, 55, 1817–1828. [Google Scholar] [CrossRef]
- Billimoria, K.; Heeley, E.L.; Parsons, N.; Figiel, Ł. An investigation into the crystalline morphology transitions in poly-L-lactic acid (PLLA) under uniaxial deformation in the quasi-solid-state regime. Eur. Polym. J. 2018, 101, 127–139. [Google Scholar] [CrossRef]
- Xu, R.-J.; Tian, Z.-Q.; Xie, J.-Y.; Lei, C.-H. The structure transformation of pre-oriented polylactic acid film during uniaxial stretching at room temperature. Polym. Cryst. 2019, 2, e10072. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E. Mechanisms of plastic deformation in biodegradable polylactide/poly(1,4-cis-isoprene) blends. J. Appl. Polym. Sci. 2011, 124, 4579–4589. [Google Scholar] [CrossRef]
- Stoclet, G. Strain-induced structural evolution of poly(L-lactide) and poly(D-lactide) blends. Polymer 2016, 99, 231–239. [Google Scholar] [CrossRef]
- Brüster, B.; Martin, A.; Bardon, J.; Koutsawa, Y.; Bernstorff, S.; Raquez, J.-M.; André, S.; Dubois, P.; Addiego, F. In situ multiscale study of deformation heterogeneities in polylactide-based materials upon drawing: Influence of initial crystallinity and plasticization. J. Polym. Sci. Part B Polym. Phys. 2018, 66, 1452–1468. [Google Scholar] [CrossRef]
- Goutianos, S.; Van der Schueren, L.; Beauson, J. Failure mechanisms in unidirectional self-reinforced biobased composites based on high stiffness PLA fibres. Compos. Part A 2019, 117, 169–179. [Google Scholar] [CrossRef]
- Park, S.D.; Todo, M.; Arakawa, K.; Koganemaru, M. Effect of crystallinity and loading-rate on mode I fracture behavior of poly(lactic acid). Polymer 2006, 47, 1357–1363. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D.; Li, H. Extrusion foaming of semi-crystalline PLA and PLA/thermoplastic starch blends. Macromol. Biosci. 2007, 7, 907–920. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]


| Approach | Tensile Strength (MPa) | Elongation at Breakup (%) | Impact Strength (kJ/m2) | Tg (°C) | Ref. |
|---|---|---|---|---|---|
| Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) | 31 | 157 | n.d. | 30 | [33] |
| Multiblock copolymer of poly(L-lactide-co-ε-caprolactone) (80/20 (w/w)) (reactive mixing with hexamethylene diisocyanate) | 12 | 57 | n.d. | n.d. | [24] |
| Poly(L-lactide-co--ethylene-glycidyl methacrylate) (95/5 (w/w)) (reactive mixing with dicumyl peroxide) | 67 | 270 | 6 | 66 | [29] |
| Poly(L-lactide) and block copolymer of poly(L-lactide) and poly(caprolactone) (85/15 (w/w)) | 47 | 210 | n.d. | 61 | [23] |
| Poly(lactic acid) and poly(butylene adipate-co-terephthalate) blend (85/15 (w/w)) | 24 | 485 | n.d. | 61 | [39] |
| Poly(lactic acid) and poly(butylene succinate adipate) blend (80/20 (w/w)) | 47 | 25 | 5 | 59 | [50] |
| Poly(L-lactide) and poly(ethylene glycol) 400 (90/10 (w/w)) | 32.5 | 140 | n.d. | 50 | [61] |
| Poly(L-lactide) and L-lactic acid oligomer (75/25 (w/w)) | n.d. | 300 | n.d. | 31 | [89] |
| Poly(lactic acid) with epoxidized sunflower oil (80/20 (w/w)) | 18 | 230 | n.d. | n.d. | [95] |
| Poly(lactic acid) (Bioflex (BF), trade name Bio-Flex® F2110) + carvacrol + montmorillonite (90/5/5 (w/w/w)) | 8 | 300 | n.d. | n.d. | [122] |
| Poly(lactic acid) in n-pentanol (AR grade) (uniaxial stretching) | 12.5 | 570 | n.d. | n.d. | [131] |
| Pre-oriented poly(lactic acid) (uniaxial stretching) | n.d. | 270 | n.d. | 62 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalygina, E.E.; Aleksanyan, K.V. Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review). Polymers 2024, 16, 87. https://doi.org/10.3390/polym16010087
Mastalygina EE, Aleksanyan KV. Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review). Polymers. 2024; 16(1):87. https://doi.org/10.3390/polym16010087
Chicago/Turabian StyleMastalygina, Elena E., and Kristine V. Aleksanyan. 2024. "Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review)" Polymers 16, no. 1: 87. https://doi.org/10.3390/polym16010087
APA StyleMastalygina, E. E., & Aleksanyan, K. V. (2024). Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review). Polymers, 16(1), 87. https://doi.org/10.3390/polym16010087








