Preparation of Polyoxymethylene/Exfoliated Molybdenum Disulfide Nanocomposite through Solid-State Shear Milling
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Sample Preparation
2.2.1. Preparation of POM/MoS2 Composite
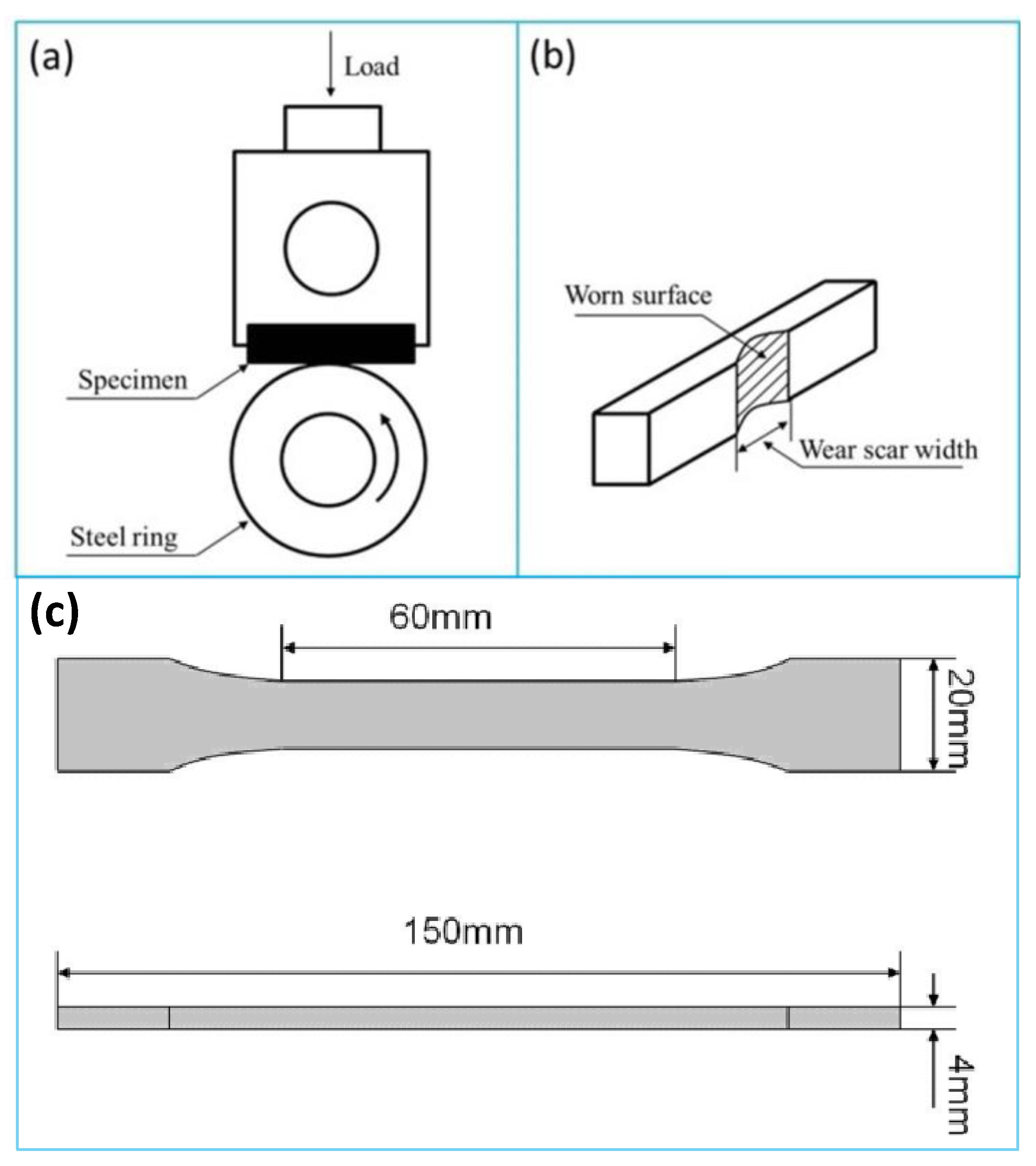
2.2.2. Preparation of POM/MoS2 Composite Samples for Tensile and Tribological Tests
2.3. Characterization
3. Results and Discussion
3.1. Preparation and Structure Characterization of POM/MoS2 Nanocomposite
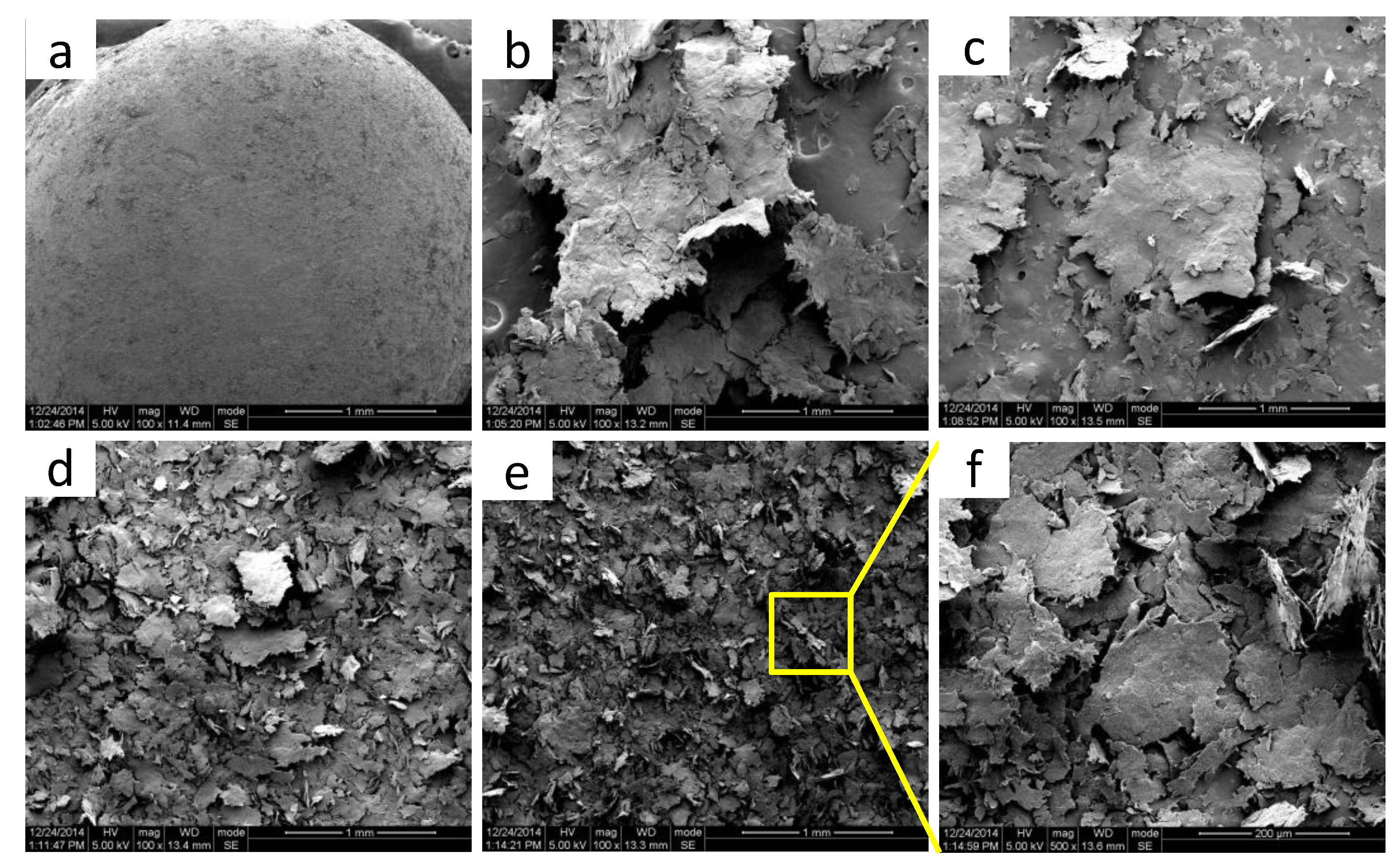
3.1.1. Morphology Evolution of S3M POM/MoS2 Co-Powders
3.1.2. The Dispersion of MoS2 Particles in POM Matrix
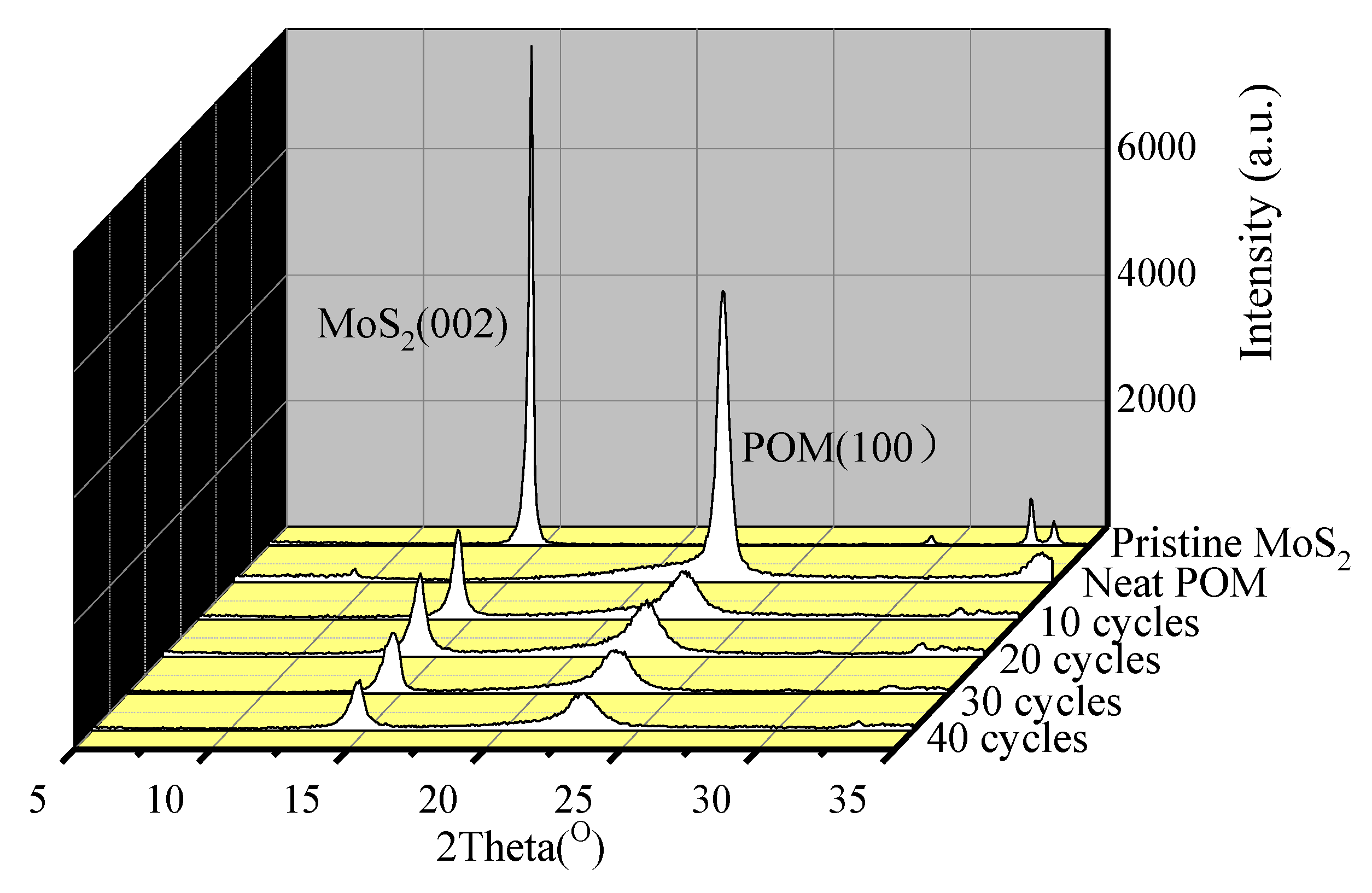
3.1.3. Crystal Structure of S3M POM/MoS2 Co-Powders
3.1.4. The Microscopic Morphology of MoS2 in the POM Matrix
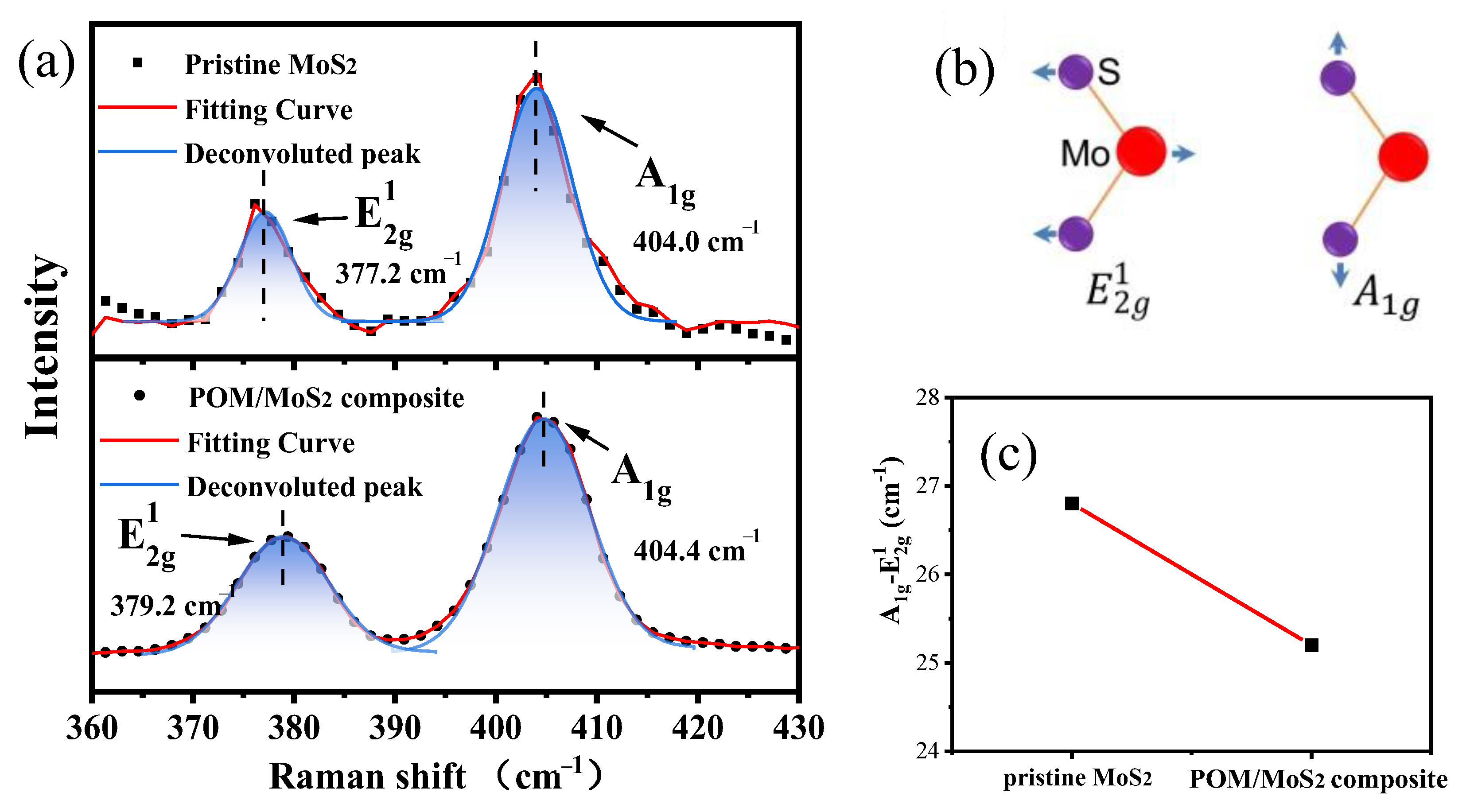
3.1.5. Raman Spectra of Pristine MoS2 and S3M MoS2/POM Nanocomposite
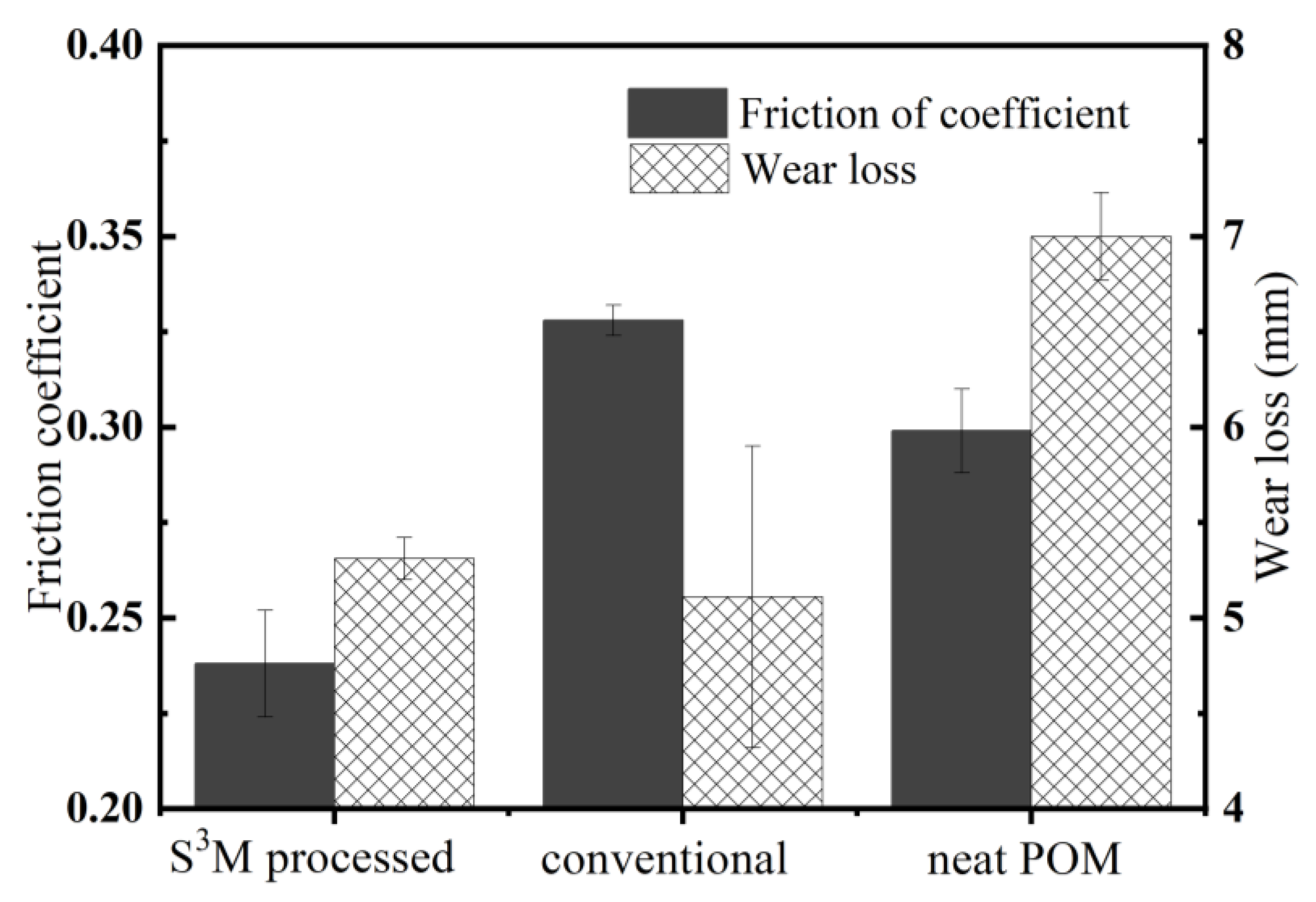
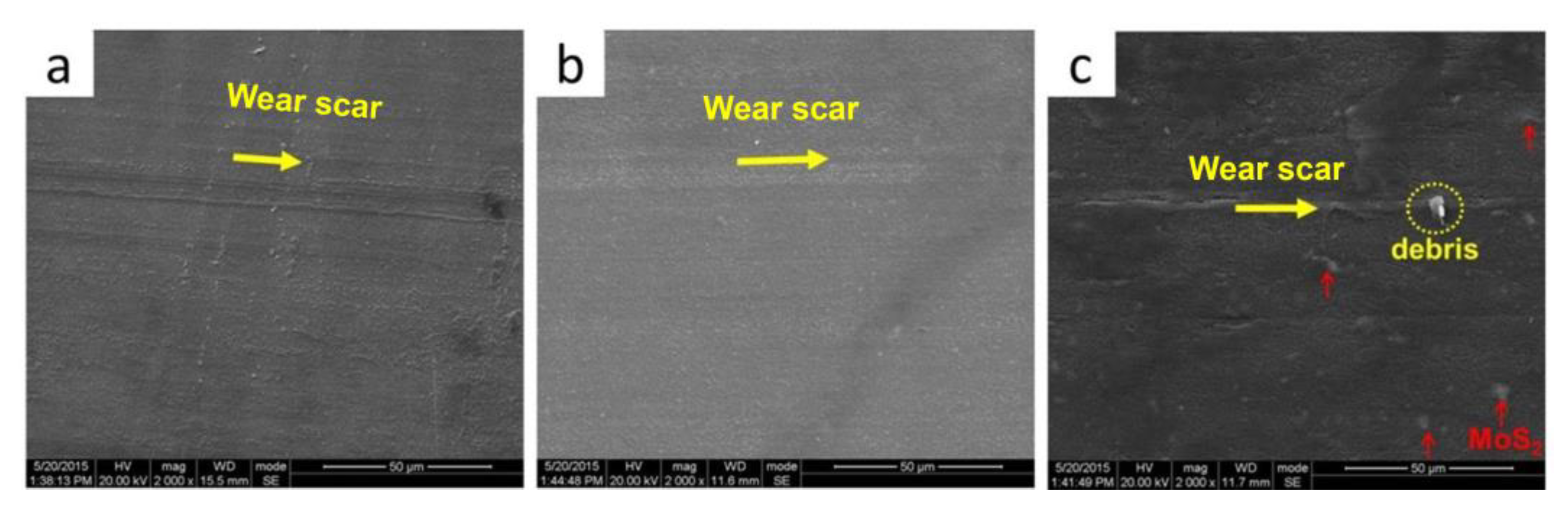
3.2. Tribological Performance of POM/MoS2 Nanocomposite
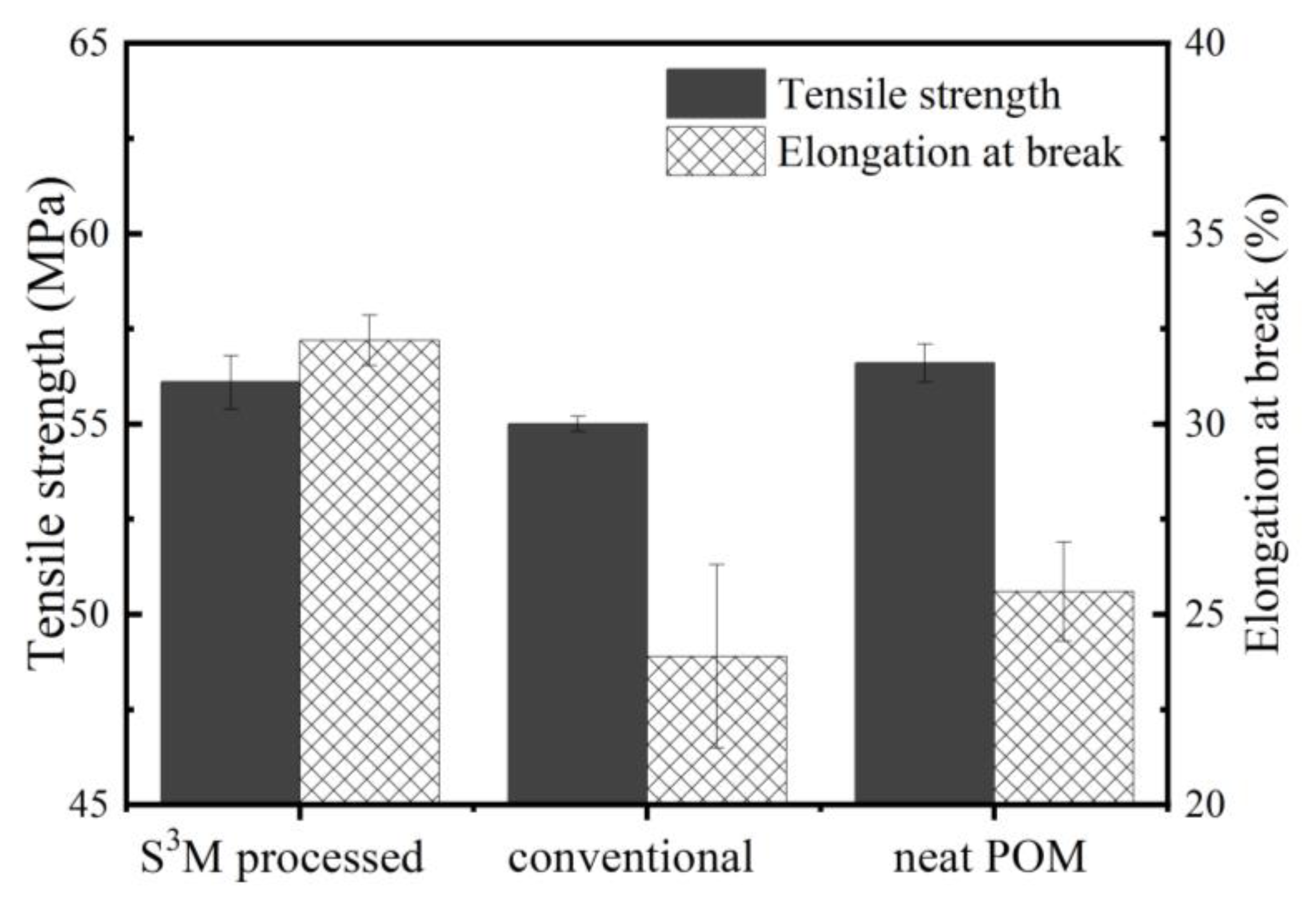
3.3. Mechanical Performance of POM/MoS2 Nanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ao, D.; Fan, X.; Zeng, Z.; Zhu, M.; Ye, X.; Shang, L. Tribological properties of graphene quantum dot hybrid polyethylene glycol lubricated molybdenum disulfide films. Tribol. Int. 2024, 193, 109437. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Hu, K.H.; Han, C.L.; Hu, X.G.; Xu, Y.F. Morphological Influence of Molybdenum Disulfide on the Tribological Properties of Rapeseed Oil. Tribol. Lett. 2013, 49, 513–524. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Y.; Liu, B.; Yu, L.; Tong, Y.; Yan, M.; Yang, Z.; Hao, Y.; Shangguan, L.; Zhang, S. Research progresses of nanomaterials as lubricant additives. Friction 2024, 1–45. [Google Scholar] [CrossRef]
- Rapoport, L.; Moshkovich, A.; Perfilyev, V.; Laikhtman, A.; Lapsker, I.; Yadgarov, L.; Rosentsveig, R.; Tenne, R. High Lubricity of Re-Doped Fullerene-Like MoS2 Nanoparticles. Tribol. Lett. 2011, 45, 257–264. [Google Scholar] [CrossRef]
- Chhowalla, M.; Amaratunga, G.A.J. Thin films of fullerene-like MoS2 nanoparticles with ultra-low friction and wear. Lett. Nat. 2000, 407, 164–167. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Huang, F.; Liu, J.S. The Effect of Morphology on the Tribological Properties of MoS2 in Liquid Paraffin. Tribol. Lett. 2010, 40, 155–165. [Google Scholar] [CrossRef]
- Bao, W.; Cai, X.; Kim, D.; Sridhara, K.; Fuhrer, M.S. High mobility ambipolar MoS2 field-effect transistors: Substrate and dielectric effects. Appl. Phys. Lett. 2013, 102, 042104. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Cui, Y.L.S.; Zhang, Y.Z.; Cao, Z.J.; Gu, J.N.; Du, Z.G.; Li, B.; Yang, S.B. A perspective on highentropy twodimensional materials. SusMat 2022, 2, 65–75. [Google Scholar] [CrossRef]
- Enyashin, A.; Gemming, S.; Seifert, G. Nanosized allotropes of molybdenum disulfide. Eur. Phys. J. Spec. Top. 2007, 149, 103–125. [Google Scholar] [CrossRef]
- Visic, B.; Dominko, R.; Gunde, M.K.; Hauptman, N.; Skapin, S.D.; Remskar, M. Optical properties of exfoliated MoS2 coaxial nanotubes—Analogues of graphene. Nanoscale Res. Lett. 2011, 6, 593. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, C.; Yan, B.; Lin, L.; Wang, S.; Gong, T.; Guo, J.; Huang, W.; Zhang, X. A highly sensitive MoS2/MoTe2 heterostructure enhanced by localized surface plasmon effect for broad-spectrum photodetection. Scr. Mater. 2024, 245, 115985. [Google Scholar] [CrossRef]
- Guo, H.; Montes-García, V.; Peng, H.; Samorì, P.; Ciesielski, A. Molecular Connectors Boosting the Performance of MoS2 Cathodes in Zinc-Ion Batteries. Small 2024, 2310338. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-H.; Yang, Z.-G.; Li, X.-H. Study on the friction and wear behavior of POM/Al2O3 nanocomposites. Wear 2008, 264, 693–700. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Wang, Q.; Zhu, L.; Yang, L.; Xu, D. Solid-State Utilization of Chitin to Construct Flexible Films with Enhanced Biocompatibility and Mechanical Performance. ACS Sustain. Chem. Eng. 2024, 12, 4497–4505. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Wang, Q. The experiment and simulation of enhanced mechanical performance for polyethylene terephthalate/high-density polyethylene composites via domain size control. Polym. Adv. Technol. 2023, 34, 3356–3369. [Google Scholar] [CrossRef]
- Pei, H.; Shi, S.; Chen, Y.; Xiong, Y.; Lv, Q. Combining Solid-State Shear Milling and FFF 3D-Printing Strategy to Fabricate High-Performance Biomimetic Wearable Fish-Scale PVDF-Based Piezoelectric Energy Harvesters. ACS Appl. Mater. Interfaces 2022, 14, 15346–15359. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhu, H.; Liu, Z.; Li, L. Poly(vinyl alcohol)/Kaolin Barrier Films with Superior Dispersion Fabricated by Solid-State Shear Milling and Biaxial Stretching. Ind. Eng. Chem. Res. 2022, 61, 10106–10116. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, S.; Wang, Q. Recycling of waste wind turbine blades for high-performance polypropylene composites. J. Appl. Polym. Sci. 2024, e55474. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Pu, Z.; He, J.; Yang, S. Transforming waste to treasure: Superhydrophobic coatings from recycled polypropylene for high-value application. Prog. Org. Coat. 2024, 188, 108248. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Wang, F.; Chen, Y. The cutting of multi-walled carbon nanotubes and their strong interfacial interaction with polyamide-6 in the solid state. Carbon 2006, 44, 2708–2714. [Google Scholar] [CrossRef]
- Evangelista, I.; Wencel, D.; Beguin, S.; Zhang, N.; Gilchrist, M.D. Influence of Surface Texturing on the Dry Tribological Properties of Polymers in Medical Devices. Polymers 2023, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P.; De Baets, P.; Schoukens, G.; Quintelier, J. Wear transitions and stability of polyoxymethylene homopolymer in highly loaded applications compared to small-scale testing. Tribol. Int. 2007, 40, 819–833. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, S.; Nie, M.; Wang, Q. Effect of blend composition on crystallization behavior of polyoxymethylene/poly(ethylene oxide) crystalline/crystalline blends. J. Polym. Res. 2011, 19, 9787. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, M.; Lu, C. Morphological and structural development of hardwood cellulose during mechanochemical pretreatment in solid state through pan-milling. Cellulose 2007, 14, 447–456. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, H.; Wu, X. Layer-by-Layer Thinning of MoS2 by Plasma. ACS Nano 2013, 7, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Plechinger, G.; Heydrich, S.; Eroms, J.; Weiss, D.; Schuller, C.; Korn, T. Raman spectroscopy of the interlayer shear mode in few-layer MoS2 flakes. Appl. Phys. Lett. 2012, 101, 101906. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Li, C. Thermal conductivity and tribological properties of POM-Cu composites. Polym. Eng. Sci. 2010, 50, 2153–2159. [Google Scholar] [CrossRef]
- Sun, L.-H.; Yang, Z.-G.; Li, X.-H. Mechanical and tribological properties of polyoxymethylene modified with nanoparticles and solid lubricants. Polym. Eng. Sci. 2008, 48, 1824–1832. [Google Scholar] [CrossRef]
- Tannous, J.; Dassenoy, F.; Lahouij, I.; Le Mogne, T.; Vacher, B.; Bruhács, A.; Tremel, W. Understanding the Tribochemical Mechanisms of IF-MoS2 Nanoparticles Under Boundary Lubrication. Tribol. Lett. 2010, 41, 55–64. [Google Scholar] [CrossRef]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and Breaking of Ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef]
- Hu, K.H.; Wang, J.; Schraube, S.; Xu, Y.F.; Hu, X.G.; Stengler, R. Tribological properties of MoS2 nano-balls as filler in polyoxymethylene-based composite layer of three-layer self-lubrication bearing materials. Wear 2009, 266, 1198–1207. [Google Scholar] [CrossRef]
- Liang, Y.N.; Li, S.Z.; Li, D.F.; Li, S. Some developments for single-pass pendulum scratching. Wear 1996, 199, 66–73. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, G. Polyethylene Toughened by Rigid Inorganic Particles. Polym. Eng. Sci. 1992, 32, 94–97. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Zhou, X.; Yang, S.; Tan, J.; Chen, M.; Chen, Y.; Zhang, H.; Zhu, X.; Wu, S.; Gu, H. Preparation of Polyoxymethylene/Exfoliated Molybdenum Disulfide Nanocomposite through Solid-State Shear Milling. Polymers 2024, 16, 1334. https://doi.org/10.3390/polym16101334
Feng S, Zhou X, Yang S, Tan J, Chen M, Chen Y, Zhang H, Zhu X, Wu S, Gu H. Preparation of Polyoxymethylene/Exfoliated Molybdenum Disulfide Nanocomposite through Solid-State Shear Milling. Polymers. 2024; 16(10):1334. https://doi.org/10.3390/polym16101334
Chicago/Turabian StyleFeng, Shuo, Xinwen Zhou, Sen Yang, Jiayu Tan, Meiqiong Chen, Yinghong Chen, Huarong Zhang, Xu Zhu, Shulong Wu, and Haidong Gu. 2024. "Preparation of Polyoxymethylene/Exfoliated Molybdenum Disulfide Nanocomposite through Solid-State Shear Milling" Polymers 16, no. 10: 1334. https://doi.org/10.3390/polym16101334
APA StyleFeng, S., Zhou, X., Yang, S., Tan, J., Chen, M., Chen, Y., Zhang, H., Zhu, X., Wu, S., & Gu, H. (2024). Preparation of Polyoxymethylene/Exfoliated Molybdenum Disulfide Nanocomposite through Solid-State Shear Milling. Polymers, 16(10), 1334. https://doi.org/10.3390/polym16101334









